Introduction
Squamous cell carcinoma is the most common type of
malignant neoplasm of the oral cavity. Due to the extent of recent
investigation of its pathogenesis and management, the five-year
survival rate for patients with oral squamous cell carcinoma (OSCC)
has marginally improved within the last 15 years, yet it remains as
low as 60% (1). The presence of
cervical lymph node metastasis is the foremost reliable, adverse
prognostic factor in patients with OSCC. The presence of metastatic
spread to the regional lymph nodes correlates strongly with a poor
overall prognosis, an increased risk of distant metastasis and a
reduction in the five-year survival rate by ~50% (2).
Epithelial-mesenchymal transition (EMT) is a key
step toward cancer metastasis. EMT is marked the by loss of
epithelial characteristics (such as cell polarity and cell-cell
junctions) and the gain of mesenchymal characteristics (including
fibroblastic spindle-shaped morphology and increased motility). It
has been proposed that EMT may provide a link between cancer
metastasis and stem cell properties (3).
The tumor microenvironment appears to play a
prominent role in affecting EMT changes. For example, the
E-cadherin transcriptional repressor, TWIST, is positively
regulated by hypoxia-induced factor 1α (HIF-1α) (4). In a previous study, a number of
different types of cancer cells were exposed to carefully
controlled hypoxic conditions and investigated for EMT changes
(5).
Notch signaling influences a variety of cellular
processes, including cell fate specification, proliferation,
differentiation, apoptosis and the maintenance of stem cells
(6). To date, four vertebrate Notch
genes have been identified: Notch1-4. In addition, five ligands,
Dll1, Dll3, Dll4 and Jagged1/2, have been identified in mammals.
Aberrant Notch signaling is detected in a range of human tumor
types. Upregulated expression of Notch receptors and their ligands
has been identified in cervical, lung, colon, renal and pancreatic
cancer, as well as in acute myeloid leukemia and Hodgkin’s and
large-cell lymphomas (7,8).
Certain studies have revealed links between hypoxia
and activation of Notch in solid tumors (9,10). Low
oxygen content has been observed to potentiate Notch signaling in
melanocytes through stabilization of HIF-1α (11). In addition, Notch signaling and Wnt
pathways have been demonstrated to be required for conversion of
the hypoxic stimulus into EMT, increased motility and invasiveness
(12).
However, the molecular mechanisms by which hypoxia
affects EMT and the Notch signaling pathway, in order to increase
the invasiveness and metastatic potential of OSCC, are unclear. In
this study, hypoxia potentiated EMT, which was induced by Notch
signal activation, thus enhancing motility and invasiveness in
human OSCC cell lines.
Materials and methods
Cell culture
Human OSCC cell lines, HSC-2, HSC-4 and Ca99-2, were
purchased from Health Science Research Resources Bank (Osaka,
Japan). Cell lines were cultured in Minimum Essential Medium Eagle
(Sigma-Aldrich, St. Louis, MO, USA), supplemented with 10% fetal
bovine serum (CCB, Nichirei Bioscience, Tokyo, Japan), penicillin
(100 U/ml), streptomycin (100 μg/ml) and amphotericin B (0.25
μg/ml) (Invitrogen, Carlsbad, CA, USA). The cells were placed under
hypoxia (1% O2) or normoxia (21% O2) for 24
h. Notch activity was blocked by 5 μM DAPT, a γ-secretase inhibitor
(GSI; Calbiochem, Basel, Switzerland) in immunohistochemical,
wound-healing and invasion assays.
qPCR
Total RNAs were isolated using TRIzol (Invitrogen),
and cDNAs were synthesized with a High-Capacity cDNA Reverse
Transcription kit (pplied Biosystems, Foster City, CA, USA) from 1
μg of total RNA. qPCR was performed with the cDNA samples and 2X
SYBR-Green PCR master mix (PE Applied Biosystems), using a Step
One™ Real-Time PCR system (Applied Biosystems). The formation of
the PCR product was monitored using SYBR-Green (Applied
Biosystems). All samples were amplified in duplicate. The relative
changes in the levels of the transcripts in each sample were
determined by normalization with the β-actin mRNA levels. The
sequences of the primers used in the real-time PCR are listed in
Table I.
 | Table IPCR primer sequences. |
Table I
PCR primer sequences.
| Target molecules | Primer sequence |
|---|
| Notch1 | Forward:
5′-CAATGTGGATGCCGCAGTTGTG-3′
Reverse: 5′-CCATCCTGGGACTTCTTCCT-3′ |
| Notch2 | Forward:
5′-AAAAATGGGGCCAACCGAGAC-3′
Reverse: 5′-TTCATCCAGAAGGCGCACAA-3′ |
| Notch3 | Forward:
5′-TCTTGCTGCTGGTCATTCTC-3′
Reverse: 5′-TGCCTCATCCTCTTCAGTTG-3′ |
| Notch4 | Forward:
5′-CACTGAGCCAAGGCATAGAC-3′
Reverse: 5′-ATCTCCACCTCACACCACTG-3′ |
| Jagged1 | Forward:
5′-CGGGATTTGGTTAATGGTTATC-3′
Reverse: 5′-ATAGTCACTGGCACGGTTGTAGCAC-3′ |
| Dll4 | Forward:
5′-TGACCACTTCGGCCACTATG-3′
Reverse: 5′-AGTTGGAGCCGGTGAAGTTG-3′ |
| HES1 | Forward:
5′-AGGCGGACATTCTGGAAATG-3′
Reverse: 5′-CGGTACTTCCCCAGCACACTT-3′ |
| HEY1 | Forward:
5′-CGAGGTGGAGAAGGAGAGTG-3′
Reverse: 5′-CTGGGTACCAGCCTTCTCAG-3′ |
| Snail | Forward:
5′-CATCCTTCTCACTGCCATGGA-3′
Reverse: 5′-AGGCAGAGGACACAGAACCAGA-3′ |
| β-actin | Forward:
5′-AAGAGATGGCCACGGCTG-3′
Reverse: 5′-GAACCGCTCATTGCCAATG-3′ |
Immunohistochemical examination
Cells were fixed with 4% paraformaldehyde
(Sigma-Aldrich) in phosphate buffered saline (PBS) and subjected to
immunofluorescence staining. Cells were blocked with PBS containing
10% fetal calf serum and 0.1% Triton X-100 (Sigma-Aldrich). The
primary rabbit polyclonal antibody used was anti-E-Cadherin
(diluted 1:200; Calbiochem, Basel, Switzerland). A fluorescent
rhodamine-conjugated donkey anti-rabbit IgG antibody (diluted
1:200; Chemicon, Temecula, CA, USA) was used as the secondary
antibody.
Wound-healing assay
Cell migration was analyzed by a scratch
wound-healing assay. Cells were grown to confluence and a scratch
wound was made in the monolayer by dragging a pipette tip across
it. Detached cells were washed away with PBS, and fresh medium was
used for culture under hypoxia or normoxia. Photomicrographs of
three areas were simultaneously captured under a phase contrast
microscope (CKX41; Olympus, Tokyo, Japan), and migrated cells were
counted in three scratched areas at magnification, ×400.
Invasion assay
Cell invasion was analyzed using the BD BioCoat™
Matrigel™ Invasion Chamber (BD, Franklin Lakes, NJ, USA), according
to the manufacturer’s instructions. Individual cells were plated in
the upper insert, at a density of 1.5×105 cells/ml for a
24-well chamber, in serum-free medium. Medium containing 10% fetal
bovine serum as a chemoattractant was added to the well. The cells
were placed under hypoxia (1% O2) or normoxia (21%
O2) for 24 and 48 h. Invaded cells were stained by
Diff-Quik kit (Sysmex, Kobe, Japan) according to the manufacturer’s
instructions. Invaded cells were counted in three suitable areas by
stereoscopic microscope (BH-2; Olympus) at magnification,
×400.’
Statistical analyses
Data are presented as mean ± standard deviation.
Differences were analyzed to determine their statistical
significance using Student’s t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Expression of Notch signaling molecules
and Snail mRNA is upregulated by hypoxia
To analyze whether Notch receptor activity was
promoted by hypoxia in OSCC cell lines, Notch1-4 mRNA expression
was investigated using qPCR (Fig.
1A). Notch1 mRNA expression was upregulated in HSC-2 cells
(3.52-fold) and HSC-4 cells (2.45-fold) under hypoxia, compared
with the levels under normoxia. Notch2 was upregulated in HSC-2
cells (3.04-fold) and Ca9-22 cells (2.45-fold) under hypoxia.
Notch3 mRNA expression was upregulated in HSC-2 cells (6.13-fold),
and Notch-4 was upregulated in Ca9-22 cells (2.97-fold). These
results indicated that hypoxia increased the expression level of
Notch receptor mRNA in OSCC cell lines.
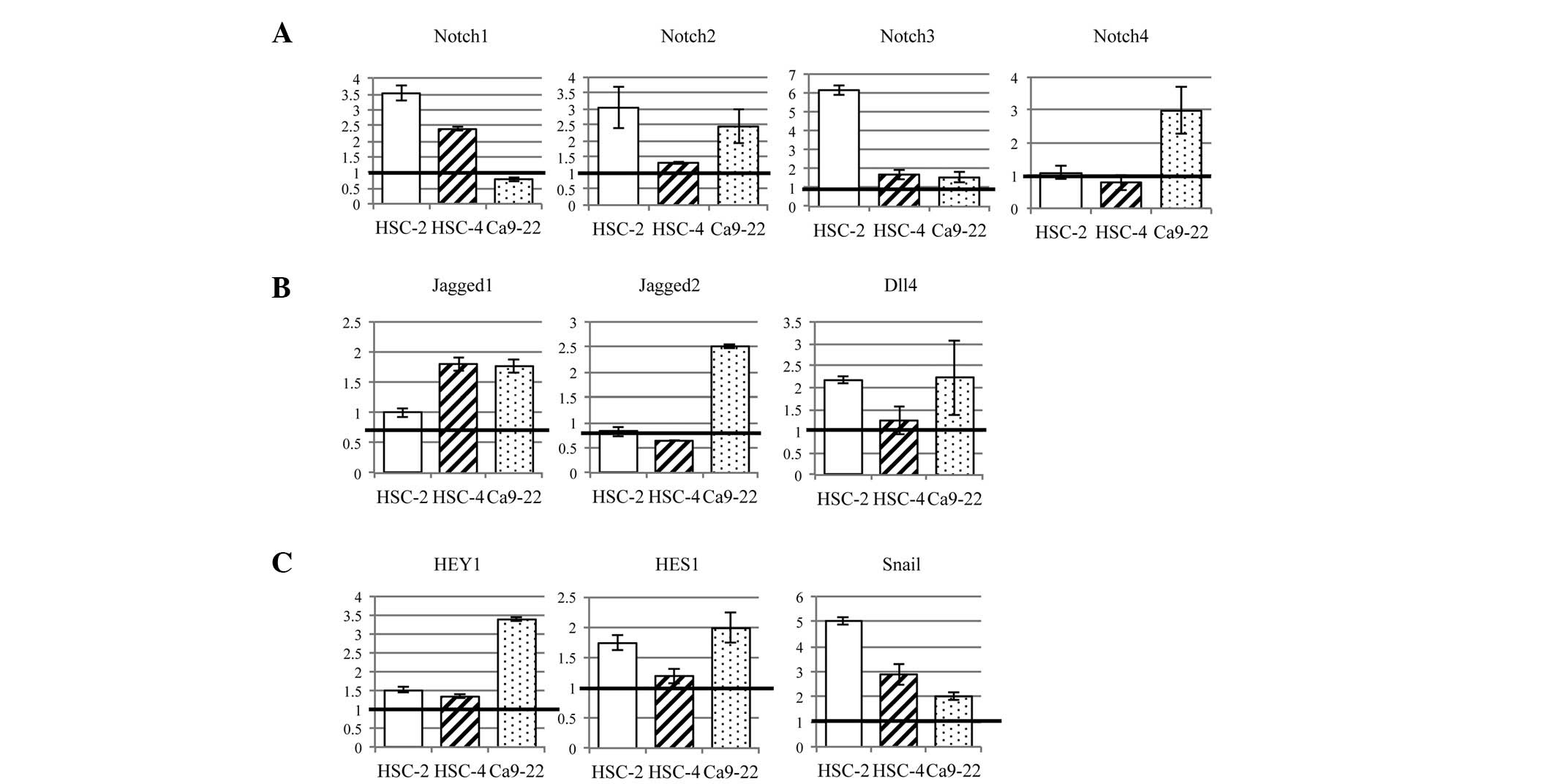 | Figure 1Expression of Notch signaling pathway
genes was increased by hypoxia, compared with the levels under
normoxia, in OSCC cell lines. (A) Notch receptors were upregulated
in all three OSCC cell lines by hypoxia; in particular, HSC-2 cells
showed a marked increase in Notch1-3 expression. (B) Three Notch
ligands, Jagged1/2 and Dll4, were examined. These ligands were
increased in OSCC cell lines by hypoxia. (C) Notch target genes,
HEY1 and HES1, were upregulated by hypoxia. Snail, a marker of EMT,
was increased by hypoxia in all OSCC cell lines. OSCC, oral
squamous cell carcinoma; EMT, epithelial-mesenchymal
transition. |
We examined three Notch ligands, Jagged1/2 and Dll4
(Fig. 1B). Expression of Jagged1
mRNA was increased in HSC-4 cells (1.80-fold) and Ca9-22 cells
(1.76-fold) by hypoxia. Jagged2 was increased in Ca9-22 cells
(2.51-fold). Dll4 was increased in HSC-2 cells (2.17-fold) and
Ca9-22 cells (2.23-fold). Thus, hypoxia promoted Notch ligand
expression in OSCC cell lines.
The expression of Notch target genes, HEY1 and HES1,
was increased in all three OSCC cell lines (1.19- to 3.38-fold)
(Fig. 1C). In particular, HEY1 and
HES1 were upregulated, 1.98- and 3.38-fold in Ca9-22 cells and
1.73- and 1.51-fold in HSC-2 cells, respectively, compared with the
levels under normoxia. In HSC-4 cells, HES1 and HEY1 were not
markedly affected by hypoxia (1.33- and 1.19-fold,
respectively).
Snail, an E-cadherin repressor, was increased in all
three OSCC cell lines by hypoxia (2.01- to 5.03-fold) (Fig. 1C). This indicated that a hypoxic
environment promoted EMT through increased expression of Snail.
Hypoxia decreases expression of
E-cadherin via activation of Notch signaling
The expression of E-cadherin was examined in a 24-h
culture under hypoxia or normoxia. In all three OSCC cell lines,
E-cadherin expression decreased upon culture under hypoxia compared
with that under normoxia (Fig. 2),
indicating that hypoxia induced EMT in OSCC cell lines. Notch
signaling-induced EMT in a hypoxic environment was subsequently
examined. GSI, a Notch inhibitor, prevented downregulation of
E-cadherin by hypoxia in all OSCC cell lines. These findings
indicated that hypoxia activated the Notch signaling pathway to
induce EMT in OSCC cell lines.
Hypoxia enhances cell motility and
invasion via Notch signaling
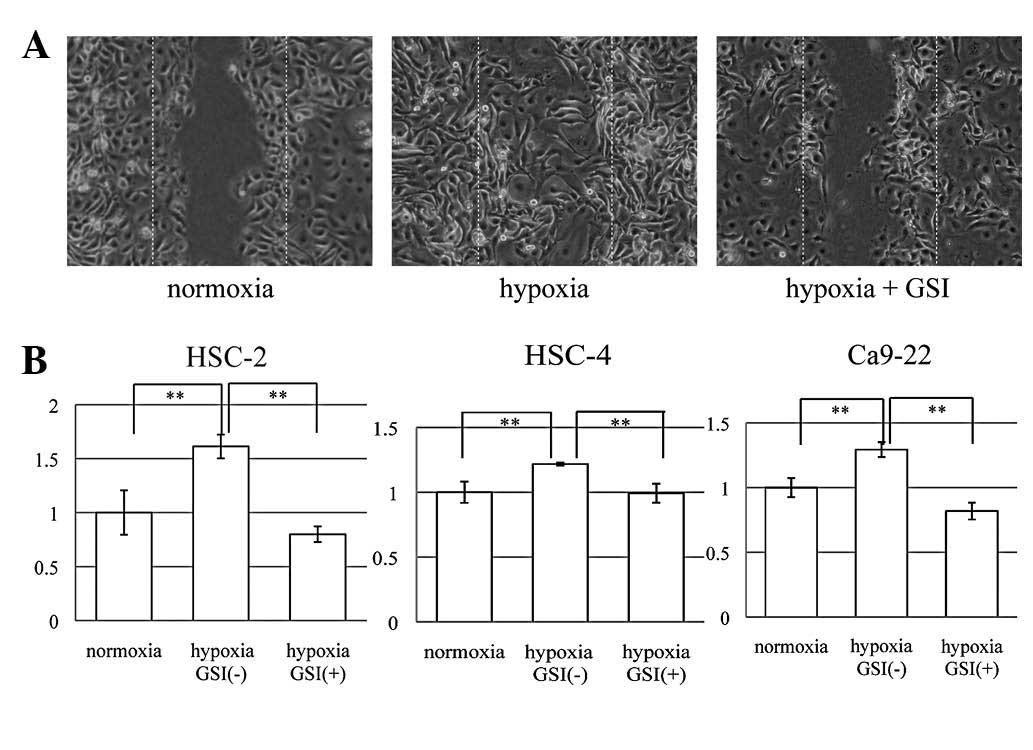
To study cell motility, we used a scratch wound
healing assay (Fig. 3A), in which
the extent of migration of cells into the scratched areas was
counted at three points. Quantification of relative closure of the
scratch wound was undertaken and compared with that of the control.
All three OSCC cell lines showed significantly increased cell
motility due to hypoxia (P<0.01), and GSI significantly
prevented this effect (P<0.01) (Fig.
3B). These results indicated that hypoxia upregulated cell
motility through activation of the Notch signaling pathway in OSCC
cell lines. To examine whether a hypoxic environment promoted the
invasiveness of OSCC cell lines, we conducted an invasion assay
cultured under hypoxia or normoxia for 24 h (Fig. 4A). All three OSCC cell lines showed
an increased cell invasion ratio under hypoxia, compared with that
under normoxia (Fig. 4B). The
number of invasive cells under hypoxia, compared with that under
normoxia, was increased 2.47-fold in HSC-2 cells (P<0.05),
4.05-fold in HSC-4 cells (P<0.05) and 1.71-fold in Ca9-22 cells
(P<0.05). Subsequently, it was examined whether hypoxia promoted
the increased invasiveness via the Notch signaling pathway. Cells
were cultured under hypoxia, with or without 5 μM GSI, for 48 h
(Fig. 4C). All three OSCC cell
lines showed a decreased cell invasion ratio with GSI. The number
of invasive cells with GSI, compared with those without GSI, was
decreased 0.56-fold in HSC-2 cells (P<0.01), 0.58-fold in HSC-4
cells (P<0.05) and 0.36-fold in Ca9-22 cells (P<0.05). These
results indicated that hypoxia enhanced tumor invasiveness through
Notch pathway activation in OSCC cell lines.
Discussion
Tumor metastasis entails a series of distinct,
sequential steps, involving local tumor growth, invasion by
transmigration through basement membranes and non-tumor host
tissue, intravasation into blood vessels, dissemination and
survival in the bloodstream, extravasation and re-establishment at
distant sites (13). EMT also
occurs during the early stages of carcinogenesis to bypass
oncogene-induced senescence (14).
Several studies have demonstrated the expression of EMT-specific
genes at the invasive front of primary tumors, thus providing
evidence that EMT may constitute a critical prerequisite for
primary tumor cells to break through the basal lamina into
non-involved adjacent host tissue (15,16).
Clinical evidence has indicated that tumor hypoxia is a poor
prognostic factor for patient outcome (17–19). A
number of different types of cancer cells were observed to respond
to hypoxic exposure within 72 h by classic EMT changes
(fibroblastoid phenotype, Snail and β-catenin nuclear
translocation, and changes in E-cadherin), and they exhibited
increased migration and invasiveness (20).
In the present study, hypoxia downregulated the
expression of E-cadherin in three OSCC cell lines, as shown by
immunohistochemical examination. Additionally, OSCC cell migration
and invasion were also facilitated by culture in 1% O2,
compared with the levels in 21% O2. These results
suggest that a hypoxic environment induces EMT and an aggressive
phenotype in OSCC cell lines.
The Notch signaling pathway exhibits different
downstream effects depending on which sub-site or microenvironment
it is influencing (21). Recently,
two studies hypothesized that Notch1 has a tumor-suppressive
function in head and neck squamous cell carcinoma (HNSCC) (22,23).
The results of the present study indicated that Notch signaling
acts as an oncogenic factor under hypoxia in OSCC cell lines.
Previously, it was suggested that inhibition of the Notch pathway
suppresses OSCC growth (24).
Notch1 has been implicated as a downstream effector of oncogenic
Ras in human mammary tumorigenesis (25). Patients with tumors expressing high
levels of Jagged1 protein exhibited a poorer outcome than those
with tumors expressing low levels of this protein in breast cancer
(26,27), HNSCC (28) and tongue squamous cell carcinoma
(29). The critical role of Notch
signaling in EMT has been demonstrated by blocking Notch signaling,
via knockdown of either HEY1 or Jagged1 expression (30), or by γ-secretase inhibitor
attenuation of EMT (31). The
present study demonstrated that hypoxia decreased the expression of
E-cadherin and increased cell motility and invasion, but GSI
inhibited these effects. These results indicate that hypoxia
induced EMT through upregulation of the Notch signaling pathway as
a form of oncogenic activation in the OSCC cell lines.
The expression of mRNA associated with Notch
signaling in OSCCs cultured in 1% O2 or 21%
O2 for 24 h was examined using qPCR. All three OSCC cell
lines showed upregulation of the mRNA levels of Notch receptors,
ligands and target genes under hypoxia, compared with the levels
under normoxia, indicating that the Notch signaling was enhanced by
the hypoxic stimulus in the OSCC cell lines. In addition, culture
of lung (9) and ovarian (31) cancer cells under hypoxia has been
observed to increase Notch signaling pathway activation. Low oxygen
content has also been demonstrated to potentiate Notch signaling in
melanocytes through stabilization of HIF-1α (11). HIF-1α is a transcription factor that
activates transcription of genes involved in anaerobic metabolism,
angiogenesis, survival, invasion, metastasis and treatment
resistance in tumor cells, thus promoting cellular adaptation and
survival under hypoxic conditions. HIF-1α is critical in
hypoxia-activated gene expression (32). Stabilization and activation of the
HIF-1α transcription complex also correlates with tumor metastasis
and poor prognosis in patients with cancer (33–35).
Upregulation of Snail correlates with metastasis and
poor prognosis, whereas silencing of Snail is critical for reducing
tumor growth and invasiveness (36,37).
Hypoxia may attenuate the expression of E-cadherin, via activation
of the lysyl oxidase (LOX)-Snail pathway, to promote tumor invasion
and metastasis. This indicates that hypoxia-induced LOX and HIFs
are important factors that regulate tumor microenvironments to
favor metastasis (38,39). Although the present study
demonstrated that the level of Snail mRNA was increased by hypoxia
in these OSCC cell lines, no clear association between Notch
signaling pathway activation and Snail expression was observed.
However, previous studies have indicated that Snail expression is
directly regulated by the Notch signaling pathway (31,40).
Further investigation is necessary to clarify this issue in
OSCC.
The current study identified that hypoxia promoted
cell motility and invasion, decreased the expression of E-cadherin
and upregulated the Notch signaling pathway, indicating that
hypoxia induced EMT in the OSCC cell lines. GSI inhibited this
upregulation of cell motility and invasion, and the decrease in
expression of E-cadherin under hypoxia. These results suggest that
hypoxia induced EMT through the upregulation of Notch signaling
activation. Elucidation of the mechanism of EMT through activation
of the Notch signaling pathway may provide novel molecular targets
and contribute to improving the prognosis of patients with
OSCC.
Acknowledgements
This study was supported by Grant-in-Aid for
Scientific Research (C; grant no. 23592968; KAKENHI).
References
|
1
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J,
Murray T and Thun MJ: Cancer statistics, 2008. CA Cancer J Clin.
58:71–96. 2008. View Article : Google Scholar
|
|
2
|
Kademani D, Bell RB, Bagheri S, Holmgren
E, Dierks E, Potter B and Homer L: Prognostic factors in intraoral
squamous cell carcinoma: the influence of histologic grade. J Oral
Maxillofac Surg. 63:1599–1605. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Weinberg RA: Twisted
epithelial-mesenchymal transition blocks senescence. Nat Cell Biol.
10:1021–1023. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang MH, Wu MZ, Chiou SH, et al: Direct
regulation of TWIST by HIF-1alpha promotes metastasis. Nat Cell
Biol. 10:295–305. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cannito S, Novo E, Compagnone A, et al:
Redox mechanisms switch on hypoxia-dependent epithelial-mesenchymal
transition in cancer cells. Carcinogenesis. 29:2267–2278. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Leong KG, Niessen K, Kulic I, et al:
Jagged1-mediated Notch activation induces epithelial-to-mesenchymal
transition through Slug-induced repression of E-cadherin. J Exp
Med. 204:2935–2948. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Miele L: Notch signaling. Clin Cancer Res.
12:1074–1079. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang Z, Zhang Y, Li Y, Banerjee S, Liao J
and Sarkar FH: Down-regulation of Notch-1 contributes to cell
growth inhibition and apoptosis in pancreatic cancer cells. Mol
Cancer Ther. 5:483–493. 2006. View Article : Google Scholar
|
|
9
|
Chen J, Imanaka N, Chen J and Griffin JD:
Hypoxia potentiates Notch signaling in breast cancer leading to
decreased E-cadherin expression and increased cell migration and
invasion. Br J Cancer. 102:351–360. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pietras A, von Stedingk K, Lindgren D,
Påhlman S and Axelson H: JAG2 induction in hypoxic tumor cells
alters Notch signaling and enhances endothelial cell tube
formation. Mol Cancer Res. 9:626–636. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bedogni B, Warneke JA, Nickoloff BJ,
Giaccia AJ and Powell MB: Notch1 is an effector of Akt and hypoxia
in melanoma development. J Clin Invest. 118:36602008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jiang YG, Luo Y, He DL, et al: Role of
Wnt/beta-catenin signaling pathway in epithelial-mesenchymal
transition of human prostate cancer induced by hypoxia-inducible
factor-1alpha. Int J Urol. 14:1034–1039. 2007. View Article : Google Scholar
|
|
13
|
Chambers AF, Groom AC and MacDonald IC:
Dissemination and growth of cancer cells in metastatic sites. Nat
Rev Cancer. 2:563–572. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ansieau S, Bastid J, Doreau AS, et al:
Induction of EMT by twist proteins as a collateral effect of
tumor-promoting inactivation of premature senescence. Cancer Cell.
14:79–89. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gavert N, Conacci-Sorrell M, Gast D, et
al: L1, a novel target of beta-catenin signaling, transforms cells
and is expressed at the invasive front of colon cancers. J Cell
Biol. 168:633–642. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hlubek F, Spaderna S, Jung A, et al:
β-catenin activates a coordinated expression of the proinvasive
factors laminin-5 γ2 chain and MT1-MMP in colorectal carcinomas.
Int J cancer. 108:321–326. 2004.
|
|
17
|
Brizel DM, Scully SP, Harrelson JM, et al:
Tumor oxygenation predicts for the likelihood of distant metastases
in human soft tissue sarcoma. Cancer Res. 56:941–943.
1996.PubMed/NCBI
|
|
18
|
Hockel M, Schlenger K, Aral B and Mitze M:
Association between tumor hypoxia and malignant progression in
advanced cancer of the uterine cervix. Cancer Res. 56:4509–4515.
1996.PubMed/NCBI
|
|
19
|
Fyles A, Milosevic M, Wong R, et al:
Oxygenation predicts radiation response and survival in patients
with cervix cancer. Radiother Oncol. 48:149–156. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hill RP, Marie-Egyptienne DT and Hedley
DW: Cancer stem cells, hypoxia and metastasis. Semin Radiat Oncol.
19:106–111. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Howard JD, Lu B and Chung CH: Therapeutic
targets in head and neck squamous cell carcinoma: Identification,
evaluation, and clinical translation. Oral Oncol. 48:10–17. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Agrawal N, Frederick MJ, Pickering CR, et
al: Exome sequencing of head and neck squamous cell carcinoma
reveals inactivating mutations in NOTCH1. Science. 333:1154–1157.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Stransky N, Egloff AM, Tward AD, et al:
The mutational landscape of head and neck squamous cell carcinoma.
Science. 333:1157–1160. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hijioka H, Setoguchi T, Miyawaki, et al:
Upregulation of Notch pathway molecules in oral squamous cell
carcinoma. Int J Oncol. 36:817–822. 2010.PubMed/NCBI
|
|
25
|
Weijzen S, Rizzo P, Braid M, et al:
Activation of Notch-1 signaling maintains the neoplastic phenotype
in human Ras-transformed cells. Nat Med. 8:979–986. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Santagata S, Demichelis F, Riva A, et al:
JAGGED1 expression is associated with prostate cancer metastasis
and recurrence. Cancer Res. 64:6854–6857. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dickson BC, Mulligan AM, Zhang H, et al:
High-level JAG1 mRNA and protein predict poor outcome in breast
cancer. Mod Pathol. 20:685–693. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lin JT, Chen MK, Yeh KT, et al:
Association of high levels of Jagged-1 and Notch-1 expression with
poor prognosis in head and neck cancer. Ann Surg Oncol.
17:2976–2983. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang TH, Liu HC, Zhu LJ, et al:
Activation of Notch signaling in human tongue carcinoma. J Oral
Pathol Med. 40:37–45. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zavadil J, Cermak L, Soto-Nieves N and
Böttinger EP: Integration of TGF-beta/Smad and Jagged1/Notch
signalling in epithelial-to-mesenchymal transition. EMBO J.
23:1155–1165. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sahlgren C, Gustafsson MV, Jin S,
Poellinger L and Lendahl U: Notch signaling mediates
hypoxia-induced tumor cell migration and invasion. Proc Natl Acad
Sci USA. 105:6392–6397. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zagzag D, Zhong H, Scalzitti JM, Laughner
E, Simons JW and Semenza GL: Expression of hypoxia-inducible factor
1alpha in brain tumors: association with angiogenesis, invasion,
and progression. Cancer. 88:2606–2618. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Harris AL: Hypoxia - a key regulatory
factor in tumour growth. Nat Rev Cancer. 2:38–47. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Schindl M, Schoppmann SF, Samonigg H, et
al: Overexpression of hypoxia-inducible factor 1alpha is associated
with an unfavorable prognosis in lymph node-positive breast cancer.
Clin Cancer Res. 8:1831–1837. 2002.PubMed/NCBI
|
|
35
|
Gupta GP and Massagué J: Cancer
metastasis: building a framework. Cell. 127:679–695. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Perl AK, Wilgenbus P, Dahl U, Semb H and
Christofori G: A causal role for E-cadherin in the transition from
adenoma to carcinoma. Nature. 392:190–193. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Moody SE, Perez D, Pan TC, et al: The
transcriptional repressor Snail promotes mammary tumor recurrence.
Cancer Cell. 8:197–209. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Erler JT, Bennewith KL, Nicolau M, et al:
Lysyl oxidase is essential for hypoxia-induced metastasis. Nature.
440:1222–1226. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pouysségur J, Dayan F and Mazure NM:
Hypoxia signalling in cancer and approaches to enforce tumour
regression. Nature. 441:437–443. 2006.PubMed/NCBI
|
|
40
|
Timmerman LA, Grego-Bessa J, Raya A, et
al: Notch promotes epithelial-mesenchymal transition during cardiac
development and oncogenic transformation. Genes Dev. 18:99–115.
2004. View Article : Google Scholar
|