Introduction
Cholangiocarcinoma (CC) is the primary epithelial
cell cancer of the biliary tract and the second most prevalent form
of primary hepatic tumor (1). Due
to a late diagnosis and no effective therapy, CC is associated with
a high rate of mortality and a poor prognosis (1). Little is known about the molecular
changes and mechanisms that are involved in the pathogenesis and
pathophysiology of CC.
Phosphorylation on serine or threonine residues that
precede proline (pSer/Thr-Pro) represents a key regulatory
mechanism for controlling the function of signaling molecules in a
cellular process. The pSer/Thr-Pro exists as two types of
conformations that are regulated by the peptidyl-prolyl isomerase,
PIN1. PIN1 changes its conformation and thereby regulates the
function of phosphoproteins (2–4).
PIN1-mediated prolyl-isomerization potentiates the progression of
the cell cycle and cell proliferation through the regulation of
target genes, including cyclin D, cyclin E, jun, myc and TP53
(3,4). Whether PIN1 acts as a tumor promoter
or suppressor remains a controversial issue. PIN1 may play a
positive or negative role in tumorigenesis, possibly in a cell-type
selective manner (4). To date,
however, no studies have examined the significance of the
expression and the role of PIN1 in the pathogenesis of extrahepatic
CC (ECC).
The present study examined PIN1 expression in
surgical human ECC specimens and its association with
clinicopathological factors. Furthermore, the associations between
PIN1 expression and the rate of cellular proliferation (Ki-67
index) and between PIN1 expression and the expression of TP53, a
tumor suppressor gene, were also examined. Finally, the effects of
silencing PIN1 using small interfering RNA (siRNA) on the growth,
migration and invasion of CC cells was investigated.
Materials and methods
Patients and specimens
The present study was approved by the Institutional
Review Board (IRB) of Chonbuk National University Hospital (Jeonju,
Chonbuk, South Korea). Written informed consent was waived by the
IRB due to the retrospective nature of the study. A total of 67 ECC
specimens and their corresponding non-neoplastic tissues that were
obtained from patients who underwent surgical resection at the
Chonbuk National University Hospital between 1998 and 2009 were
used to examine the location and expression of PIN1. For
immunohistochemical staining, 10% formalin-fixed, paraffin-embedded
tissue sections were prepared as 4-μm thick tissue samples. Of the
67 patients with ECC, 49 (73.1%) were male and 18 (26.9%) were
female. The tumors were histologically graded as 24 cases of
well-differentiated (35.8%), 38 cases of moderately-differentiated
(56.7%) and five cases of poorly-differentiated (7.5%) ECC. The
clinicopathological data were obtained from the patients who were
previously hospitalized at Chonbuk National University Hospital
using a retrospective review of the medical records. The tumors
were staged according to the 2010 American Joint Committee on
Cancer tumor-node-metastasis classification system (5).
Cell lines and culture
The human CC RBE cell line was purchased from the
RIKEN BRC cell bank (Tsukuba, Japan). The cell line was maintained
in RPMI-1640 medium (Gibco, Grand Island, NY, USA) supplemented
with 10% fetal bovine serum (FBS) and penicillin/streptomycin
(Gibco), cultured at 37°C and 5% CO2 in a humidified
incubator.
Immunohistochemistry
The immunohistochemical staining was performed by a
polymer intense detection system using the Bond-Max Automatic
Stainer (Leica, Bannockburn, IL, USA), according to the
manufacturer’s instructions. Briefly, following antigen retrieval
(microwaved at high power for 10 min in 0.01 M citrate buffer; pH
6), the samples were incubated with anti-PIN1 (Santa Cruz
Biotechnology, Santa Cruz, CA, USA), anti-Ki-67 (Novocastra,
Newcastle, UK) and anti-TP53 (Novocastra) antibodies for 30 min.
Peroxidase activity was detected using the enzyme substrate,
3-amino-9-ethyl carbazole. The samples were subjected to an
immunohistochemistry analysis and their immunohistochemical
properties were rated according to a score that was calculated by
multiplying the intensity of the stain with the area of the stain.
The intensity of the cell staining was graded as follows: 0, no
staining; 1+, weak staining; 2+, moderate staining; and 3+, strong
staining. The area of staining was evaluated as follows: 0, 0–9% of
cells stained positive; 1+, 10–29% of cells stained positive; 2+,
30–69% of cells stained positive; and 3+, >70% of cells stained
positive. The maximum combined score was nine and the minimum score
was zero. If the combined score was ≥4, the tumor was considered
positive. Otherwise, the tumor was considered negative. The samples
with nuclear Ki-67 and TP53 staining of >20% of the tumor cells
were defined as positive.
siRNA transfection
siRNA was used to silence PIN1 expression. PIN1
siRNA and negative controls were purchased from Bioneer Corporation
(Daejeon, Korea). The sequences for PIN1 specific siRNA were
forward, 5′-CCAUUUGAAGACGCCUCGU(dTdT)-3′ and reverse,
5′-ACGAGGCGUCUUCAAAUGG(dTdT)-3′. The sequences for the negative
control were forward, 5′-CCUACGCCACCA AUUUCGU(dTdT)-3′ and reverse,
5′-ACGAAAUUGGUGGCGUAGG(dTdT)-3′.
Transfection of the siRNA was performed using the
lipofectamine RNAiMAX transfection reagent (Invitrogen, Carlsbad,
CA, USA), following the manufacturer’s instructions.
Western blotting
Western blotting of PIN1 in the RBE cell line was
performed as previously described (6). Briefly, the cell lysates were
subjected to denaturing sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) followed by electroblotting and
immunoblotting for anti-PIN1 (Santa Cruz Biotechnology). The blots
were developed using horseradish peroxidase conjugated anti-mouse
IgG secondary antibodies (Santa Cruz Biotechnology) and the immune
complexes were visualized using an enhanced chemiluminescence
detection system (Amersham Biosciences, Buckinghamshire, UK). The
blots were then analyzed using an LAS-3000 luminescent image
analyzer (Fuji Film, Tokyo, Japan).
Cell growth and proliferation assay
Cell growth was determined using the colorimetric
tetrazolium-derived
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoniumbromide (MTT)
assay (Sigma, St. Louis, MO, USA). DNA synthesis of the cells was
assessed by the bromodeoxyuridine (BrdU) incorporation assay (Roche
Applied Science, Mannheim, Germany). For the cell growth and
proliferation assay, 48 h after the transfection of the siRNA, the
cells of each group were reseeded in 96-well plates at a density of
5×103 cells/well. Following 24–120 h, the MTT and
incorporated BrdU were measured colorimetrically using a Bio-Rad
model 680 microtiter plate reader (Bio-Rad, Hercules, CA, USA) at
wavelengths of 560 and 450 nm, respectively.
In vitro assay of cellular migration and
invasion
Cellular migration and invasion were assayed using a
24-transwell migration chamber (Corning Life Sciences, Acton, MA,
USA) and a 24-transwell BioCoat Matrigel invasion chamber (BD
Biosciences, San Jose, CA, USA) with an 8-μm pore size
polyvinylpyrrolidone-free polycarbonate membrane, respectively,
following the manufacturers’ instructions. The cells that migrated
to or invaded the lower surface of the filter were counted under a
light microscope at ×100 magnification in five randomly selected
fields per well.
Statistical analysis
The statistical analysis was performed using SPSS
version 15.0 (SPSS Inc., Chicago, IL, USA). Data are presented as
mean ± standard deviation. The clinicopathological characteristics
were compared with PIN1 expression using the χ2 test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
PIN1 expression in the ECC tissue
samples
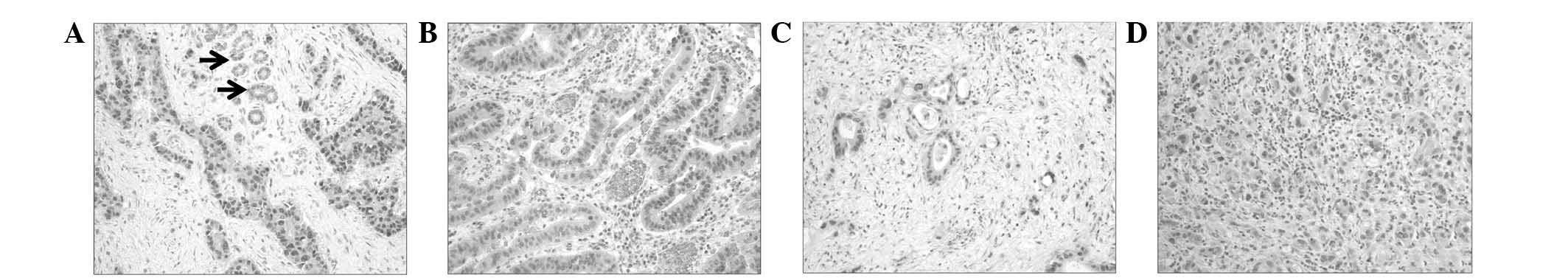
Of the 67 ECC tissue sections, 35 were PIN1-positive
(52.2%). The immunoreactive score for PIN1 was significantly higher
in the tumor cells (4.07±0.4) compared with that of the adjacent
non-neoplastic bile duct cells (1.19±0.4; P<0.001). In the CC
cells, PIN1 expression was predominantly localized to the nucleus.
However, in certain cells, it was present in the nucleus and the
cytoplasm (Fig. 1).
Correlation between PIN1 expression and
clinicopathological factors
PIN1 expression in ECC and its correlation with the
clinicopathological factors are presented in Table I. PIN1 expression was significantly
correlated with the cell proliferative rate (Ki-67 labeling index;
P=0.024). However, PIN1 expression was not significantly correlated
with other clinicopathological parameters, including tumor
differentiation, lymph node metastasis, distant metastasis, nerve
invasion, gross type and tumor stage. The positive expression of
TP53 was observed in 52.2% (35/67) of the total cases of ECC. There
was no significant correlation between PIN1 and TP53 expression in
the ECC cases.
 | Table IExpression of PIN1 in ECC and its
correlation with the clinicopathological parameters, Ki-67 labeling
index and TP53 expression. |
Table I
Expression of PIN1 in ECC and its
correlation with the clinicopathological parameters, Ki-67 labeling
index and TP53 expression.
| Category | Total, n | PIN1 expression, n
(%) | P-value |
|---|
|
|---|
| Negative | Positive |
|---|
| Differentiation | | | | 0.784 |
| Well | 24 | 12 (50.0) | 12 (50.0) | |
| Moderate | 38 | 17 (44.7) | 21 (55.3) | |
| Poor | 5 | 3 (60.0) | 2 (40.0) | |
| T category | | | | 0.834 |
| T1, T2 | 41 | 20 (48.8) | 21 (51.2) | |
| T3, T4 | 26 | 12 (46.2) | 14 (53.8) | |
| LN metastasis | | | | 0.13 |
| Absent | 51 | 27 (52.9) | 24 (47.1) | |
| Present | 16 | 5 (31.3) | 11 (68.7) | |
| Distant
metastasis | | | | 0.569 |
| Absent | 62 | 29 (46.8) | 33 (53.2) | |
| Present | 5 | 3 (60.0) | 2 (40.0) | |
| Nerve invasion | | | | 0.582 |
| Absent | 27 | 14 (51.9) | 13 (48.1) | |
| Present | 40 | 18 (45.0) | 22 (55.0) | |
| Gross type | | | | 0.285 |
| IG | 21 | 8 (38.1) | 13 (61.9) | |
| PI | 46 | 24 (52.2) | 22 (47.8) | |
| Stage | | | | 0.883 |
| I | 34 | 17 (50.0) | 17 (50.0) | |
| II | 23 | 10 (43.5) | 13 (56.5) | |
| III | 5 | 2 (40.0) | 3 (60.0) | |
| IV | 5 | 3 (60.0) | 2 (40.0) | |
| Ki-67 labeling
index, % | | | | 0.024 |
| <20 | 15 | 11 (73.3) | 4 (26.7) | |
| ≥20 | 52 | 21 (40.4) | 31 (59.6) | |
| TP53
expression | | | | 0.183 |
| Negative | 32 | 18 (56.3) | 14 (43.7) | |
| Positive | 35 | 14 (40.0) | 21 (60.0) | |
Effect of PIN1 silencing on cell growth,
proliferation, migration and invasion
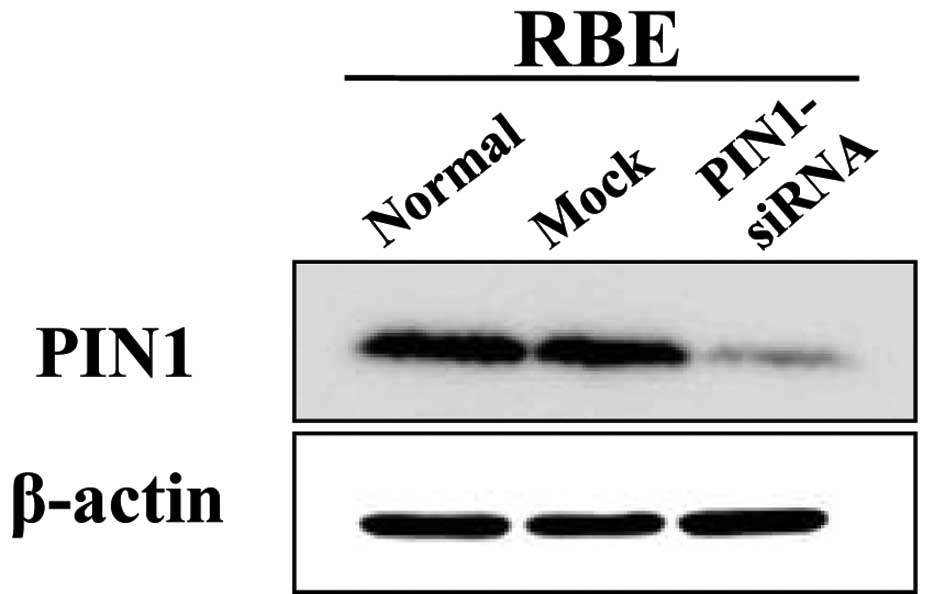
The transfection with PIN1 siRNA resulted in a
marked decrease in the expression of PIN1 at 48 h post-transfection
in the RBE cells (Fig. 2).
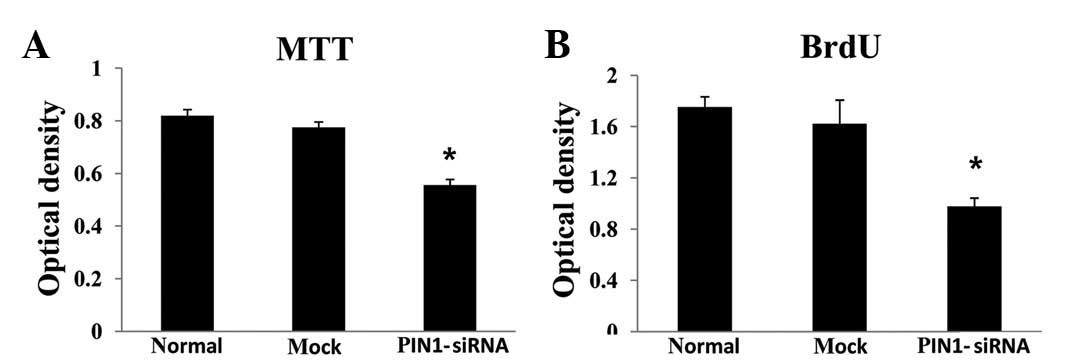
Silencing PIN1 gene expression in the RBE cells using PIN1 siRNA
resulted in a significant inhibition of cell growth compared with
the control (P<0.05; Fig. 3A).
In the PIN1 siRNA-transfected cells, there was a significant
decrease in the BrdU incorporation compared with the control
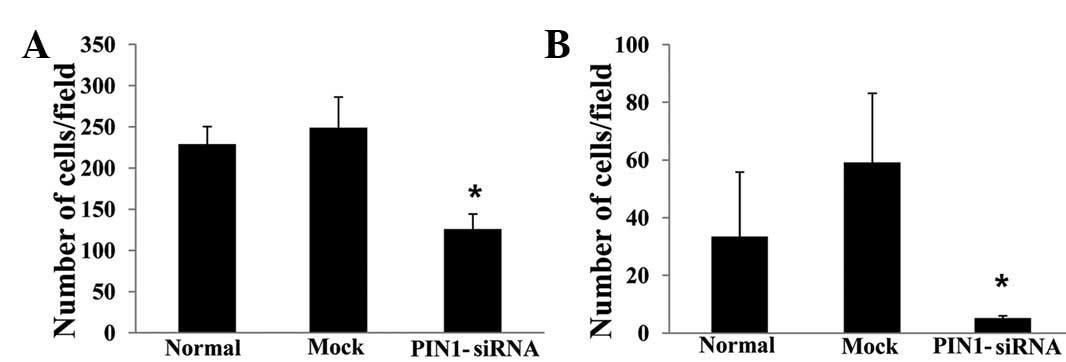
(P<0.05; Fig. 3B). Silencing
PIN1 gene expression significantly inhibited the migration
(Fig. 4A) and invasion abilities of
the RBE cells (Fig. 4B).
Discussion
pSer/Thr-Pro is a key signaling mechanism by which
cellular proliferation and transformation occur. PIN1 is a
peptidyl-prolyl isomerase that recognizes the pSer/Thr-Pro motifs
in certain proteins and catalyzes the prolyl isomerization
(2–4). The prolyl-isomerization induces
conformational changes and has a distinct effect on various target
genes, including cyclin, β-catenin, jun, myc and p53. These targets
of PIN1 play a key role in the regulation of the cell cycle and are
often deregulated in cancer (2–4,7–11).
The possible involvement of PIN1 in human carcinogenesis is based
on the observations that the overexpression of the gene is
frequently identified in human cancers (4,7–11).
Therefore, PIN1 has been of increasing interest as a potential
target in the treatment of patients with cancer. The results of the
present study demonstrated that the degree of PIN1 expression was
significantly elevated in the ECC tissues compared with the
non-tumorous bile duct cells. A significant correlation was
identified between the degree of PIN1 expression and the tumor cell
proliferation rate (Ki-67 labeling index). PIN1 silencing had a
significant inhibitory effect on the growth and proliferation of
the ECC cells and reduced their migration and invasion abilities.
However, a significant correlation between PIN1 and TP53 expression
in the ECC cells was not identified using immunohistochemistry. The
results of the present study indicated a possible role for PIN1 in
ECC development and also indicated that PIN1 expression may be
involved in the proliferation and invasion of tumor cells.
Numerous studies have indicated that PIN1 plays a
role in oncogenesis and that the depletion of this gene expression
leads to a decreased susceptibility to oncogenesis (7–13). By
contrast, several studies have shown that decreased levels of PIN1
are associated with a selective growth disadvantage due to an
increase in the time that is required for the progression of the
cell cycle (4,14,15).
PIN1 is able to promote the degradation of c-Myc and cyclin E with
the mediation of the FBXW7 E3 ligase, thus providing the evidence
that it acts as a tumor suppressor (16,17).
The present data revealed that the degree of PIN1 expression was
significantly higher in the ECC tissues compared with the non-tumor
tissues. Furthermore, there was a positive correlation between the
expression of PIN1 and the Ki-67 labeling index in the ECC cells.
The suppression of PIN1 expression decreased the degree of cellular
growth and proliferation. The degree of PIN1 expression has been
reported to increase in various types of cancer, including human
prostate (11,12), breast (10,11)
and lung (11,18) cancer and hepatocellular carcinoma
(11,13). PIN1 is also involved in increasing
tumor cell growth and colony formation through the upregulation of
the expression of β-catenin and cyclin D1 in several types of
cancer (12,13,18).
In addition, it has also been suggested that PIN1 expression may
play a role as a prognostic indicator in human cancers occurring in
the breast (10), lung (18) and prostate (19). These studies and the results of the
present study support the argument that PIN1 overexpression may
play a role in promoting the occurrence of tumors and then increase
the risk of developing cancer. However, this remains somewhat
controversial. By contrast, previous studies have also shown that
the downregulation of PIN1 expression is frequently observed in
renal cell carcinoma (RCC) and gastric and testicular cancer
(11,20). Furthermore, although the expression
of PIN1 is frequently observed in patients with Merkel cell
carcinoma, those with a higher degree of PIN1 expression have been
reported to have a significantly longer overall survival compared
with those with a lower degree of expression (21). However, the variability in the
biological and clinical effects of PIN1 depending on the types of
cancer remains unclear. Teng et al demonstrated that PIN1
had an inhibitory effect on the development of RCC where TP53
signaling remained intact. These findings are consistent with the
physiological role of PIN1 in regulating the functions of TP53
(20). Several studies have
indicated that PIN1 may inhibit the cell cycle and cell growth by
stabilizing p53, a tumor suppressor gene (4,14,15,22,23),
or by destabilizing oncoproteins (16,17).
This indicates a possible correlation between the PIN1 and TP53
expression status in tissue samples of ECC. The p53 gene product is
unstable with a short half-life. This explains the reason that
wild-type p53 is not well detected. However, a mutation in the p53
gene often results in a prolonged half-life with a loss of function
compared with the wild-type allele (25). The mutated p53 gene products tend to
accumulate in the cell nuclei and display positive nuclear staining
on immunohistochemistry, thus suggesting the presence of mutated
p53. The present study demonstrated that TP53 expression was
observed in 35 of 67 (52%) surgical ECC specimens using
immunohistochemistry. This degree of expression is consistent with
an earlier study showing that the mean degree of TP53 expression
was 51% (range, 19–86%), as observed by immunohistochemistry in
cases of ECC (26). Despite a
previous study stating that there was a correlation between PIN1
expression and TP53 in cases of lung cancer (27), the present study failed to identify
any significant correlations between the expression of the two
genes. It is noteworthy that the overexpression of PIN1 is
frequently observed in human cancers where a p53 mutation is
prevalent, including breast and liver cancer (10,13,20).
Furthermore, PIN1 cooperates with mutant p53 during ras-dependent
transformation and increases the aggressiveness through the
inhibition of antimetastatic factor p63 and the induction of a
mutant p53 transcriptional program in breast cancer cells (24). Based on a previous study
demonstrating that TP53 overexpression is frequently observed in
cases of ECC (26), we speculate
that a p53 mutation inhibits the activity of PIN1 as a tumor
suppressor gene and that this may lead to its increased activity in
promoting tumor development in ECC cells. However, further studies
are warranted to clarify the molecular mechanisms by which PIN1
plays a variable role in the development of diverse types of cancer
cells, which would be essential for disclosing its role in
association with p53.
The morbidity and mortality of cancer is
predominantly based on the invasion and metastasis of cells from
the primary occurrence. Since the invasion and metastasis of cancer
depends on the migratory and invasive potential, the present study
examined whether silencing PIN1 expression in CC cells was able to
reduce migration and invasion. The results revealed that the
migration and invasion abilities of the cancer cells were
significantly reduced with the RNAi-mediated knockdown of PIN1
expression in the RBE cells. This is consistent with a previous
study, in which the migration and invasion abilities of lung cancer
cells were significantly reduced with the inhibition of PIN1
expression using PIN-1-specific siRNA (18). With the blockage of PIN1 by stable
transfection of a microRNA (miRNA) plasmid targeting PIN1, the
proliferation and invasion of cells from the malignant melanoma
A275 cell line were significantly reduced (28). Similarly, with the depletion of PIN1
by small hairpin RNA, the transforming growth factor
(TGF)-β-mediated migration and invasion of human prostate cancer
cells (PC3) were significantly inhibited (29). Overall, the present results indicate
a potential role for PIN1 expression in the migration and invasion
of ECC cells. However, further studies are warranted to examine the
mechanisms by which the role of PIN1 in the invasion and metastasis
of the tumor is promoted.
In conclusion, the present data indicated that the
upregulation of PIN1 is involved in the development and progression
of ECC tumors. Silencing PIN1 gene expression significantly
inhibited tumor cell proliferation and decreased the migration and
invasion of the ECC cells. The high degree of TP53 expression in
ECC cells may play a role in promoting the oncogenic potential of
PIN1 in these cells.
Acknowledgements
This study was supported by the National Research
Foundation of Korea Grant funded by the Korean Government (no.
2012-0009320). The biospecimens for this study were partly provided
by the Biobank of Chonbuk National University Hospital, a member of
the National Biobank of Korea, which is supported by the Ministry
of Health, Welfare and Family Affairs.
References
|
1
|
Lazaridis KN and Gores GJ:
Cholangiocarcinoma. Gastroenterology. 128:1655–67. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Blume-Jensen P and Hunter T: Oncogenic
kinase signaling. Nature. 411:355–365. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lippens G, Landrieu I and Smet C:
Molecular mechanisms of the phospho-dependent prolyl cis/trans
isomerase Pin1. FEBS J. 274:5211–5222. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yeh ES and Means AR: PIN1, the cell cycle
and cancer. Nat Rev Cancer. 7:381–388. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: AJCC Cancer Staging Manual. 7th edition.
Springer; New York, NY: 2010
|
|
6
|
Kwon CY, Kim KR, Choi HN, et al: The role
of serum response factor in hepatocellular carcinoma: implications
for disease progression. Int J Oncol. 37:837–844. 2010.PubMed/NCBI
|
|
7
|
Finn G and Lu KP: Phosphorylation-specific
prolyl isomerase Pin1 as a new diagnostic and therapeutic target
for cancer. Curr Cancer Drug Targets. 8:223–229. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ryo A, Liou YC, Lu KP and Wulf G: Prolyl
isomerase Pin1: a catalyst for oncogenesis and a potential
therapeutic target in cancer. J Cell Sci. 116:773–783. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ryo A, Nakamura M, Wulf G, Liou YC and Lu
KP: Pin1 regulates turnover and subcellular localization of
beta-catenin by inhibiting its interaction with APC. Nat Cell Biol.
3:793–801. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wulf GM, Ryo A, Wulf GG, et al: Pin1 is
overexpressed in breast cancer and cooperates with Ras signaling in
increasing the transcriptional activity of c-Jun towards cyclin D1.
EMBO J. 20:3459–3472. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bao L, Kimzey A, Sauter G, Sowadski JM, Lu
KP and Wang DG: Prevalent overexpression of prolyl isomerase Pin1
in human cancers. Am J Pathol. 164:1727–1737. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ryo A, Uemura H, Ishiguro H, et al: Stable
suppression of tumorigenicity by Pin1-targeted RNA interference in
prostate cancer. Clin Cancer Res. 11:7523–7531. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pang RW, Lee TK, Man K, et al: PIN1
expression contributes to hepatic carcinogenesis. J Pathol.
210:19–25. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Atchison FW, Capel B and Means AR: Pin1
regulates the timing of mammalian primordial germ cell
proliferation. Development. 130:3579–3586. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Joseph JD, Daigle SN and Means AR: PINA is
essential for growth and positively influences NIMA function in
Aspergillus nidulans. J Biol Chem. 279:32373–32384. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yeh ES, Lew BO and Means AR: The loss of
PIN1 deregulates cyclin E and sensitizes mouse embryo fibroblasts
to genomic instability. J Biol Chem. 281:241–251. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yeh E, Cunningham M, Arnold H, et al: A
signalling pathway controlling c-Myc degradation that impacts
oncogenic transformation of human cells. Nat Cell Biol. 6:308–318.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tan X, Zhou F, Wan J, et al: Pin1
expression contributes to lung cancer: Prognosis and
carcinogenesis. Cancer Biol Ther. 9:111–119. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ayala G, Wang D, Wulf G, et al: The prolyl
isomerase Pin1 is a novel prognostic marker in human prostate
cancer. Cancer Res. 63:6244–6251. 2003.PubMed/NCBI
|
|
20
|
Teng BL, Hacker KE, Chen S, Means AR and
Rathmell WK: Tumor suppressive activity of prolyl isomerase Pin1 in
renal cell carcinoma. Mol Oncol. 5:465–474. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lill C, Schneider S, Pammer J, et al:
Significant correlation of peptidyl-prolyl isomerase overexpression
in Merkel cell carcinoma with overall survival of patients. Head
Neck. 33:1294–1300. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zacchi P, Gostissa M, Uchida T, et al: The
prolyl isomerase Pin1 reveals a mechanism to control p53 functions
after genotoxic insults. Nature. 419:853–857. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wulf GM, Liou YC, Ryo A, Lee SW and Lu KP:
Role of Pin1 in the regulation of p53 stability and p21
transactivation, and cell cycle checkpoints in response to DNA
damage. J Biol Chem. 277:47976–47979. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Girardini JE, Napoli M, Piazza S, et al: A
Pin1/mutant p53 axis promotes aggressiveness in breast cancer.
Cancer Cell. 20:79–91. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Harris CC: Structure and function of the
p53 tumor suppressor gene: clues for rational cancer therapeutic
strategies. J Natl Cancer Inst. 88:1442–1455. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Khan SA, Thomas HC, Toledano MB, Cox IJ
and Taylor-Robinson SD: p53 Mutations in human cholangiocarcinoma:
a review. Liver Int. 25:704–716. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
He J, Zhou F, Shao K, et al:
Overexpression of Pin1 in non-small cell lung cancer (NSCLC) and
its correlation with lymph node metastases. Lung Cancer. 56:51–58.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jin J, Zhang Y, Li Y, et al:
RNA-interference-mediated downregulation of Pin1 suppresses
tumorigenicity of malignant melanoma A375 cells. Neoplasma.
60:92–100. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Matsuura I, Chiang KN, Lai CY, et al: Pin1
promotes transforming growth factor-beta-induced migration and
invasion. J Biol Chem. 285:1754–1764. 2010. View Article : Google Scholar : PubMed/NCBI
|