Introduction
Nasopharyngeal carcinoma (NPC) is a relatively
uncommon type of malignant tumor that is distinct from other types
of head and neck cancer with regard to epidemiology, method of
treatment and prognosis (1,2). NPC has been identified as particularly
prevalent in Southeast Asian populations (3). The anatomical location of NPC limits
surgery to biopsies only (4) and
the primary choice of treatment is radiotherapy (RT). However,
conventional 2-dimensional RT has been shown to correlate with high
rates of local recurrence and metastasis, particularly in patients
with locally advanced NPC (5,6).
Although radiotherapy is the cornerstone treatment for NPC,
adjunctive chemotherapy has shown promise for improving tumor
control and, possibly, the survival rate in cases of advanced NPC
(7). The expression of E-cadherin
and vimentin has also been demonstrated to correlate with
metastasis formation in head and neck squamous cell carcinoma
patients (8). Therefore, it is
necessary to elucidate the effects of radiation on metastasis and
recurrence and identify the relevant molecular mechanisms involved.
An improved understanding of the mechanisms underlying the
metastasis and recurrence of cancer following IR is likely to
highlight and improve the therapeutic potential of radiotherapy for
these types of cancer.
Epithelial-mesenchymal transition (EMT) is important
for tumor progression and metastasis (9,10).
Previous results have also demonstrated that ionizing radiation
(IR) may enhance the invasiveness of cancer cells by inducing EMT
(11). The loss of E-Cadherin
expression has been shown to correlate with EMT and promote the
radioresistance of human tumor cells (12). In addition, the activation of AKT
has been demonstrated to correlate with the metastasis of NPC
(13). In clinical practice, a
marked correlation has been identified between the expression of
active AKT and the outcome of treatment for breast cancer (14). Previous studies have also shown the
importance of AKT inhibition for enhancing the efficacy of
radiotherapy in head and neck cancer (15).
Since the activation of AKT is a critical element
for cancer metastasis, recurrence and drug/radio-resistance,
targeting the AKT pathway is a rational approach for cancer
therapy. To understand the role of AKT in EMT and its association
with radioresistance in NPC, a pan-AKT kinase inhibitor currently
in clinical development for patients with various malignancies
(16) was used to prevent the
activation of AKT following radiation. GSK690693 is a novel
ATP-competitive inhibitor of AKT that inhibits proliferation and
induces apoptosis in a subset of tumor cells with a potency
consistent with the intracellular inhibition of AKT kinase activity
in vitro and in vivo(16–18).
The results of the present study demonstrate that GSK690693 may
block the phosphorylation of AKT and lead to the inhibition of cell
metastasis and radioresistance via the regulation of
ZEB1/E-cadherin levels in NPC cell lines.
Materials and methods
Cell culture and reagents
The NPC cell lines, CNE1 and CNE2, were purchased
from American Type Culture Collection (ATCC, Manassas, VA, USA) and
were maintained in RPMI-1640 medium supplemented with 10% newborn
calf serum. The cells were maintained at 37°C in a humidified
atmosphere of 5% CO2 and 95% air as recommended. For the
EMT models, the cells were seeded and grown for 72 h, followed by
being exposed to 5 Gy IR. The AKT inhibitor, GSK690693, was
obtained from Selleck Chemicals (Houston, TX, USA).
Western blotting
Whole cell extracts were prepared by washing the
cells in cold PBS and lysis in a buffer containing 50 mM Tris-HCl
(pH 7.5), 150 mM NaCl, 1% Nonidet P-40, 0.25% sodium deoxycholate,
1 mM Na3VO4 and 1 mM NaF, in addition to
complete protease inhibitor cocktail tablets (Roche Diagnostics
GmbH, Mannheim, Germany). Protein concentrations were determined
using bicinchoninic protein assay reagents according to the
manufacturer’s instructions (Pierce Biotechnology, Inc., Rock-ford,
IL, USA). Antibodies against ZEB1, E-cadherin, vimentin, snail,
GAPDH (1:500; Santa Cruz Biotechnology, Santa Cruz, CA, USA), AKT
and phospho-AKT (1:1,000; Ser473; Cell Signaling Technology,
Danvers, MA, USA) were used.
Cell invasion assay
Matrigel invasion chambers (BD Biosciences, Franklin
Lakes, NJ, USA) were rehydrated in DMEM for 2 h and then placed in
0.75 ml DMEM supplemented with 5% fetal calf serum. Following 72 h
of IR or drug treatment for 48 h, 1.5×104 cells
suspended in 0.5 ml DMEM were seeded onto Matrigel chambers and
allowed to invade for 12 or 24 h. The cells on the upper surface
were gently removed with a cotton bud and the cells that had
migrated through the 8-mm pores were fixed with 4% paraformaldehyde
for 15 min and stained with 0.1% crystal violet for 10 min. The
membranes were washed, removed and mounted onto a glass slide, and
the level of invasion was quantified by visual counting using a
microscope (magnification, ×20).
Immunofluorescence
The cells were plated onto glass cover slips in
6-well culture plates, then fixed with 4% paraformaldehyde for 10
min, permeabilized with 0.2% Triton X-100 for 5 min and blocked
with 1% BSA in PBS for 30 min. E-cadherin and vimentin (1:100) and
AKT and phospho-AKT (p-AKT; Thr308; 1:200) were applied to sections
overnight at 4°C. Nuclei were visualized by staining with DAPI
(Sigma-Aldrich, St Louis, MO, USA) and images were captured on an
inverted phase/fluorescence microscope (Nikon Eclipse 80i; Nikon,
Amsterdam, Netherlands).
Xenograft experiments
Animal studies were conducted in strict accordance
with the principles and procedures approved by the Committee on the
Ethics of Animal Experiments of Southern Medical University
(Guangzhou, Guangdong, China). Nude mice (BALB/C nu/nu) were
provided with autoclaved water and laboratory rodent chow. A volume
of 100 μl culture medium mixed with Matrigel (BD Biosciences)
containing 3×106 CNE2 cells was transplanted into the
flanks of the mice by subcutaneous injection. The tumor volume was
monitored every 5 days and calculated as follows: Volume
(mm3) = (a × b2) / 2, where a indicates the
largest diameter and b the perpendicular diameter. Once tumors had
reached ~70 mm3, the mice were randomly distributed into
four groups (three mice/group) and treated with 30 mg/kg GSK690693
AKT inhibitor for 3 weeks (weekend rest) or 3×5 Gy IR. The tumor
volume was monitored at various times for up to 24 days.
Patient and primary NPC samples
Established protocols were followed with regard to
written informed consent and anonymity. Tissues were obtained
retrospectively from 22 NPC and 7 normal patients (chronic
inflammation only) under strict anonymity from historical
collections in the Department of Pathology, The First Affiliated
Hospital of Zhengzhou University (Zhengzhou, China). All samples
were fresh-frozen in liquid nitrogen following surgery and stored
at −80°C. Frozen tissue samples were homogenized using the Tissue
Ruptor (Qiagen, Hilden, Germany) prior to RNA extraction. Total RNA
was extracted using TRI Reagent (MRC, Inc., Cincinnati, OH, USA)
according to the manufacturer’s instructions. To determine the AKT
and ZEB1 status of the primary NPC samples, PCR products of AKT and
ZEB1 were used from genomic DNA. Written informed consent was
obtained from each patient and the study was approved by the Ethics
Committee of Nanfang Hospital (Guangzhou, China).
Side population (SP) cell analysis by
flow cytometry
The cells were analyzed by FACS when they had
reached the alogarithmic growth phase (24 h after replating). The
cells were digested with 0.25% trypsin (Giboc, Carlsbad, CA, USA),
washed twice with calcium/magnesium-free PBS, re-suspended in
ice-cold RPMI-1640 culture (supplemented with 2.5% FBS) at a
concentration of 1×106 cells/ml and incubated at 37°C in
a 5% CO2 incubator for 10 min. The DNA binding dye,
Hoechst 33342 (Sigma-Aldrich), was then added at a final
concentration of 5 mg/ml and the samples were incubated for 90 min
in the dark with periodic mixing. The cells were washed twice with
PBS, then 1 mg/ml propidium iodide (Sigma-Aldrich) was added and
the cells were kept at 4°C in the dark prior to analysis by a FACS
Aria Flow Cytometer (Becton-Dickinson, Franklin Lakes, NJ, USA).
Since Hoechst 33342 is extruded from cells treated with verapamil
(a calcium ion channel antagonist)-sensitive ABC transporters, a
subset of the cells were incubated with 50 mmol/l verapamil for 30
min at 37°C prior to the addition of Hoechst 33342.
Colony formation assays
The cells were counted, plated in triplicate at 200
cells per well in six-well plates and cultured with RPMI-1640
complete culture for 12 h. Next, the cells were treated with 5 Gy
irradiation or the addition of 10 μM AKT inhibitor (GSK690693) into
the growth media at the indicated concentrations and allowed to
form colonies for 16 days. Once the majority of the cell colonies
had expanded to >50 cells in the control group, they were washed
twice with PBS, fixed in methanol for 10 min and stained with
crystal violet for 10 min at room temperature. Subsequent to
washing out the dye, the colonies that contained >50 cells were
counted and the results were compared. The colony-forming
efficiency (CFE) was the ratio of the colony number to the
transplanted cell number.
Tumorsphere formation assay
Single-cell suspensions of cell lines or cells
isolated from pleural effusions were suspended at a density of
4×104 cells/ml in Dulbecco’s modified Eagle’s
medium/F-12 containing 5 mg/ml insulin, 0.5 mg/ml hydrocortisone,
2% B27 (Invitrogen Ltd., Paisley, Scotland) and 20 ng/ml epidermal
growth factor, and seeded into six-well plates (2.5 ml per plate)
or T80 tissue culture flasks (10 ml per flask) coated with 1.2%
polyhema. Cultures were fed weekly and passaged every 2 weeks.
Tumorspheres were measured using Zeiss Axiovision software (Carl
Zeiss, Jena, Germany). When passaged, the tumorspheres were
harvested, incubated with trypsin for 3 min at 37°C and dispersed
by pipetting with a 23-gauge needle. Subsequent to checking for
single cells, the cells were pelleted and suspended in tumorsphere
culture medium at 4×104 cells/ml prior to replating in
non-adherent plates or flasks.
shRNAs
The pLKO.1 lentiviral shRNA vector and control shRNA
targeting against GFP were purchased from Sigma-Aldrich. The Zeb1
targeting sequence in the shZeb1 plasmid was
5′-GGUUACGAACUAAGCUAUA-3′. The sense and antisense oligonucleotides
were annealed and ligated into the pLKO.1 lentiviral vector.
Statistical analysis
All experiments were repeated at least three times.
Data are presented as the mean ± SD and P<0.05 was considered to
indicate a statistically significant difference. All statistical
analyses were performed by the v5.0 GraphPad Prism Program
(GraphPad Software Inc., San Diego, CA, USA).
Results
IR-induced morphological changes and
increased invasion of cancer cells are consistent with AKT
activation in NPC
IR induces morphological changes and EMT in various
types of carcinoma (19–21). To verify the changes in NPC cells
following exposure to IR, the NPC cell lines, CNE1 and CNE2, were
irradiated with 5 Gy shortly following attachment. The morphology
of the irradiated cells was spindle-shaped and elongated when
compared with that of the control group (Fig. 1A). To investigate whether IR may
promote the migration and invasiveness of carcinoma cells, cell
invasion assays using untreated and irradiated NPC cells were
conducted. Irradiated cells exhibited increased motility and
invasiveness (Fig. 1B). Western
blot analysis of the irradiated group revealed the increased
expression of vimentin, snail and ZEB1 and the downregulation of
E-cadherin (Fig. 1C). To identify
whether the phosphorylation of AKT is involved in IR-induced cell
invasion, the expression of AKT and p-AKT (Ser473) was primarily
examined in the NPC cells treated with various doses of IR. Total
AKT and p-AKT protein expression levels in the IR-treated and
untreated groups were determined by western blot analysis (Fig. 1D).
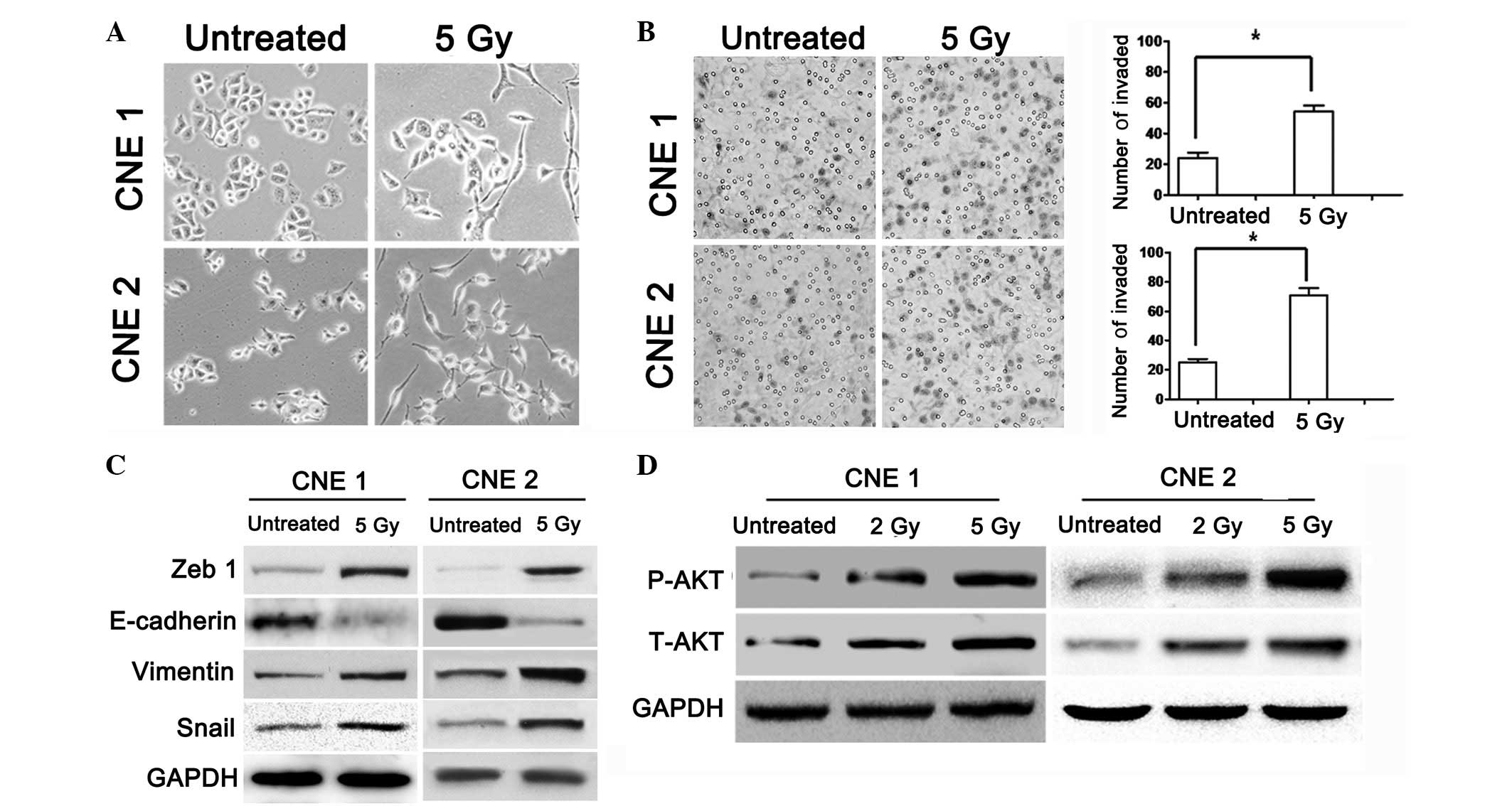 | Figure 1AKT suppresses EMT phenotype and stem
cell properties. (A) Morphology of CNE1 and CNE2 cells 3 days after
treatment with 5 Gy IR. (B) Representative immunohistochemical
staining of pAKT and AKT and immunofluorescence using confocal
microscopy. The differential expression of pAKT and AKT at the
protein level in the CNE1 and CNE2 cells is shown. The cells were
seeded in Matrigel-coated transwell inserts ands irradiated with
two doses of IR (0 and 5 Gy). After 18 h, images of the cells that
had invaded the membranes were captured under a light microscope
and counted. The difference between the two groups was determined
by Student’s t-test, as shown in the graphs. (C) Expression of
ZEB1, vimentin, E-cadherin, snail and GAPDH was detected by western
blot analysis in the untreated and irradiated CNE1 and CNE2 cells.
(D) The CNE1 and CNE2 cells were cultured following irradiation
with various doses (0, 2 and 5 Gy) and maintained in RPMI-1640
medium supplemented with 10% newborn calf serum for 72 h. Total
lysates were run on SDS-PAGE, blotted and stained with antibodies
against pAkt and Akt. Anti-GAPDH served as an internal control.
*P<0.05 vs. untreated (magnification, ×1,000; scale
bar, 20 μm). EMT, epithelial-mesenchymal transition. |
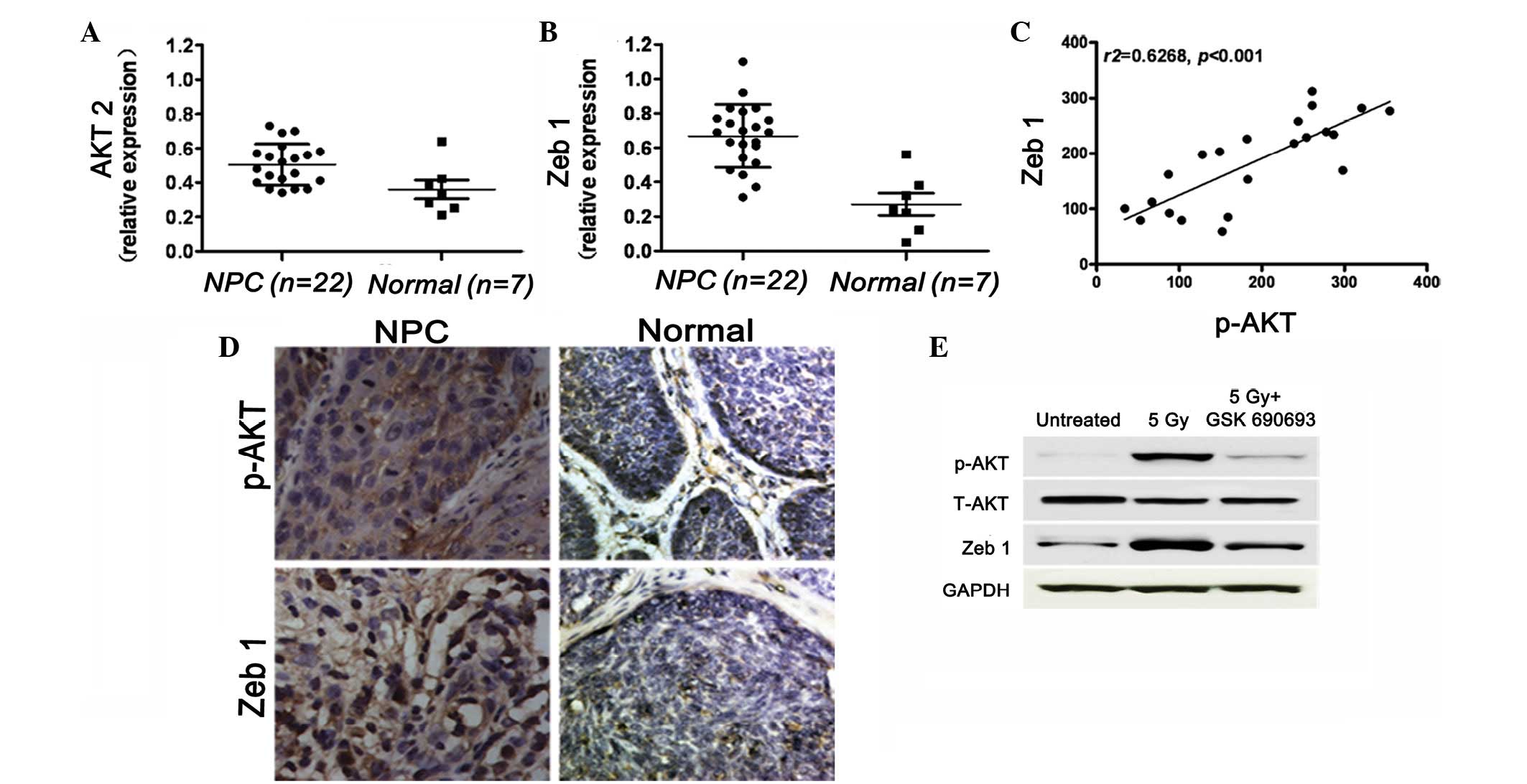
Correlation between the expression of AKT
and ZEB1 in human primary and metastatic NPC samples
Notably, previous studies have demonstrated that the
phosphorylation of AKT correlates with poor prognosis and
radioresistance (22). The current
study examined the expression of AKT and ZEB1 using qPCR in 22 NPC
cases and 7 normal cases (chronic inflammation only) as controls.
The results showed that the expression of AKT was higher in the NPC
group when compared with that of the control group. The expression
pattern for ZEB1 was similar to that of AKT in the 22 NPC and 7
normal cases (Fig. 2A and B). The
correlation between p-AKT (Ser473) and ZEB1 expression was
determined using immunohistochemistry and a marked correlation was
identified (r2=0.6268; P<0.05; Fig. 2C and D). Based on these results, it
may be hypothesized that patients with high levels of p-AKT and
ZEB1 expression are at a higher risk of metastasis. GSK690693 is
selective for AKT isoforms compared with the majority of other
kinase families and has been shown to reduce the levels of p-AKT
substrates in vivo(16,23).
The results of the present study showed that IR induces AKT
phosphorylation and the expression of ZEB1 in NPC cells. The NPC
cells cotreated with shZEB1 and IR showed no change in expression
when compared with the AKT and IR groups. Next, the GSK690693 AKT
inhibitor was applied to the NPC cells, which were then treated
with 5 Gy radiation (Fig. 2E).
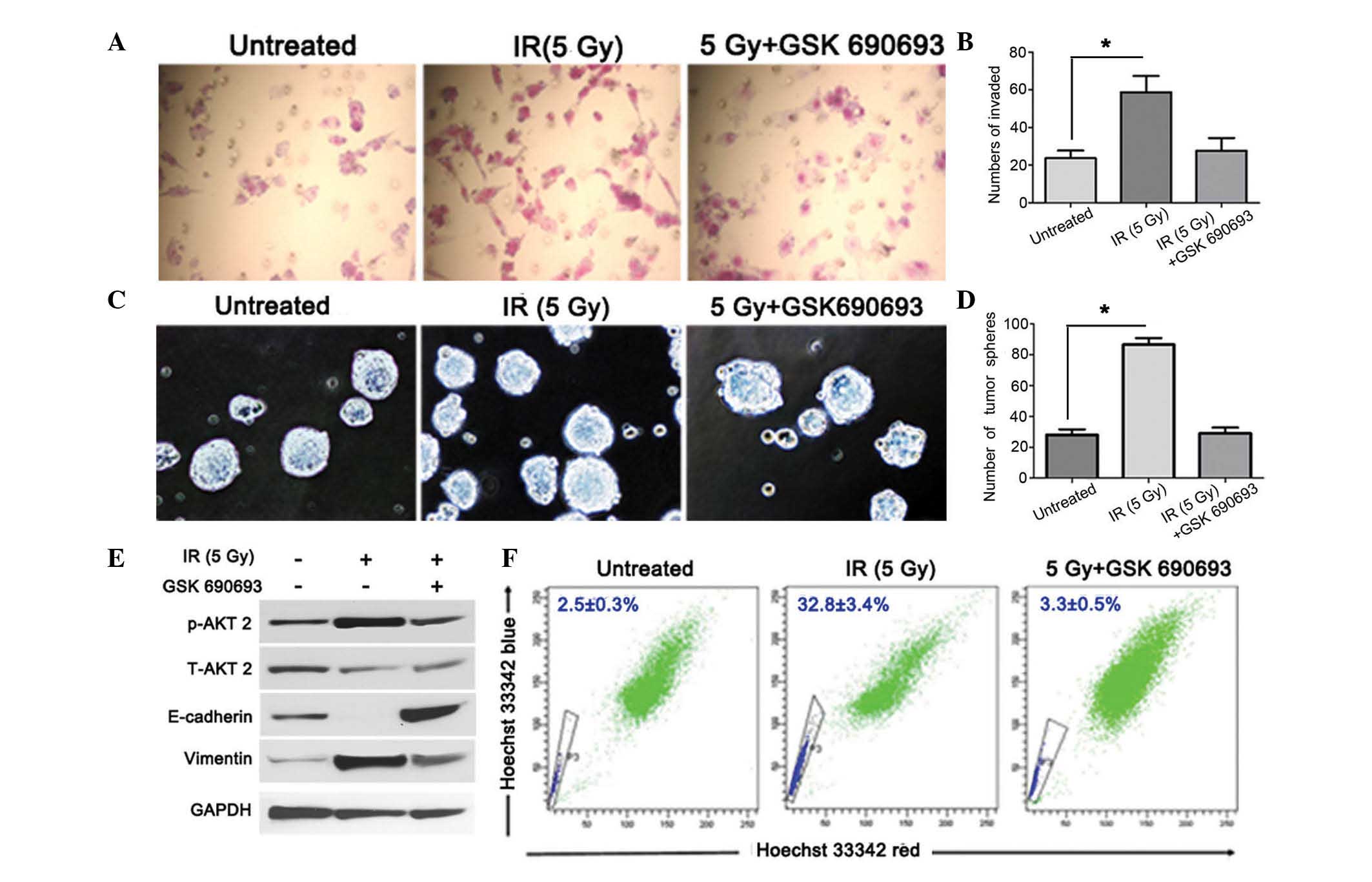
GSK690693 AKT inhibitor blocks IR-induced
EMT and stemness in NPC
To clarify the efficacy of GSK690693 in the
irradiated NPC cells, the invasion of the irradiated cells treated
with the GSK690693 AKT inhibitor was compared with that of cells
treated with IR only using a cell invasion assay. Cell invasion was
identified to be higher in the irradiated NPC cells compared with
the untreated cells (Fig. 3A and
B), indicating that the AKT inhibitor may block IR-triggered
cell invasion by inhibiting AKT phosphorylation. Side population
(SP) cells and tumor sphere cells have stem cell characteristics
that were hypothesized to be enriched of cancer stem cells in NPC
(24). The rate of tumor sphere
formation was ~24 spheres/1,000 cells in the untreated group and
was identified to increase to ~83 spheres/1,000 cells following
treatment with IR (Fig. 3C and D).
To examine the correlation between AKT phosphorylation and the EMT
activator (vimentin) and inhibitor (E-cadherin) in IR, the
expression levels of these EMT-associated markers in IR-treated NPC
cells were compared with that of cells cotreated with IR and
GSK690693. IR-induced p-AKT activation in NPC cells was shown to
correlate with a reduction in the levels of E-cadherin and an
increase in vimentin expression. In the NPC cells cotreated with IR
and GSK690693, the phosphorylation of AKT was inhibited compared
with that of cells treated with IR only (Fig. 3E). In the present study, the
treatment of irradiated NPC cells with GSK690693 reduced the
percentage of SP cells (Fig.
3F).
GSK69069 AKT inhibitor modulates
radioresistance in NPC cancer cells in vivo
To examine the effects of the GSK690693 AKT
inhibitor on NPC cells during radiotherapy in vivo, clone
formation was compared in the untreated, GSK69069- or IR-treated
and IR and GSK690693-cotreated NPC cell groups (Fig. 4A and B). Following treatment with
GSK690693 and normalization against the control, ~62% of clones
formed in the GSK690693 group and ~38% of clones were viable in the
IR-treated group. However, following cotreatment with IR and
GSK690693, >95% of the clones were killed. To determine the
contribution of GSK690693 to the increased sensitivity of NPC cells
to IR administration in vivo, the mice with tumors (70
mm3) that arose 10 days after injection were treated
with 30 mg/kg GSK690693 every 3 days for 3 weeks (weekend rest),
3×5 Gy IR or combinations of GSK690693 with IR (Fig. 4C and D). As predicted, IR treatment
caused significant regression of the tumor, but relapse of the
disease occurred after 40 days. In the cells treated with GSK690693
only, an extremely small effect was noted on tumor growth and this
was hypothesized to be due to the insensitivity of NPC to GSK690693
in vivo. Notably, cotreatment with GSK690693 and IR resulted
in marked regression of tumor growth and relapse was prevented.
Therefore, these results indicate that the GSK690693 AKT inhibitor
potentiates radiotherapy in vivo.
 | Figure 4(A) Colony formation assay of CNE2
cells that were untreated, treated with 5 Gy IR or GSK690693 and
cotreated with IR and GSK690693. (B) Colonies were counted in each
well following the indicated treatment. (C and D) Tumor volume
(mm3) in xenografts treated with saline, 5 Gy IR and
GSK690693 or cotreated with IR and GSK690693 at days 3, 6, 9, 12,
15, 18, 21, 24, 27 and 31. Data are presented as the mean ± SD.
P<0.05. |
Discussion
Previous studies have reported that IR may enhance
the metastatic potential of residual cancer (25). In addition, the activation of AKT in
NPC cells during radiotherapy has been identified to correlate with
cancer cell metastasis (13). The
present study identified that the progeny of irradiated NPC cells
are notably sensitized to undergo AKT phosphorylation-induced EMT.
IR-induced AKT phosphorylation caused a phenotypic transition that
occurred in the progeny of cells irradiated once and continued to
persist during the activation of AKT. This resulted in increased
motility, enhanced invasion, disrupted epithelial morphogenesis and
an increased expression of mesenchymal markers.
AKT has previously been hypothesized to function as
a positive and negative effector of mammary tumorigenesis, a tumor
suppressor at early stages and a stimulator of tumor invasion at
later stages (26). In addition,
the phosphorylation of AKT has been demonstrated to correlate with
a poor prognosis in a number of types of human cancer (27). According to the results of the
present study, AKT exhibited an intimate correlation with ZEB1 and
was upregulated in residual NPC cells following IR. Activation of
ZEB1 by IR induced the mesenchymal markers, vimentin and snail, but
the epithelial marker, E-cadherin, was suppressed. The aberrant
expression of vimentin and snail in epithelial tumors has been
hypothesized to promote the migration and invasion of carcinoma
cells (28). In the present study,
the expression of ZEB1 and AKT was compared and the correlation
between p-AKT (Ser473) and ZEB1 was examined in clinical specimens.
The levels of ZEB1 expression were demonstrated to correlate with
p-AKT levels in NPC, however, GSK69069 prevented the activation of
ZEB1 following IR. Supplementation of the NPC cells with low
concentrations of GSK69069 was sufficient to prevent IR-induced EMT
in vitro. The AKT inhibitor was used to knockdown the AKT
gene in the NPC cells, and using immunohistochemical analyses of
tumor xenografts previous studies have identified that repeated
doses of GSK690693 reduce the number of pAKT substrates in
vivo(16).
Consistent with the in vitro results, the
expression of AKT and ZEB1 in the tissue from the untreated group
increased following IR, but was shown to decrease in the groups
treated with IR and GSK69069. IR led to the activation of
mesenchymal markers, including vimentin and snail, and the
suppressed expression of E-cadherin. It has been previously
demonstrated that snail and slug are critical for cancer cells to
acquire stem cell and EMT characteristics, including
radioresistance and drug resistance (29). The loss of E-cadherin has been
demonstrated to correlate with EMT and promote radioresistance in
human tumor cells (12).
Constitutively-activated AKT in BDC cells has also been shown to
correlate with radioresistance (30) and AKT has been hypothesized to be
important in the feedback loop whereby the IR-induced activation of
AKT increases the radioresistance of GBM cells (31). Targeting the AKT signaling pathway
may therefore have important therapeutic implications when combined
with IR in the treatment of a subset of brain tumor patients.
Increased AKT activation has been shown to correlate with
radioresistance in various types of tumor and, in the present
study, AKT activation was observed in residual cells following IR.
Using NPC cells treated with the GSK69069 AKT inhibitor, the
inhibition of IR-induced AKT activation was shown to increase
radiosensitivity.
In conclusion, the observations of the current study
have led to a number of hypotheses. Firstly, that IR-induced EMT
activation of AKT occurs via the ZEB1 pathway and secondly, that
activation of AKT is involved in radioresistance and EMT following
IR in NPC. In addition, the novel AKT inhibitor, GSK69069, may
block the AKT/ZEB1/E-cadherin/vimentin pathway, increase
radiosensitivity and prevent recurrence and metastasis following IR
therapy in NPC patients.
Acknowledgements
The current study was supported by a grant from the
Key Project of Joint Fund of Natural Science Foundation of China
and Guangdong Province (no. 1060006). The authors would like to
thank the First Affiliated Hospital of Zhengzhou University for
providing the human NPC specimens.
References
|
1
|
Erkal HS, Serin M and Cakmak A:
Nasopharyngeal carcinomas: analysis of patient, tumor and treatment
characteristics determining outcome. Radiother Oncol. 61:247–256.
2001. View Article : Google Scholar
|
|
2
|
Roychowdhury DF, Tseng A Jr, Fu KK,
Weinburg V and Weidner N: New prognostic factors in nasopharyngeal
carcinoma. Tumor angiogenesis and C-erbB2 expression. Cancer.
77:1419–1426. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu MT, Hsieh CY, Chang TH, Lin JP, Huang
CC and Wang AY: Prognostic factors affecting the outcome of
nasopharyngeal carcinoma. Jpn J Clin Oncol. 33:501–508. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Geara FB, Glisson BS, Sanguineti G, et al:
Induction chemotherapy followed by radiotherapy versus radiotherapy
alone in patients with advanced nasopharyngeal carcinoma: results
of a matched cohort study. Cancer. 79:1279–1286. 1997. View Article : Google Scholar
|
|
5
|
Sanguineti G, Geara FB, Garden AS, et al:
Carcinoma of the nasopharynx treated by radiotherapy alone:
determinants of local and regional control. Int J Radiat Oncol Biol
Phys. 37:985–996. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yeh SA, Tang Y, Lui CC, Huang YJ and Huang
EY: Treatment outcomes and late complications of 849 patients with
nasopharyngeal carcinoma treated with radiotherapy alone. Int J
Radiat Oncol Biol Phys. 62:672–679. 2005. View Article : Google Scholar
|
|
7
|
Wei WH, Cai XY, Xu T, et al: Concurrent
weekly docetaxel chemotherapy in combination with radiotherapy for
stage III and IVA-B nasopharyngeal carcinoma. Asian Pac J Cancer
Prev. 13:785–789. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nijkamp MM, Span PN, Hoogsteen IJ, van der
Kogel AJ, Kaanders JH and Bussink J: Expression of E-cadherin and
vimentin correlates with metastasis formation in head and neck
squamous cell carcinoma patients. Radiother Oncol. 99:344–348.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kang Y and Massagué J:
Epithelial-mesenchymal transitions: twist in development and
metastasis. Cell. 118:277–279. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou YC, Liu JY, Li J, et al: Ionizing
radiation promotes migration and invasion of cancer cells through
transforming growth factor-beta-mediated epithelial-mesenchymal
transition. Int J Radiat Oncol Biol Phys. 81:1530–1537. 2011.
View Article : Google Scholar
|
|
12
|
Theys J, Jutten B, Habets R, et al:
E-Cadherin loss associated with EMT promotes radioresistance in
human tumor cells. Radiother Oncol. 99:392–397. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu Y, Chen LH, Yuan YW, Li QS, Sun AM and
Guan J: Activation of AKT is associated with metastasis of
nasopharyngeal carcinoma. Tumour Biol. 33:241–245. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Castaneda CA, Cortes-Funes H, Gomez HL and
Ciruelos EM: The phosphatidyl inositol 3-kinase/AKT signaling
pathway in breast cancer. Cancer Metastasis Rev. 29:751–759. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bussink J, van der Kogel AJ and Kaanders
JH: Activation of the PI3-K/AKT pathway and implications for
radioresistance mechanisms in head and neck cancer. Lancet Oncol.
9:288–296. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rhodes N, Heerding DA, Duckett DR, et al:
Characterization of an Akt kinase inhibitor with potent
pharmacodynamic and antitumor activity. Cancer Res. 68:2366–2374.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Altomare DA, Zhang L, Deng J, et al:
GSK690693 delays tumor onset and progression in genetically defined
mouse models expressing activated Akt. Clin Cancer Res. 16:486–496.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Heerding DA, Rhodes N, Leber JD, Clark TJ,
et al: Identification of
4-(2-(4-amino-1,2,5-oxadiazol-3-yl)-1-ethyl-7-{[(3S)-3-piperidinylmethyl]oxy}-1H-imidazo[4,5-c]pyridin-4-yl)-2-methyl-3-butyn2-ol
(GSK690693), a novel inhibitor of AKT kinase. J Med Chem.
51:5663–5679. 2008.PubMed/NCBI
|
|
19
|
Andarawewa KL, Erickson AC, Chou WS, et
al: Ionizing radiation predisposes nonmalignant human mammary
epithelial cells to undergo transforming growth factor beta induced
epithelial to mesenchymal transition. Cancer Res. 67:8662–8670.
2007. View Article : Google Scholar
|
|
20
|
Li T, Zeng ZC, Wang L, et al: Radiation
enhances long-term metastasis potential of residual hepatocellular
carcinoma in nude mice through TMPRSS4-induced
epithelial-mesenchymal transition. Cancer Gene Ther. 18:617–626.
2011. View Article : Google Scholar
|
|
21
|
Santisteban M, Reiman JM, Asiedu MK, et
al: Immune-induced epithelial to mesenchymal transition in vivo
generates breast cancer stem cells. Cancer Res. 69:2887–2895. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fernandez-L A, Squatrito M, Northcott P,
et al: Oncogenic YAP promotes radioresistance and genomic
instability in medulloblastoma through IGF2-mediated Akt
activation. Oncogene. 31:1923–1937. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Carol H, Morton CL, Gorlick R, et al:
Initial testing (stage 1) of the Akt inhibitor GSK690693 by the
pediatric preclinical testing program. Pediatr Blood Cancer.
55:1329–1337. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang J, Guo LP, Chen LZ, Zeng YX and Lu
SH: Identification of cancer stem cell-like side population cells
in human nasopharyngeal carcinoma cell line. Cancer Res.
67:3716–3724. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
von Essen CF: Radiation enhancement of
metastasis: a review. Clin Exp Metastasis. 9:77–104. 1991.
|
|
26
|
Jeong JW, Jin CY, Park C, et al:
Inhibition of migration and invasion of LNCaP human prostate
carcinoma cells by cordycepin through inactivation of Akt. Int J
Oncol. 40:1697–1704. 2012.PubMed/NCBI
|
|
27
|
Setsu N, Yamamoto H, Kohashi K, et al: The
Akt/mammalian target of rapamycin pathway is activated and
associated with adverse prognosis in soft tissue leiomyosarcomas.
Cancer. 118:1637–1648. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Radisky DC: Epithelial-mesenchymal
transition. J Cell Sci. 118:4325–4326. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kurrey NK, Jalgaonkar SP, Joglekar AV, et
al: Snail and slug mediate radioresistance and chemoresistance by
antagonizing p53-mediated apoptosis and acquiring a stem-like
phenotype in ovarian cancer cells. Stem Cells. 27:2059–2068. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tanno S, Yanagawa N, Habiro A, et al:
Serine/threonine kinase AKT is frequently activated in human bile
duct cancer and is associated with increased radioresistance.
Cancer Res. 64:3486–3490. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li HF, Kim JS and Waldman T:
Radiation-induced Akt activation modulates radioresistance in human
glioblastoma cells. Radiat Oncol. 4:432009. View Article : Google Scholar : PubMed/NCBI
|