Introduction
Surgical resection is the only known curative option
for pancreatic cancer. However, the majority of pancreatic cancers
are usually diagnosed at advanced stages and only 15–20% of
patients are candidates for a gross margin-negative pancreatectomy
(R0) (1). Following a curative
resection, distant metastases, particularly in the liver, local
recurrence and peritoneal dissemination frequently occur and these
patients succumb to their diseases (2,3) The
reported five-year survival rate following surgical resection is
12.1–25.0% (1–5) and the overall survival rate is
considered to be <5%, suggesting that pancreatic cancer is one
of the most lethal gastrointestinal malignancies.
According to a previous survival analysis of
resected pancreatic cancers, an R0 was shown to be an independent
favorable prognostic factor (6–8). Over
the past few decades, surgeons have attempted to achieve ideal R0
resections by extending the surgical margins in the hopes that
clearing the surrounding soft tissue that contains malignant cells
may improve the survival outcome. However, several significant
prospective randomized control studies revealed that extending the
margins did not result in an additional survival benefit over the
standard surgery in resectable pancreatic cancer (9–12). It
has been proposed that a possible underestimation of microscopic
cancer spreading beyond the surgical field may contribute to a poor
prognosis following a potentially curative surgical treatment
(13).
Lymph node metastasis is common in pancreatic cancer
(14–16) and para-aortic lymph nodes (PALNs)
are considered to be the final nodes in the systemic lymphatic
circulation in periampullary cancer (17,18).
Although there have only been a few studies on the oncological
outcome according to PALN staging (19–21),
PALN involvement is known to be a poor prognostic factor in
periampullary tumors (18).
However, pancreatic surgeons may encounter clinical cases of
potentially resectable pancreatic tumors with unexpected PALN
metastasis that are only identified on intraoperative frozen
section biopsies. Considering the expected poor prognosis in
patients with unexpected PALN metastasis and the potential curative
role of an R0 in pancreatic cancer, the decision to resect must be
promptly determined in the operating theatre.
The present study aimed to develop an approach to
this clinical dilemma. The oncological outcomes in patients with
PALN metastasis that were detected by hematoxylin and eosin (HE)
staining were analyzed in resected pancreatic tumors.
Immunohistochemistry (IHC) with antibodies against cytokeratin
(CK)-19 was used to detect the presence of PALN micrometastasis in
resected pancreatic ductal adenocarcinoma. The role of curative
surgery in resectable pancreatic cancer with incidentally
identified PALN metastasis was further investigated using
intraoperative frozen section biopsies.
Materials and methods
Study design
The present study retrospectively investigated
patients who underwent a surgical resection for pancreatic ductal
adenocarcinoma between January 1999 and December 2009 at Yonsei
University Health System (Seoul, South Korea). During the study
period, a total of 1,119 patients were diagnosed with pancreatic
ductal adenocarcinoma and 171 patients (15.3%) underwent grossly
curative pancreatectomies. Of these patients, 99 with available
healthy paraffin-embedded tissue blocks of PALN were re-evaluated
using IHC. This study was approved by the ethics committee of
Yonsei University Health System (Seoul, Korea).
Surgery and staging
A pancreaticoduodenectomy or distal pancreatectomy
with splenectomy was performed, which included a resection of the
main pancreatic tumor, the associated regional lymph nodes, the
retroperitoneal soft tissue and the PALNs, which allowed for
pathological staging. The surgical margins, including the bile
duct, pancreatic duct, peripancreatic soft tissue adjacent to the
superior mesenteric artery (retroperitoneal margin), duodenum or
stomach, were evaluated grossly and microscopically in order to
determine their status. These surgical margins, with the exception
of the retroperitoneal margin, were analyzed using frozen-sections.
If the margin was positive for invasive carcinoma, an additional
resection was performed. A pancreatic resection margin without
evidence of invasive carcinoma was considered an R0. The final
margin status was noted in the permanent pathology report. The TMN
stage was evaluated based on the American Joint Committee on Cancer
(AJCC) Cancer Staging Manual, 7th edition (23).
IHC
Three serial sectional cuts from formalin-fixed and
paraffin-embedded blocks were studied for IHC. Each of the 4-μm
sections with 6-μm intervals was prepared for IHC staining with
CK-19 (M0888 mouse, monoclonal, 1:100; Dako, Copenhagen, Denmark).
The IHC was detected using a dextran polymer-based, biotin-free
visualization system (Envision kit; Dako). Previous HE-stained
sections were re-evaluated for metastasis and the results were
compared with the IHC-stained sections by an experienced
pathologist. Lymph node micrometastasis was defined as metastatic
tumor cells that were detected by IHC evaluation using antibodies
against CK, but were missed by routine histological examination
using HE staining.
Statistical analysis
The primary goal of the study was to investigate the
clinical significance of PALN micrometastasis in resected
pancreatic ductal adenocarcinoma. The cumulative survival rates
according to overall lymph node metastasis (pN stage) and PALN
metastasis (LN16) were analyzed. The clinicopathological
characteristics of the patients with PALN metastasis were
summarized. By reviewing the medical records, recurrence and
survival data were obtained. Overall survival was defined as the
interval between surgery and mortality or the final follow-up
visit. The cumulative survival rate was calculated using the
Kaplan-Meier method. A log-rank test was used to ascertain the
statistically significant differences. P<0.05 was considered to
indicate a statistically significant difference.
Results
Characteristics of patients and resected
ductal adenocarcinoma
Among the 99 patients (39 females and 60 males), the
mean age was 61.3 years (range, 39–78). Ductal adenocarcinoma was
confirmed by microscopic examination in all patients. A total of 21
(21.2%) patients underwent a conventional pancreaticoduodenectomy,
64 (64.6%) underwent a pylorus-preserving pancreaticoduodenectomy,
13 (13.1%) underwent a distal pancreatectomy with splenectomy and
one (1%) was treated with a total pancreatectomy. Three patients
were diagnosed with pT1 pancreatic cancer, six with pT2, 85 with
pT3 and five with pT4. The median tumor size was 2.6 cm (range,
0.5–8.5). There were no R2 resections recorded in the medical
records. An R1 resection was performed on 18 patients (18.2%) and
an R0 was performed on 81 patients (81.8%).
Retrieved PALN assessment in resected
pancreatic cancer
A total of 484 PALNs (mean, 4.9 nodes per patient;
range, 1–19) were evaluated from the available PALN blocks in 99
patients (Fig. 1). A total of 13
PALNs (2.7%) demonstrated metastasis with HE staining in nine
patients (eight patients in frozen sections and one patient in a
permanent section). All the eight patients who demonstrated PALN
metastasis on the HE staining of frozen sections showed a pattern
of clustered gland formation and desmoplasia, which occupied the
entire involved node (Fig. 2A). The
histology of the patient who had PALN metastasis confirmed by HE
staining of the permanent section, which was not detected in the
frozen section, revealed a pattern of scattered small gland
formation without desmoplasia (Fig.
2B). IHC reassessment for all 484 PALNs revealed that only one
additional patient immunohistochemically demonstrated
micrometastasis in a PALN that was not detected otherwise. The
CK-19 staining revealed micrometastases in an ~46-μm isolated
pattern (Fig. 2C).
Oncological outcomes of pancreatic cancer
with PALN metastasis
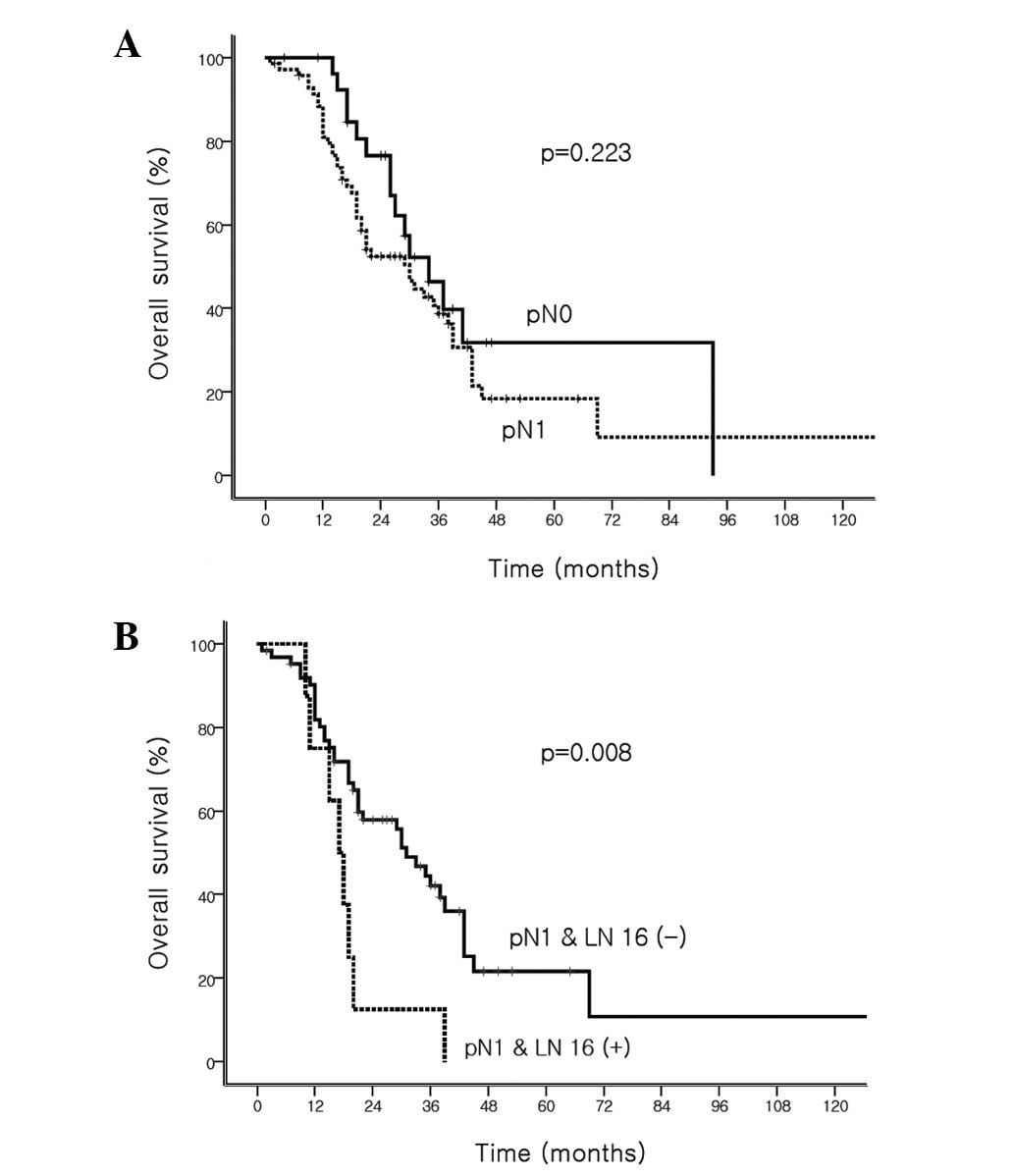
The survival rate did not significantly differ based
on the pN staging in the present study. The median survival time
was 34 months (95% CI, 24.3–43.7) for the pN0 tumors and 30 months
(95% CI,: 20.3–39.7) for the pN1 tumors (P=0.223; Fig. 3A). However, a statistically
significant difference was observed in the survival time based on
PALN involvement, confirmed by routine HE evaluation. The median
survival time was 31 months (95% CI, 23.9–38.1) in patients without
PALN metastases versus 17 months (95% CI, 12.8–21.6) for patients
with PALN metastases (P=0.008; Fig.
3B).
Table I shows the
characteristics of the patients with PALN metastasis. Cases 1–8
were the patients who showed PALN metastasis on HE staining of the
frozen sections. These patients exhibited large metastatic tumors
in the PALNs (median size, 2 mm; range, 0.8–12 mm) with clustered
gland patterns and extensive desmoplasia. Case 9, who had PALN
metastasis confirmed by HE staining of the permanent section, had
relatively small metastatic tumors in the PALNs with a pattern of
scattered small gland formation and no desmoplasia. Lastly, case
10, who exhibited PALN detected only by IHC staining, possessed
extremely small isolated metastatic tumors in the PALNs. All
patients who exhibited PALN metastasis confirmed by HE staining
(cases 1–9) had other aggressive tumor characteristics, including
other regional lymph node metastasis (N1) and lymphovascular and/or
perineural invasions, and one patient demonstrated positive
resection margins (R1). Case 10 exhibited less aggressive adverse
pathological features. The median survival time of cases 1–8 was
17.5 months (range, 10–39). The survival times of cases 9 and 10
were 34 and 41 months, respectively.
 | Table ISummary of patients with PALN
metastasis. |
Table I
Summary of patients with PALN
metastasis.
| Case | Age, years
/gender | Surgery | R status | Initial stage | Tumor size, cm | PALN,
positive/total | PALN size and
character, mm | LVI/PNI | Recurrence time,
months | Recurrence
pattern | Survival, months |
|---|
| 1 | 60/M | PD | R1 | IIB (T3N1M0) | 2.0 | 1/5 (F) | 2 GL+D | +/+ | 4 | Liver, Rp,
ureter | 18 |
| 2 | 53/M | PPPD | R0 | IIB (T3N1M0) | 4.0 | 2/4 (F) | 1.3, 1.2 GL+D | −/+ | 11 | Bone, lung
mesentery | 19 |
| 3 | 66/M | PPPD | R0 | IIB (T3N1M0) | 2.2 | 4/5 (F) | 10, 2, 1, 0.9
GL+D | +/− | 12 | Rp, lung | 17 |
| 4 | 63/F | PD | R0 | IIB (T3N1M0) | 2.5 | 1/6 (F) | 2 GL+D | −/+ | 9 | Rp | 20 |
| 5 | 78/F | DP | R0 | IIB (T3N1M0) | 7.0 | 2/3 (F) | 2.3, 1.2 GL+D | +/− | 8 | Para-aortic area | 39 |
| 6 | 73/F | PPPD | R0 | IIB (T3N1M0) | 2.0 | 1/3 (F) | 8 GL+D | −/+ | 8 | Liver, para-aortic
area | 10 |
| 7 | 62/M | PPPD | R0 | IIB (T3N1M0) | 3.2 | 1/3 (F) | 12 GL+D | +/+ | 4 | Liver, para-aortic
area | 15 |
| 8 | 75/F | PPPD | R0 | IIB(T3N1M0) | 2.5 | 1/5 (F) | 0.8 GL+D | +/+ | 6 | Peritoneal seeding,
Rp | 11 |
| 9 | 55/F | PPPD | R0 | IIA (T3N1M0) | 1.3 | 1/11 (P) | 0.3 scattered
GL | +/− | 18 |
Para-aorticarea | 34 |
| 10 | 51/F | PPPD | R0 | IIA (T3N0M0) | 1.8 | 1/4 (I) | 0.046 isolated | − | 24 | Lung, spine,
Rp | 41 |
Discussion
Based on the results of the present study,
additional IHC using CK-19 antibodies to detect PALN
micrometastases in resected pancreatic ductal adenocarcinoma is not
an appropriate method to predict prognosis. While the rate of PALN
metastasis has been reported to be 6–26% (24–27),
there may be a surgical selection bias that is affecting these
numbers. For example, pancreatectomies are generally not performed
when PALN metastasis is strongly suggested in pre-operative imaging
studies, with observations that include large and conglomerated
lymph nodes in the retroperitoneal para-aortic area. As a result,
only nine patients (9.1%) exhibited PALN metastases in routine
intraoperative frozen section biopsies and permanent pathological
reports, and only one additional patient (1.0%) was shown to have
PALN micrometastasis upon CK-19 staining. Therefore, the incidence
of PALN metastasis was observed to be 10.1%. An accurate staging of
PALN metastasis conducted by a careful pathological examination
with routine HE staining is considered to be sufficient for tumor
staging and is useful in predicting prognosis.
Due to the infrequency of resectable pancreatic
ductal adenocarcinoma, only a few studies have demonstrated the
clinical significance of PALN metastasis in resected pancreatic
cancer. Doi et al(20)
analyzed the clinicopathological factors in patients with
short-term survival who underwent margin-negative radical extended
pancreaticoduodenectomy. PALN metastasis was concluded to be the
only independent factor for poor prognosis and ~85% of patients
with PALN metastasis succumbed within one year. Shimada et
al(28) reported that PALN
metastasis was the definitive predictor of recurrence and a shorter
survival outcome (<12 months). Yoshida et al(27) also identified the
clinicopathological features and surgical outcomes of PALN-positive
periampullary adenocarcinoma, and recommended performing
intraoperative PALN sampling for frozen section biopsies. The study
concluded that radical pancreatectomy with extended soft tissue
clearance should not be performed in PALN-positive patients due to
a poor oncological outcome. According to the data set of the
present study, all eight patients whose frozen section biopsies
confirmed PALN metastasis eventually developed tumor recurrences
within 12 months of surgery and seven succumbed to the disease
within two years (median survival, 17.5 months; Table I). These eight patients had
significantly shorter survival periods compared with those patients
with regional lymph node metastasis without PALN involvement
(P<0.008; Fig. 2B).
Notably, a few cases of long-term survival in
patients with PALN metastasis in resected pancreatic cancer have
been reported (21,29). Although, as noted previously,
studies suggest a poor outcome in PALN-positive resected pancreatic
cancer, the majority do not consider the associated characteristics
of PALN metastasis. Shimada et al(28), however, observed that PALN
metastasis was notably associated with elevated CA 19–9 levels, a
larger tumor size and positive surgical margins. Peritoneal
cytology was correlated with PALN metastasis in the study (P=0.09).
Furthermore, Yamada, et al(30) concluded that radical surgery may
still have value for certain populations of patients with PALN
metastasis, such as those aged 60 years or older, patients with
tumors of <4 cm and those without portal vein involvement. The
study also suggested that patients with one lymph node positive for
PALN metastasis tended to have an improved prognosis compared with
those with two or more positive PALN metastases (P=0.14).
Although the present data set was rather small and
selection bias may have been a factor, it suggests a potential role
for curative surgery in certain patient groups with resectable
pancreatic cancer and PALN involvement. In spite of the poor
prognosis in patients who demonstrated PALN metastases observed in
routine HE staining, relatively longer survivals were noted in
several patients (Table I).
Considering a poor median survival time of 5–11 months for
unresectable pancreatic tumors without distant metastases (31), the oncological outcomes of the
patients included in the present study are thought to be
significant, given that curative surgery is the only intervention
that may lead to long-term survival in pancreatic cancer. Given the
current state of continually improving surgical techniques,
perioperative management and adjuvant therapies for pancreatic
cancer, the findings of the present study may expand the role of
surgery in managing pancreatic cancer, even when PALN metastasis is
unexpectedly identified intraoperatively.
According to the present results, all eight patients
with PALN metastasis confirmed in frozen section biopsies showed
large metastatic tumor sizes and clustered gland patterns with
extensive desmoplasia. However, one patient exhibited PALN
metastasis that was only identified in routine HE staining of the
permanent section, but was missed in the frozen section. This
section demonstrated a relatively small size gland pattern without
desmoplasia or extensive involvement of the node (Fig. 2). In spite of these histological
differences, all of the patients possessed other adverse
pathological factors including regional lymph node metastasis,
perineural invasion and lymphovascular invasion (Table I). The patient whose PALN metastasis
was only detected with IHC (case 10) demonstrated a small, isolated
metastasis with less aggressive adverse pathological features. This
patient had a relatively long-term survival of 41 months. Notably,
despite a small sample size, a correlation was observed between the
tumor burden of PALN metastasis and the survival outcome. A small
PALN metastasis, which is either undetectable in frozen biopsy or
only noticeable in immunostaining, may lead to a longer survival
time.
The present study defined lymph node micrometastasis
as metastatic tumor cells that are only detectable by IHC staining.
However, a universal definition and clinical significance of
micrometastasis for pancreatic cancer is still lacking. There are
more established characterizations of micrometastasis in breast
(32), esophageal (33,34),
stomach (35) and colon cancers
(36,37) Therefore, the micrometastasis of
pancreatic cancer requires further study.
In summary, PALN metastasis that is undetected by
routine HE staining in frozen section biopsy, but identified using
CK-19 immunostaining, may indicate a relatively lower tumor burden.
This may be associated with less aggressive behavior and a
favorable prognosis in pancreatic cancer. Therefore, routine HE
staining is thought to be sufficient for predicting prognosis.
Additionally, in cases where PALN metastasis is unexpectedly
identified in intraoperative frozen section biopsies, patients may
benefit from curative radical surgery with aggressive adjuvant
chemotherapy. However, further large volume investigations are
warranted to validate this issue.
Acknowledgements
This study was supported by the Institute of
Gastroenterology, Yonsei University Health System, Seoul, South
Korea.
References
|
1
|
Kayahara M, Nagakawa T, Ueno K, Ohta T,
Takeda T and Miyazaki I: An evaluation of radical resection for
pancreatic cancer based on the mode of recurrence as determined by
autopsy and diagnostic imaging. Cancer. 72:2118–2123. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sener SF, Fremgen A, Menck HR and
Winchester DP: Pancreatic cancer: a report of treatment and
survival trends for 100,313 patients diagnosed from 1985–1995,
using the National Cancer Database. J Am Coll Surg. 189:1–7.
1999.PubMed/NCBI
|
|
3
|
Sperti C, Pasquali C, Piccoli A and
Pedrazzoli S: Recurrence after resection for ductal adenocarcinoma
of the pancreas. World J Surg. 21:195–200. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Han SS, Jang JY, Kim SW, Kim WH, Lee KU
and Park YH: Analysis of long-term survivors after surgical
resection for pancreatic cancer. Pancreas. 32:271–275. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sohn TA, Yeo CJ, Cameron JL, et al:
Resected adenocarcinoma of the pancreas-616 patients: results,
outcomes, and prognostic indicators. J Gastrointest Surg.
4:567–579. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Howard TJ, Krug JE, Yu J, et al: A
margin-negative R0 resection accomplished with minimal
postoperative complications is the surgeon’s contribution to
long-term survival in pancreatic cancer. J Gastrointest Surg.
10:1338–1346. 2006.PubMed/NCBI
|
|
7
|
Raut CP, Tseng JF, Sun CC, et al: Impact
of resection status on pattern of failure and survival after
pancreaticoduodenectomy for pancreatic adenocarcinoma. Ann Surg.
246:52–60. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Winter JM, Cameron JL, Campbell KA, et al:
1423 pancreaticoduodenectomies for pancreatic cancer: A
single-institution experience. J Gastrointest Surg. 10:1199–1211.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Riall TS, Cameron JL, Lillemoe KD, et al:
Pancreaticoduodenectomy with or without distal gastrectomy and
extended retroperitoneal lymphadenectomy for periampullary
adenocarcinoma - part 3: update on 5-year survival. J Gastrointest
Surg. 9:1191–1206. 2005.
|
|
10
|
Farnell MB, Pearson RK, Sarr MG, et al;
Pancreas Cancer Working Group. A prospective randomized trial
comparing standard pancreatoduodenectomy with pancreatoduodenectomy
with extended lymphadenectomy in resectable pancreatic head
adenocarcinoma. Surgery. 138:618–630. 2005. View Article : Google Scholar
|
|
11
|
Pedrazzoli S, DiCarlo V, Dionigi R, et al:
Standard versus extended lymphadenectomy associated with
pancreatoduodenectomy in the surgical treatment of adenocarcinoma
of the head of the pancreas: a multicenter, prospective, randomized
study. Lymphadenectomy Study Group. Ann Surg. 228:508–517. 1998.
View Article : Google Scholar
|
|
12
|
Iqbal N, Lovegrove RE, Tilney HS, et al: A
comparison of pancreaticoduodenectomy with extended
pancreaticoduodenectomy: a meta-analysis of 1909 patients. Eur J
Surg Oncol. 35:79–86. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kurahara H, Takao S, Maemura K, et al:
Impact of lymph node micrometastasis in patients with pancreatic
head cancer. World J Surg. 31:483–492. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Geer RJ and Brennan MF: Prognostic
indicators for survival after resection of pancreatic
adenocarcinoma. Am J Surg. 165:68–73. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Trede M, Schwall G and Saeger HD: Survival
after pancreatoduodenectomy. 118 consecutive resections without an
operative mortality. Ann Surg. 211:447–458. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yeo CJ, Cameron JL, Lillemoe KD, et al:
Pancreaticoduodenectomy for cancer of the head of the pancreas. 201
patients. Ann Surg. 221:721–733. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sobin LH and Wittekind C: TNM
Classification of Malignant Tumours (UICC). 5th edition. John Wiley
& Sons; New York, NY: 1997
|
|
18
|
Japanese Pancreas Society. Classification
of Pancreatic Carcinoma. 1st English edition. Kanehara huppan;
Tokyo, Japan: 1996
|
|
19
|
Yoshiaki M, Kenichiro U, Takeshi S, et al:
Prognostic impact of para-aortic lymph node metastasis in
pancreatic ductal adenocarcinoma. World J Surg. 34:1900–1907. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Doi R, Kami K, Ito D, et al: Prognostic
implication of para-aortic lymph node metastasis in resectable
pancreatic cancer. World J Surg. 31:147–154. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sakai M, Nakao A, Kaneko T, et al:
Para-aortic lymph node metastasis in carcinoma of the head of the
pancreas. Surgery. 137:606–611. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Andersen HB, Baden H, Brahe NE and
Burcharth F: Pancreaticoduodenectomy for periampullary
adenocarcinoma. J Am Coll Surg. 179:545–552. 1994.PubMed/NCBI
|
|
23
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: AJCC cancer Staging Manual. 7th edition.
Springer; New York, NY: 2010
|
|
24
|
Ishikawa O, Ohigashi H, Sasaki Y, et al:
Practical grouping of positive lymph nodes in pancreatic head
cancer treated by an extended pancreatectomy. Surgery. 121:244–249.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nakao A, Harada A, Nonami T, et al: Lymph
node metastases in carcinoma of the head of the pancreas region. Br
J Surg. 82:399–402. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yoshida T, Aramaki M, Matsumoto T, Morii
Y, Sasaki A and Kitano S: The pattern of lymphatic spread in
carcinoma of the distal bile duct. Int Surg. 83:124–127.
1998.PubMed/NCBI
|
|
27
|
Yoshida T, Matsumoto T, Sasaki A, Shibata
K, Aramaki M and Kitano S: Outcome of paraaortic node-positive
pancreatic head and bile duct adenocarcinoma. Am J Surg.
187:736–740. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shimada K, Sakamoto Y, Sano T and Kosuge
T: The role of paraaortic lymph node involvement on early
recurrence and survival after macroscopic curative resection with
extended lymphadenectomy for pancreatic carcinoma. J Am Coll Surg.
203:345–352. 2006. View Article : Google Scholar
|
|
29
|
Kayahara M, Nagakawa T, Ohta T, et al:
Analysis of paraaortic lymph node involvement in pancreatic
carcinoma: a significant indication for surgery? Cancer.
85:583–590. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yamada S, Nakao A, Fujii T, et al:
Pancreatic cancer with paraaortic lymph node metastasis: a
contraindication for radical surgery? Pancreas. 38:e13–e17. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Moertel CG, Frytak S, Hahn RG, et al:
Therapy of locally unresectable pancreatic carcinoma: a randomized
comparison of high dose (6000 rads) radiation alone, moderate dose
radiation (4000 rads + 5-fluorouracil), and high dose radiation +
5-fluorouracil: The Gastrointestinal Tumor Study Group. Cancer.
48:1705–1710. 1981.PubMed/NCBI
|
|
32
|
McGuckin MA, Cummings MC, Walsh MD, Hohn
BG, Bennett IC and Wright RG: Occult axillary node metastases in
breast cancer: their detection and prognostic significance. Br J
Cancer. 73:88–95. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jiao X, Eslami A, Ioffe O, et al:
Immunohistochemistry analysis of micrometastasis in pretreatment
lymph nodes from patients with esophageal cancer. Ann Thorac Surg.
76:996–1000. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tanabe T, Nishimaki T, Watanabe H, et al:
Immunohistochemically detected micrometastasis in lymph nodes from
superficial esophageal squamous cell carcinoma. J Surg Oncol.
82:153–159. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ishigami S, Natsugoe S, Tokuda K, et al:
Clinical impact of micrometastasis of the lymph node in gastric
cancer. Am Surg. 69:573–577. 2003.PubMed/NCBI
|
|
36
|
Bilchik AJ, Hoon DS, Saha S, et al:
Prognostic impact of micrometastases in colon cancer: interim
results of a prospective multicenter trial. Ann Surg. 246:568–577.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bosch RC: Interview with Dr. Ramón Colomer
Bosch, President of the Spanish Society of Medical Oncology (SEOM)
by Enrique Garcia Jorda. Clin Transl Oncol. 10:310–312. 2008.
|