Introduction
Esophageal carcinoma is one of the most common
malignant gastrointestinal tumors (1). The incidence of esophageal carcinoma
is high in China and it is a great threat to human health.
Chemotherapy is a combined modality therapy that is used in the
treatment of esophageal carcinoma, however, multidrug resistance
(MDR) is a major barrier to the success of chemotherapy,
particularly in recurrent tumors. MDR is the resistance of cancer
cells to multiple classes of chemotherapeutic drugs that may be
structurally and mechanistically unrelated. When tumor cells
develop drug resistance, they become resistant not only to the
treated drug, but also to a variety of structurally and
functionally unrelated drugs. Since the therapeutic effect is
determined by this resistance, MDR has become a focus for research.
There is accumulating evidence that the active export of anticancer
drugs from cells is one of the major mechanisms of MDR (2,3). Tumor
cells often gain drug resistance through the overexpression of
membrane transport proteins that effectively efflux anticancer
drugs (4,5). It has been convincingly documented
that several ATP-dependent drug transporters may cause drug
resistance in cancer cells by actively extruding clinically
administered chemotherapeutic drugs (6). The increased transmembrane efflux of
antitumor drugs is one of the best-characterized mechanisms of MDR
and is mediated through the overexpression of ATP-binding cassette
(ABC) transporter superfamily members. The well-known major drug
transporters, ABCB1 (P-glycoprotein or MDR1) and ABCG2
(BCRP/MXR/ABCP), have been characterized in detail with respect to
their structures and functions (7).
These drug transporters belong to the human ABC transporter gene
family that consists of at least 48 gene members. The
overexpression of ABCG2 has been reported to confer drug resistance
upon malignant cells to various chemotherapeutic drugs (8,9).
Artesunate (Art) is a remarkable antimalarial agent,
particularly in severe and drug-resistant cases (10–12).
Art has also been demonstrated to exert profound anticancer
activity (13–15). Art, a powerful antimalarial herbal
compound, has been shown to inhibit the growth of various tumor
cell lines in vitro and xenografted carcinoma in mice in
vivo. Art has also been shown to inhibit the growth of
esophageal cancer cells. Art may have anticancer effects on
drug-resistant cells, indicating that the compound may reverse the
drug resistance of cancer cells (16–18).
Art has few adverse effects, so it may be developed into a drug to
reverse MDR.
In the present study, the gene and protein
expression of ABCG2 was detected by various experimental methods,
to study the correlation between ABCG2 expression and the
resistance of esophageal carcinoma. An Eca109/ABCG2 MDR cell was
established by transfecting the ABCG2 gene into Eca109 cells. The
ABCG2 expression level and drug efflux of the Eca109/ABCG2 cells
was assessed using RT-PCR, western blot analysis and flow
cytometry.
The mechanism for the reversal of multidrug
resistance by Art in esophageal carcinoma was analyzed using
cellular experiments. The correlation between ABCG2 expression in
esophageal carcinoma and MDR, and the reversal of MDR by Art were
investigated in the present study. These results may be beneficial
to the chemotherapy of esophageal carcinoma in the clinic.
Materials and methods
Chemicals and reagents
Geneticin (G418), dimethyl sulfoxide (DMSO),
trypsin, RPMI-1640 and a
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT)
kit were purchased from Sigma-Aldrich (St. Louis, MO, USA). The
Lipofectamine™ 2000 kit was purchased from Invitrogen (Carlsbad,
CA, USA). Fluorescein isothiocyanate (FITC)-conjugated ABCG2
antibodies were purchased from Biolegend (San Diego, CA, USA).
ABCG2 gene transfection
PCDNA3.1-ABCG2 plasmids containing ABCG2 cDNA were
purchased from Jing Sai Co. (Wuhan, China). Lipofectamine 2000
(Invitrogen) was used as a transfection reagent, according to the
manufacturer’s instructions, and positive cell clones were selected
using 600 mg/l G418 subsequent to being transfected for 72 h. The
Eca109 cells that were transfected with PCDNA3.1 served as the
control group. The Eca109 cells that were transfected with
PCDNA3.1-ABCG2 and PCDNA3.1 were termed the Eca109/ABCG2 and
Eca109/PCDNA3.1 cells, respectively. To ascertain the efficacy and
specificity of the transfection, ABCG2 mRNA and protein levels were
monitored using RT-PCR, western blot analysis and flow cytometry,
respectively.
Cells and cell culture
The Eca109 esophageal cancer cell line was obtained
from the Tumor Institute of the Fourth Hospital of Hebei Medical
University (Shijiazhuang, China). The Eca109 cells were maintained
in RPMI-1640 supplemented with 10% fetal bovine serum (FBS), 5%
penicillin (100 U/ml) and streptomycin (100 mg/ml) in a humidified
atmosphere of 95% air and 5% CO2 at 37°C. The medium was
changed three times a week. The Eca109/ABCG2 cells were maintained
in RPMI-1640 supplemented with 10% FBS and 300 mg/l G418.
Cytotoxicity assay
The sensitivity of the Eca109, Eca109/ABCG2 and
Eca109/PCDNA3.1 cells to the anticancer drugs [adriamycin (ADM),
daunorubicin (DNR) and mitoxantrone (MIT)] was determined using the
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT)
assay, which is based on the capacity of viable cells to metabolize
a yellow tetrazolium salt, MTT, using mitochondrial succinate
dehydrogenase, into purple formazan crystals when dissolved in
acidified propan-2-ol; the resulting purple solution is then
spectrophotometrically measured at 490 nm. The cells were seeded
into 96-well culture plates at a density of 5×104
cells/ml. The serial concentrations of the anticancer drugs, ADM,
DNR and MIT, were added in a final volume of 200 μl/well. Following
the drug treatment for 72 h, the medium was replaced with an equal
volume of fresh medium containing 0.5 mg/ml MTT and incubated for 4
h. The medium was removed and 180 μl DMSO was added and incubated
for 10 min at room temperature. The cytotoxic effects of the drugs
were determined according to the optical density (OD) values using
a microplate reader at an absorption wavelength of 490 nm. The cell
viability is expressed as the relative formazan formation in the
treated samples compared with the control cells (A490 treated cells
/ A490 control cells × 100%).
Flow cytometry
The level of ABCG2 protein expression, the apoptosis
rate and the ADM content were analyzed in the Eca109 cells using
flow cytometry.
The cells were harvested with trypsin-EDTA (1:20),
washed with phosphate-buffered saline (PBS) and centrifuged for 5
min at 1,200 × g. The FITC-conjugated anti-ABCG2 antibody was added
and the cells were incubated at room temperature for 30 min in the
dark. The labeled cells were washed in PBS, centrifuged for 5 min
at 1,200 × g and kept at 4°C until they were used.
The cells were dyed in 1 ml fluorescence liquor
containing 50 μg/ml propidium iodide (PI) following incubation for
30 min in the dark at 4°C, then kept at 4°C until they were
used.
The ADM in the Eca109 cells exhibited red
fluorescence, which was detected by a 488 nm laser and analyzed
using flow cytometry.
The cells were analyzed using a Beckman Coulter
Epics-XL II type cytometer equipped with a 488 nm argon ion laser
(Beckman Coulter, Miami, FL, USA). For each sample, 10,000 events
selected in the living cell gate were measured. Forward scatter
(FSC) and side scatter (SSC) data were used to establish a gate
excluding the dead cells and debris.
ADM accumulation and efflux by flow
cytometry
The cellular accumulation and efflux of ADM were
analyzed by flow cytometry. The Eca109, Eca109/PCDNA3.1 and
Eca109/ABCG2 cells (4×105) were incubated with 0.02
μg/ml ADM at 37°C for 2 h and washed twice with ice-cold PBS. The
cells were resuspended in ADM-free RPMI-1640 for 1 h. The cells
were washed with ice-cold PBS and the ADM that was retained in the
cells was detected by flow cytometry.
In the drug efflux studies, 4×105
Eca109/ABCG2 cells were incubated with 0.02 μg/ml ADM at 37°C for 2
h and washed twice with ice-cold PBS. The cells were resuspended in
ADM-free RPMI-1640 in the absence or presence of 1 μmol/l Art for 1
h. The cells were washed with ice-cold PBS and the ADM that was
retained in the cells was detected by flow cytometry.
Total mRNA isolation and RT-PCR
Total RNA was extracted using TRIzol according to
the manufacturer’s instructions and then treated with AMV reverse
transcriptase to form cDNA (Promega Corporation, Madison, WI, USA).
The cDNA was amplified by PCR using TaqDNA polymerase, which was
performed by denaturation at 94°C for 30 sec, annealing at 57°C for
30 sec and extension at 72°C for 30 sec. This was performed for 35
cycles. The PCR-amplified products were run on a 1.5% agarose gel
and visualized by ethidium bromide staining. The expression
intensities of the optimized bands were quantified using Quantity
One software (Bio-Rad, Mississauga, ON, Canada) and expressed as a
ratio [ABCG2 vs. glyceraldehyde 3-phosphate dehydrogenase (GAPDH)].
The PCR primers were as follows: ABCG2 forward, 5′-GGT CAG AGT GTG
GTT TCT GTA GCA-3′ and reverse, 5′-GTG AGA GAT CGA TGC CCT GCT
TTA-3′ (product, 280 bp); and GAPDH forward, 5′-ACC ACA GTC CAT GCC
ATC AC-3′ and reverse, 5′-TCC ACC ACC CTG TTG CTG TA-3′ (product,
452 bp).
Western blotting
The ABCG2 protein expression level was detected by
western blotting. Each cell line was grown to confluence,
trypsinized, transferred to eppendorf tubes and rinsed with
ice-cold PBS. The contents of each tube were suspended in 200 μl
lysis buffer in the presence of protease inhibitors. The cell
suspension was incubated for 20 min at 4°C and centrifuged for 10
min at 32,000 × g to obtain a clear supernatant. The protein
concentration was measured by the Bio-Rad protein assay with bovine
serum albumin (BSA) as a standard (Bio-Rad Laboratories,
Mississauga, ON, Canada). For the western blot analysis, the
protein extract was analyzed using SDS gel electrophoresis on
appropriate polyacrylamide gels (5%) with 50 μg protein loaded on
each lane. The proteins on the gels were then transferred to
polyvinylidene difluoride (PVDF) membranes by a semi-dry transfer
method. Subsequent to blocking with Tris-buffered saline (TBS)
containing 5% skimmed milk and 0.1% Tween-20, the membranes were
probed with a primary antibody against ABCG2 (mouse monoclonal
antibody, clone no. sc-18841; Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA). The antibody was used at a dilution of 1:1,000. The
membrane blots were then reacted with a secondary antibody
(horseradish peroxidase anti-mouse immunoglobulin G), washed
extensively with TBS (0.1% Tween-20) and submerged in
3,3′-diaminobenzidine (DAB), then developed to visualize the
antibody-antigen complexes.
Statistical analysis
The statistical analysis was performed using SPSS
11.5 software (SPSS, Inc., Chicago, IL, USA). The data are
presented as the mean ± SD and three individual experiments were
performed in triplicate. A one-way ANOVA and an LSD test were used
to compare the data between three or more groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
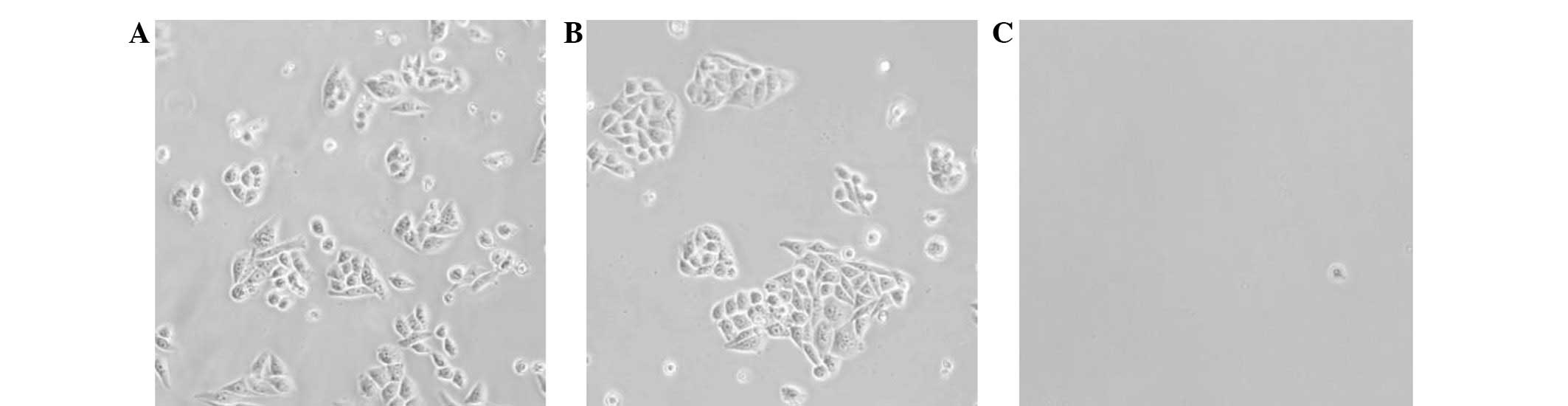
Establishment of Eca109/ABCG2 cell
line
The Eca109 cells that were transfected with
PCDNA3.1-ABCG2 plasmids were selected by G418 for 14 days. The
positive clones, i.e. the Eca109/ABCG2 cells, survived, but the
negative clones died (Fig. 1). The
Eca109 cells that were transfected with PCDNA3.1, i.e. the
Eca109/PCDNA3.1 cells, were used as the control cells.
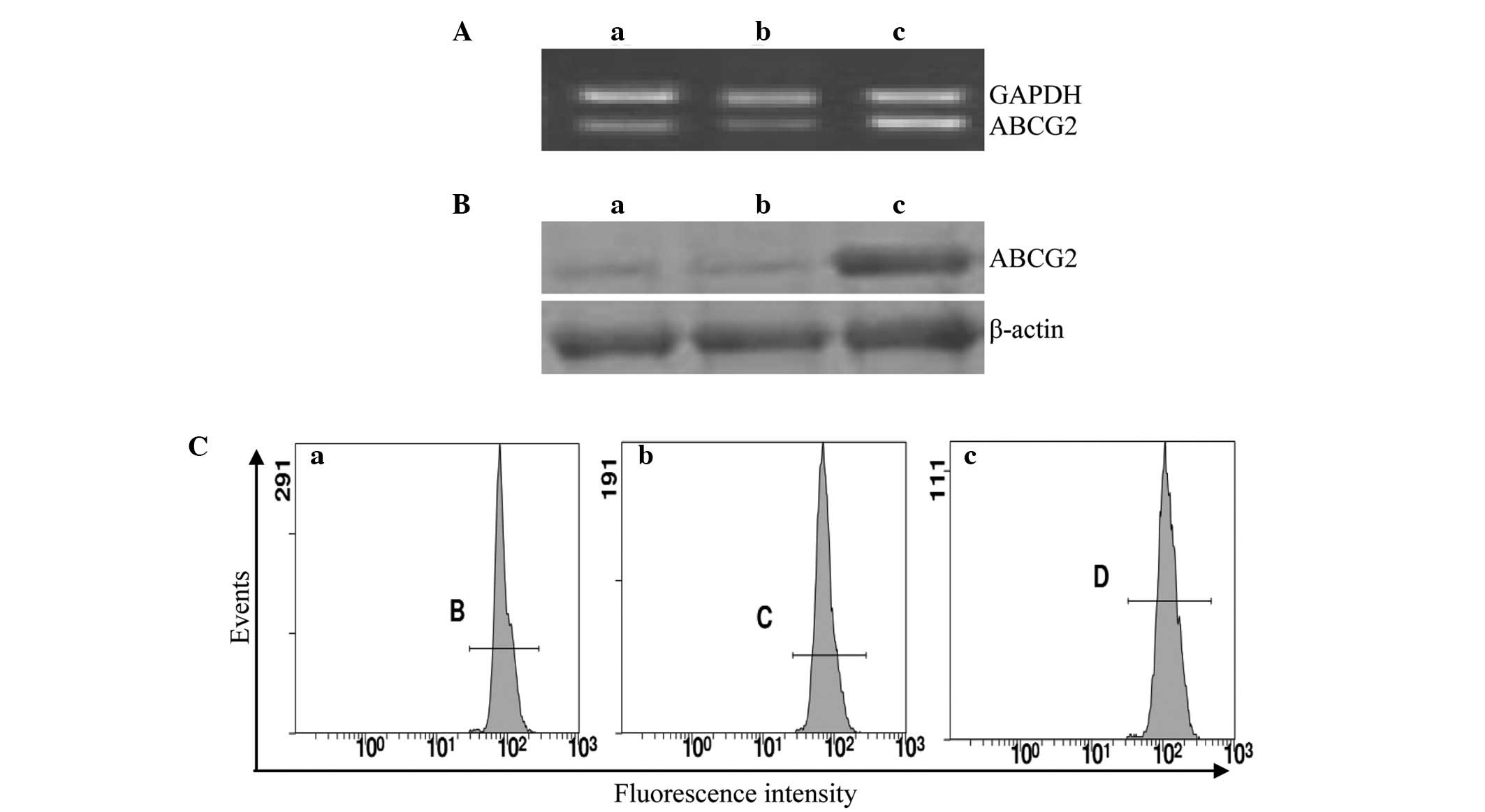
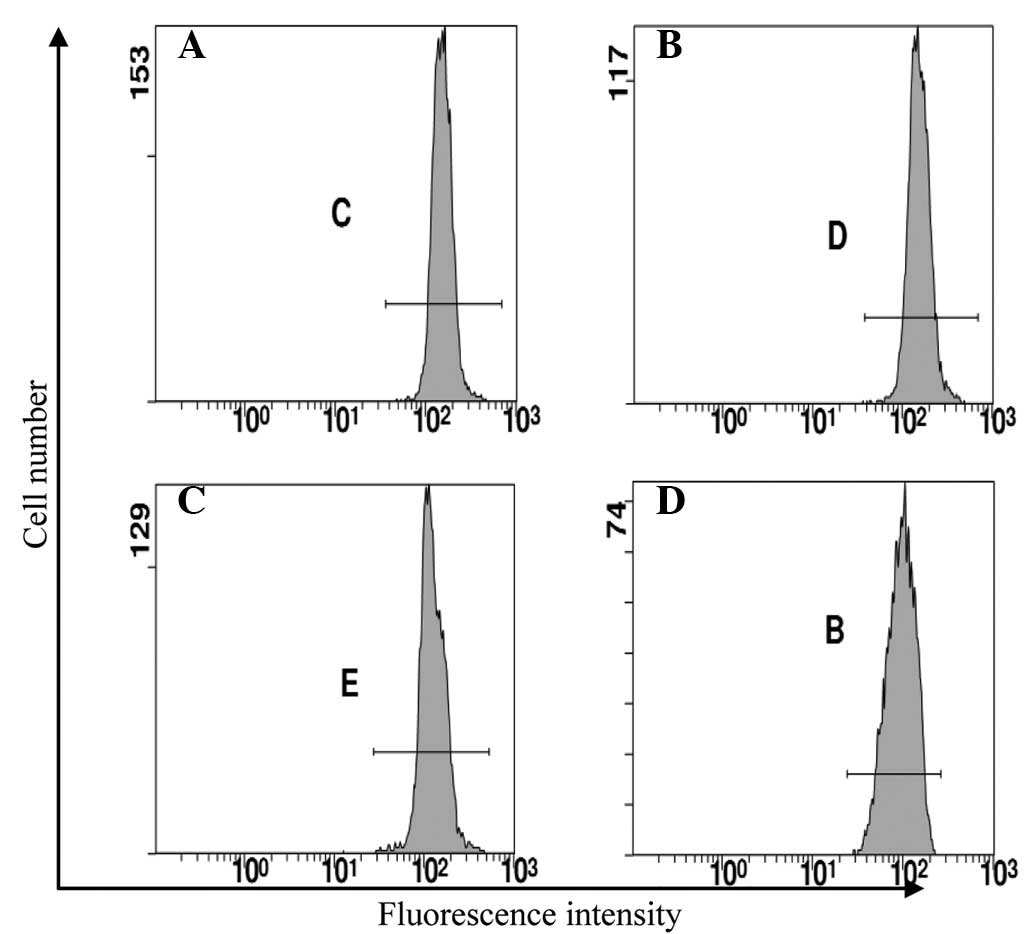
The expression of ABCG2 in the Eca109, Eca109/ABCG2
and Eca109/PCDNA3.1 cells was examined using RT-PCR, western blot
analysis and flow cytometry. The ABCG2 expression level in the
Eca109/ABCG2 cells was higher than that in the Eca109 and
Eca109/PCDNA3.1 cells (P<0.05; Fig.
2; Table I).
 | Table IExpression level of ABCG2 in various
cells detected by RT-PCR, western blot analysis and FCM. |
Table I
Expression level of ABCG2 in various
cells detected by RT-PCR, western blot analysis and FCM.
| Cell | ABCG2 mRNAa | ABCG2 proteinb | ABCG2 proteinc |
|---|
| Eca109 | 0.68±0.04d | 0.33±0.04d | 628.25±12.49d |
| Eca109/PCDNA3.1 | 0.67±0.04d | 0.36±0.05d | 627.59±9.62d |
| Eca109/ABCG2 | 0.93±0.05 | 0.74±0.05 | 689.93±10.15 |
Resistance of Eca109/ABCG2 cells to
anticancer agents
Following the anticancer drug treatment using ADM,
DNR and MIT for 72 h, the IC50 was detected by MTT
assay. The Eca109/ABCG2 cells were resistant to ADM, DNR and MIT
compared with the Eca109 and Eca109/PCDNA3.1 cells, and the
resistance to ADM was similar to the resistance to DNR and MIT
(Table II).
 | Table IIResistance of Eca109/ABCG2 cells to
anticancer agents. |
Table II
Resistance of Eca109/ABCG2 cells to
anticancer agents.
| IC50 |
|---|
|
|
|---|
| Drug | Eca109 | Eca109/PCDNA3.1 | Eca109/ABCG2 |
|---|
| ADM | 1.14±0.06
(1.00)a | 1.16±0.06
(1.02)a | 6.80±0.07 (5.96) |
| DNR | 0.41±0.07
(1.00)a | 0.39±0.07
(0.95)a | 2.32±0.07 (5.66) |
| MIT | 0.49±0.06
(1.00)a | 0.47±0.06
(0.96)a | 2.71±0.06 (5.53) |
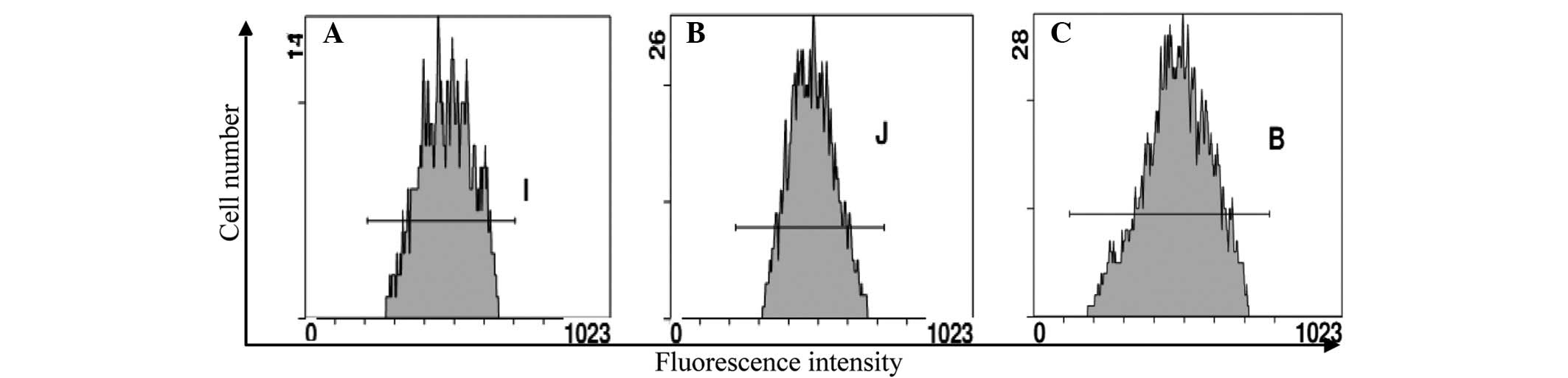
ADM efflux effect of Eca109/ABCG2 cells
assayed by flow cytometry
To investigate how the Eca109/ABCG2 cells resisted
the anticancer agents, the ADM efflux effect was investigated using
flow cytometry (Fig. 3). The cells
were incubated at 37°C with 0.02 μg/ml ADM for 2 h and then without
ADM for 1 h. The level of ADM decreased more in the Eca109/ABCG2
cells than in the Eca109 and Eca109/PCDNA3.1 cells. The ADM efflux
effect of the Eca109/ABCG2 cells was more than that of the Eca109
and Eca109/PCDNA3.1 cells.
Reversal of drug resistance by Art in
Eca109/ABCG2 cells
The ability of Art to reverse the drug resistance of
the Eca109/ABCG2 cells was examined using an MTT assay (Table III). Art alone at 0.01, 0.1 and 1
μmol/l had no cytotoxic effects on the Eca109, Eca109/PCDNA3.1 and
Eca109/ABCG2 cells. Therefore, 0.01, 0.1 and 1 μmol/l Art was used
in this experiment. Art at 0.01 and 0.1 μmol/l moderately reversed
the resistance of the Eca109/ABCG2 cells to ADM, while Art at 1
μmol/l completely reversed the resistance of the Eca109/ABCG2 cells
to ADM.
 | Table IIIEffects of Art on the cytotoxicity of
ADM for the Eca109, Eca109/PCDNA3.1 and Eca109/ABCG2 cells. |
Table III
Effects of Art on the cytotoxicity of
ADM for the Eca109, Eca109/PCDNA3.1 and Eca109/ABCG2 cells.
| IC50 |
|---|
|
|
|---|
| Drug | Eca109 | Eca109/PCDNA3.1 | Eca109/ABCG2 |
|---|
| ADM | 1.14±0.06 (1.00) | 1.16±0.06 (1.02) | 6.80±0.07 (5.96) |
| +Art (0.01
μmol/l) | 1.13±0.07 (1.00) | 1.14±0.06 (1.01) | 4.64±0.07
(4.11)a |
| +Art (0.1
μmol/l) | 1.10±0.06 (1.00) | 1.13±0.07 (1.03) | 2.45±0.06
(2.23)a |
| +Art (1 μmol/l) | 1.08±0.07 (1.00) | 1.11±0.07 (1.03) | 1.21±0.06
(1.12)a |
Effect of Art on the apoptosis of
Eca109/ABCG2 cells induced by ADM
To investigate the reversal of the drug resistance
caused by Art in the Eca109/ABCG2 cells, the effect of Art on the
apoptosis of the Eca109/ABCG2 cells induced by ADM was
investigated. The apoptosis rate of the Eca109/ABCG2 cells
following the treatment with ADM and Art was higher than that
following the treatment with ADM alone (P<0.05; Fig. 4; Table
IV).
 | Table IVApoptosis rate of Eca109/ABCG2 cells
following various drug treatments for various times, as detected by
FCM. |
Table IV
Apoptosis rate of Eca109/ABCG2 cells
following various drug treatments for various times, as detected by
FCM.
| Apoptosis rate,
% |
|---|
|
|
|---|
| Drug | 24 h | 48 h | 72 h |
|---|
| ADM (0 mg/l) | 1.29±0.60 | 1.17±0.33 | 1.28±0.31 |
| ADM (0.2 mg/l) | 3.78±0.13 | 7.22±0.35 | 9.94±0.08 |
| +Art (0.01
μmol/l) | 4.18±0.27 | 8.69±0.50 | 13.43±0.52 |
| +Art (0.1
μmol/l) | 7.18±0.32a | 13.01±0.46a | 17.81±0.24a |
| +Art (1
μmol/l) | 9.99±0.29a | 17.15±0.89a | 22.95±0.08a |
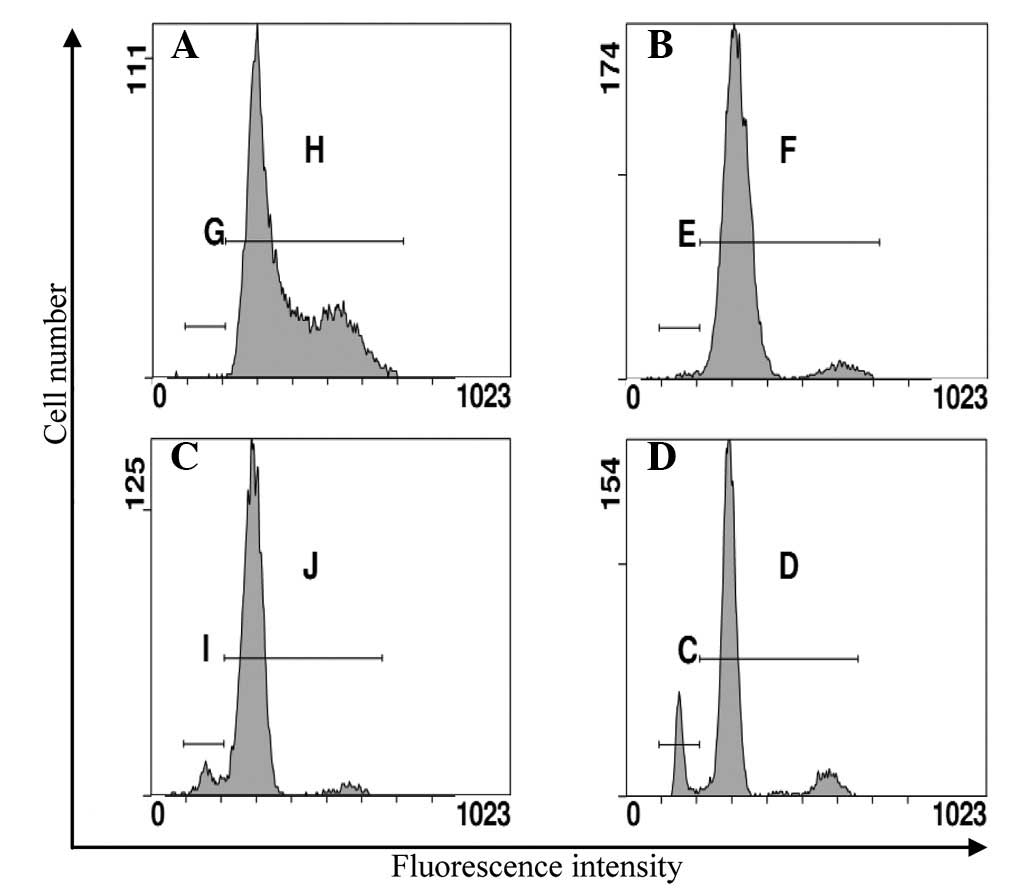
Effect of Art on the cellular
accumulation and efflux of ADM
To investigate how Art reversed the resistance of
the Eca109/ABCG2 cells to the anticancer agents, the effect on the
accumulation of ADM was investigated using flow cytometry. The
increased accumulation of ADM in the Eca109/ABCG2 cells caused by
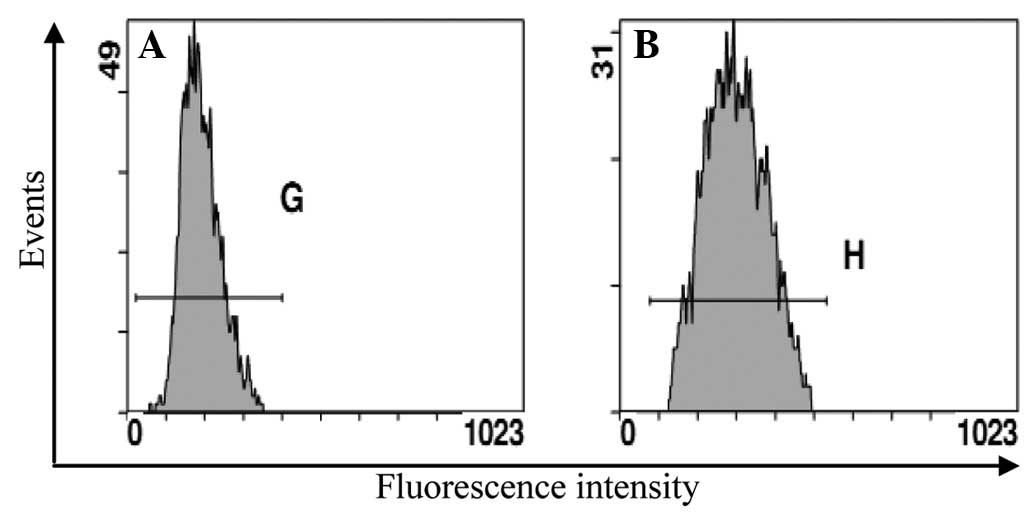
Art was due to the inhibition of the ADM efflux (Fig. 5). When the cells were incubated at
37°C with 0.02 μg/ml ADM for 2 h and then without ADM and with or
without 1 μmol/l Art for 1 h, the level of ADM in the Eca109/ABCG2
cells with Art showed a smaller decrease than that without Art. Art
inhibited the efflux of ADM from the Eca109/ABCG2 cells.
Effect of Art on the expression level of
ABCG2 in Eca109/ABCG2 cells
To investigate the mechanism of the reversal of
resistance caused by Art in the Eca109/ABCG2 cells the effect of
Art on the expression level of ABCG2 in the Eca109/ABCG2 cells was
investigated. Art inhibited the expression level of ABCG2 protein
in the Eca109/ABCG2 cells (Fig.
6).
Discussion
Chemotherapy is the most common treatment for
esophageal carcinoma, particularly for advanced esophageal cancer
and recurrent cancer. However, the response from patients with
recurrent esophageal cancer remains poor. Drug resistance is major
obstacle for chemotherapy results and is attributable to several
processes that occur in neoplastic cells. One of these processes is
the decreased accumulation of anticancer drugs within the cancer
cells due to drug efflux, which is mediated by ABC transporters
(19,20). Overexpression of these transporters
results in drug resistance in a number of tumors (21,22).
However, the significance of the expression of the ABC protein in
esophageal cancer has not yet been reported. The present study
investigated a potential correlation between ABCG2 expression and
MDR in esophageal cancer.
ABCG2 is a member of the ABC family and functions as
a ABC discharge pump. ABCG2 was initially isolated from a
doxorubicin-resistant MCF-7/AdrVP breast cancer cell line (21). ABCG2 is a 655-amino acid, 72-kDa
protein and the gene is located on chromosomal locus 4q22. The
majority of ABC transporters, including P-gp and MRPs, have two
ATP-binding domains and two sets of transmembrane domains (7). In contrast, ABCG2 has a single
ATP-binding domain at the amino terminus and a single set of
transmembrane domains at the carboxyl terminus. Thus, ABCG2 is a
half ABC transporter. Previous studies revealed that ABCG2 is
expressed in a variety of tumor cells and human solid tumors
(9,22). ABCG2 was demonstrated to be
expressed in numerous normal human tissues (23,24),
including placental syncytiotrophoblasts, brain blood capillaries,
testes, small intestine, colon, kidneys and liver. Among these
tissues, ABCG2 has been identified to be localized in the apical
border of a variety of secretory epithelia, indicating its role in
the protection of the cells against exogenous toxins, including
anticancer agents (23,24). ABCG2 prevents the intracellular
accumulation of substrate compounds, including anticancer drugs, by
limiting the influx into and facilitating the efflux out of cells.
In normal tissues, ABCG2 may play a significant role in the
protection of the fetus or organism from toxic xenobiotics
(23). In cancer cells, a high
expression of ABCG2 prevents the intracellular accumulation of
anticancer drugs, which results in drug resistance (21). The present study discussed the
association between ABCG2 expression and MDR in esophageal
cancer.
In order to investigate the correlation between drug
resistance in esophageal cancer and ABCG2 expression, the ABCG2
gene was transfected into the Eca109 esophageal cancer cell line to
establish Eca109/ABCG2 cells. The expression level of ABCG2 in the
Eca109/ABCG2 cells was higher than that in the Eca109 and
Eca109/PCDNA3.1 cells (Fig. 2). The
Eca109/ABCG2 cells showed cross-resistance to ADM, DNR and MIT
compared with the Eca109 and Eca109/PCDNA3.1 cells (Table I). The drug efflux effect of the
Eca109/ABCG2 cells was stronger than that of the Eca109 and
Eca109/PCDNA3.1 cells (Fig. 3). The
Eca109/ABCG2 cell line was multidrug resistant. A high expression
of ABCG2 in the cells enhanced the drug efflux effect of ADM, DNR
and MIT. Therefore increasing the expression level of ABCG2
resulted in MDR.
Resistance reversal agents for the ABC protein have
been studied and due to the greater toxicity, few agents are used
in clinical applications. The identification of a reversal agent
with low toxicity and efficient resistance is required. Art is a
derivative of artemisinin. Studies have identified that artemisinin
and its derivatives are anti-inflammatory, antifibrosis, fight
schistosomiasis and antitumor agents. Art is a good antimalarial
drug, particularly for heavy and drug-resistant malaria, however,
studies have paid more attention to the antitumor function of Art
(13–16). The antitumor effect of Art has not
produced cross resistance (17),
indicating that Art confers a reversal to drug resistance. The
present study investigated the function and mechanism of Art in the
reversal of esophageal cancer drug resistance. The esophageal
cancer drug resistant Eca109/ABCG2 cell line was established by
transfecting the ABCG2 gene into Eca109 cells. The Eca109/ABCG2
cells had a high expression of the ABCG2 gene and protein compared
with the Eca109 cells (Fig. 2).
High ABCG2 expression in the cells resulted in MDR. The
Eca109/ABCG2 cells were resistant to ADM, DNR and MIT (Table I). The mechanism of MDR that was
induced by ABCG2 was detected for further investigation. The ADM
content in the Eca109/ABCG2, Eca109/PCDNA3.1 and Eca109 cells was
identified using flow cytometry following the treatment with ADM.
The ADM content in the Eca109/ABCG2 cells was lower than in the
Eca109/PCDNA3.1 and Eca109 cells (Fig.
3), indicating that the drug efflux effect of Eca109/ABCG2 was
stronger than that of the other two types of cells. The
Eca109/ABCG2 cells were a cell line with an ABCG2 drug-resistant
phenotype. ABCG2-mediated drug secretion reduced the level of
anticancer drugs in the Eca109/ABCG2 cells, which caused MDR.
The inhibitory effect of ADM on the Eca109/ABCG2
cells was enhanced by combining the drug with Art (Table II). The apoptosis rate of the
Eca109/ABCG2 cells that was induced by ADM was also enhanced by
combining the drug with Art (Fig.
4). These results suggested that Art had a role in the reversal
of drug resistance. For the next level of investigation, the
mechanism behind the reversal of drug resistance by Art was
investigated. Art reduced the level of ABCG2 expression (Fig. 6) and inhibited the drug efflux
effect (Fig. 5) of the Eca109/ABCG2
cells. Art was able to reverse drug resistance by reducing ABCG2
expression and increasing the anticancer drug concentration in the
cancer cells.
In summary, ABCG2 expression participated in the MDR
of esophageal cancer. Art was able to reverse the drug resistance
by reducing ABCG2 expression and increasing the anticancer drug
concentration in the cancer cells. Art is expected to be associated
with low toxic side-effects and may be a highly efficient
resistance reversal agent in the clinic.
Acknowledgements
This study was supported by the Natural Science
Foundation of Hebei, China (no. H2012206107) and the Medical
Science Research Key Issue of Hebei, China (no. 20110131).
References
|
1
|
Gamliel Z: Incidence, epidemiology, and
etiology of esophageal cancer. Chest Surg Clin N Am. 10:441–450.
2000.PubMed/NCBI
|
|
2
|
Mehta NG and Mehta M: Overcoming
multidrug-resistance in cancer: statins offer a logical candidate.
Med Hypotheses. 74:237–239. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Katayama R, Koike S, Sato S, Sugimoto Y,
Tsuruo T and Fujita N: Dofequidar fumarate sensitizes cancer
stem-like side population cells to chemotherapeutic drugs by
inhibiting ABCG2/BCRP-mediated drug export. Cancer Sci.
100:2060–2068. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gillet JP and Gottesman MM: Mechanisms of
multidrug resistance in cancer. Methods Mol Biol. 596:47–76. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fojo T and Coley HM: The role of efflux
pumps in drug-resistant metastatic breast cancer: new insights and
treatment strategies. Clin Breast Cancer. 7:749–756. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gottesman MM, Fojo T and Bates SE:
Multidrug resistance in cancer: role of ATP-dependent transporters.
Nat Rev Cancer. 2:48–58. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gottesman MM, Ambudkar SV and Xia D:
Structure of a multidrug transporter. Nat Biotechnol. 27:546–547.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mao Q and Unadkat JD: Role of the breast
cancer resistance protein (ABCG2) in drug transport. AAPS J.
7:E118–E133. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu HG, Pan YF, You J, Wang OC, Huang KT
and Zhang XH: Expression of ABCG2 and its significance in
colorectal cancer. Asian Pac J Cancer Prev. 11:845–848.
2010.PubMed/NCBI
|
|
10
|
Shanks GD: For severe malaria, artesunate
is the answer. Lancet. 376:1621–1622. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zoller T, Junghanss T, Kapaun A, Gjorup I,
Richter J, Hugo-Persson M, Mørch K, Foroutan B, Suttorp N, Yürek S
and Flick H: Intravenous artesunate for severe malaria in
travelers, Europe. Emerg Infect Dis. 17:771–777. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bello SO: Pre-referral artesunate in
severe malaria. Lancet. 373:1762–1763. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zeng Y, Ni X, Meng WT, Wen Q and Jia YQ:
Inhibitive effect of artesunate on human lymphoblastic
leukemia/lymphoma cells. Sichuan Da Xue Xue Bao Yi Xue Ban.
40:1038–1043. 2009.(In Chinese).
|
|
14
|
Steinbrück L, Pereira G and Efferth T:
Effects of artesunate on cytokinesis and G2/M cell cycle
progression of tumour cells and budding yeast. Cancer Genomics
Proteomics. 7:337–346. 2010.PubMed/NCBI
|
|
15
|
Hamacher-Brady A, Stein HA, Turschner S,
Toegel I, Mora R, Jennewein N, Efferth T, Eils R and Brady NR:
Artesunate activates mitochondrial apoptosis in breast cancer cells
via iron-catalyzed lysosomal reactive oxygen species production. J
Biol Chem. 286:6587–6601. 2011. View Article : Google Scholar
|
|
16
|
Michaelis M, Kleinschmidt MC, Barth S,
Rothweiler F, Geiler J, Breitling R, Mayer B, Deubzer H, Witt O,
Kreuter J, Doerr HW, Cinatl J and Cinatl J Jr: Anti-cancer effects
of artesunate in a panel of chemoresistant neuroblastoma cell
lines. Biochem Pharmacol. 79:130–136. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Efferth T, Giaisi M, Merling A, Krammer PH
and Li-Weber M: Artesunate induces ROS-mediated apoptosis in
doxorubicin-resistant T leukemia cells. PLoS One. 2:e6932007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Reungpatthanaphong P and Mankhetkorn S:
Modulation of multidrug resistance by artemisinin, artesunate and
dihydroartemisinin in K562/adr and GLC4/adr resistant cell lines.
Biol Pharm Bull. 25:1555–1561. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Haufroid V: Genetic Polymorphisms of
ATP-binding cassette transporters ABCB1 and ABCC2 and their impact
on drug disposition. Curr Drug Targets. 12:631–646. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shukla S, Ohnuma S and Ambudkar SV:
Improving cancer chemotherapy with modulators of ABC drug
transporters. Curr Drug Targets. 12:621–630. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Doyle LA, Yang W, Abruzzo LV, Krogmann T,
Gao Y, Rishi AK and Ross DD: A multidrug resistance transporter
from human MCF-7 breast cancer cells. Proc Natl Acad Sci USA.
95:15665–15670. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li J, Li ZN, Du YJ, Li XQ, Bao QL and Chen
P: Expression of MRP1, BCRP, LRP, and ERCC1 in advanced
non-small-cell lung cancer: correlation with response to
chemotherapy and survival. Clin Lung Cancer. 10:414–421. 2009.
View Article : Google Scholar
|
|
23
|
Ni Z and Mao Q: ATP-binding cassette
efflux transporters in human placenta. Curr Pharm Biotechnol.
12:674–685. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Poller B, Wagenaar E, Tang SC and Schinkel
AH: Double-transduced MDCKII cells to study human P-glycoprotein
(ABCB1) and breast cancer resistance protein (ABCG2) interplay in
drug transport across the blood-brain barrier. Mol Pharm.
8:571–582. 2011. View Article : Google Scholar
|