Introduction
Due to the anatomical proximity, urothelial
carcinoma (UC) of the bladder may infiltrate and involve the
prostate. High-grade urothelial and prostatic carcinomas have
overlapping morphological characteristics and clinical
manifestations. Therefore, differentiating between the diagnoses of
carcinoma of the urothelium and adenocarcinoma of the prostate may
be difficult. Usually, adenocarcinoma may be distinguished from
transitional cell carcinoma exclusively by its histological aspect.
However, if the histopathological features of the adenocarcinoma of
the prostate are similar to those of UC, the differential diagnosis
is difficult (1). The present study
reported a novel subtype of prostatic adenocarcinoma (PAC) with
rare histopathological features of pure UC. To the best of our
knowledge, this is the first study to report PAC with rare
histopathological features of pure UC. Written informed consent was
obtained from the patient.
Case report
An 81-year-old male presented to the Urological
Outpatient Department with complaints of a recent onset of gross
hematuria and obstructive symptoms. The patient denied smoking and
the consumption of alcohol. The past medical history included a
myocardial infarction and psoriasis. Subsequently, an enlarged
prostate gland, indicative of a neoplasm, was identified using
urological ultrasound. A transrectal ultrasound revealed an
irregular prostatic enlargement of 6.6×6.4×7.6 cm. The serum
prostate-specific antigen (PSA) and free PSA levels were >120
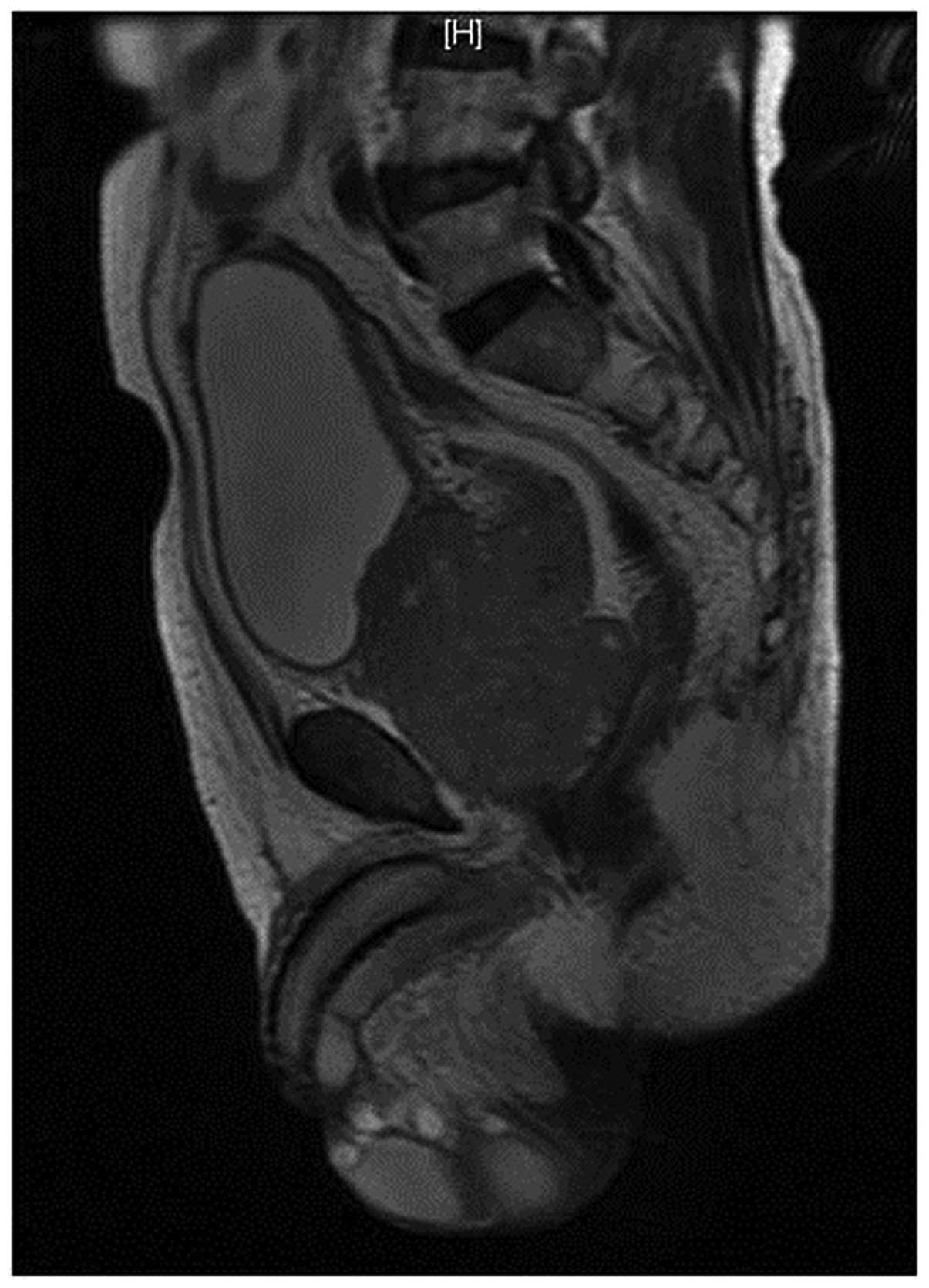
ng/ml (normal range, <4.0 ng/ml). An MRI scan (Fig. 1) revealed a prostatic neoplasm and
pelvic lymph node enlargement, with invasion of the seminal
vesicle. There was no neoplasm of the bladder or other areas of the
urethra, but the patient was positive for skeletal metastases. A
transrectal ultrasound-guided prostatic biopsy was executed and the
specimens were submitted for histopathological evaluation. The
pathological examination revealed a pure UC (Fig. 2) with the presence of solid nests of
cells associated with dense or abundant cytoplasm and striking
nuclear pleomorphism, and the absence or rarity of glandular
lumina. No other differentiation (typical adenocarcinoma or
squamous differentiation) was observed. In order to define the
origin of the tumor cells and reach a diagnosis,
immunohistochemical analyses were performed for PSA, high molecular
weight cytokeratin (HMWCK; clone 34βE12), α-methylacyl coenzyme A
racemase (AMACR/P504S), cytokeratin (CK)-7, CK 20 and p63 (Fig. 2).
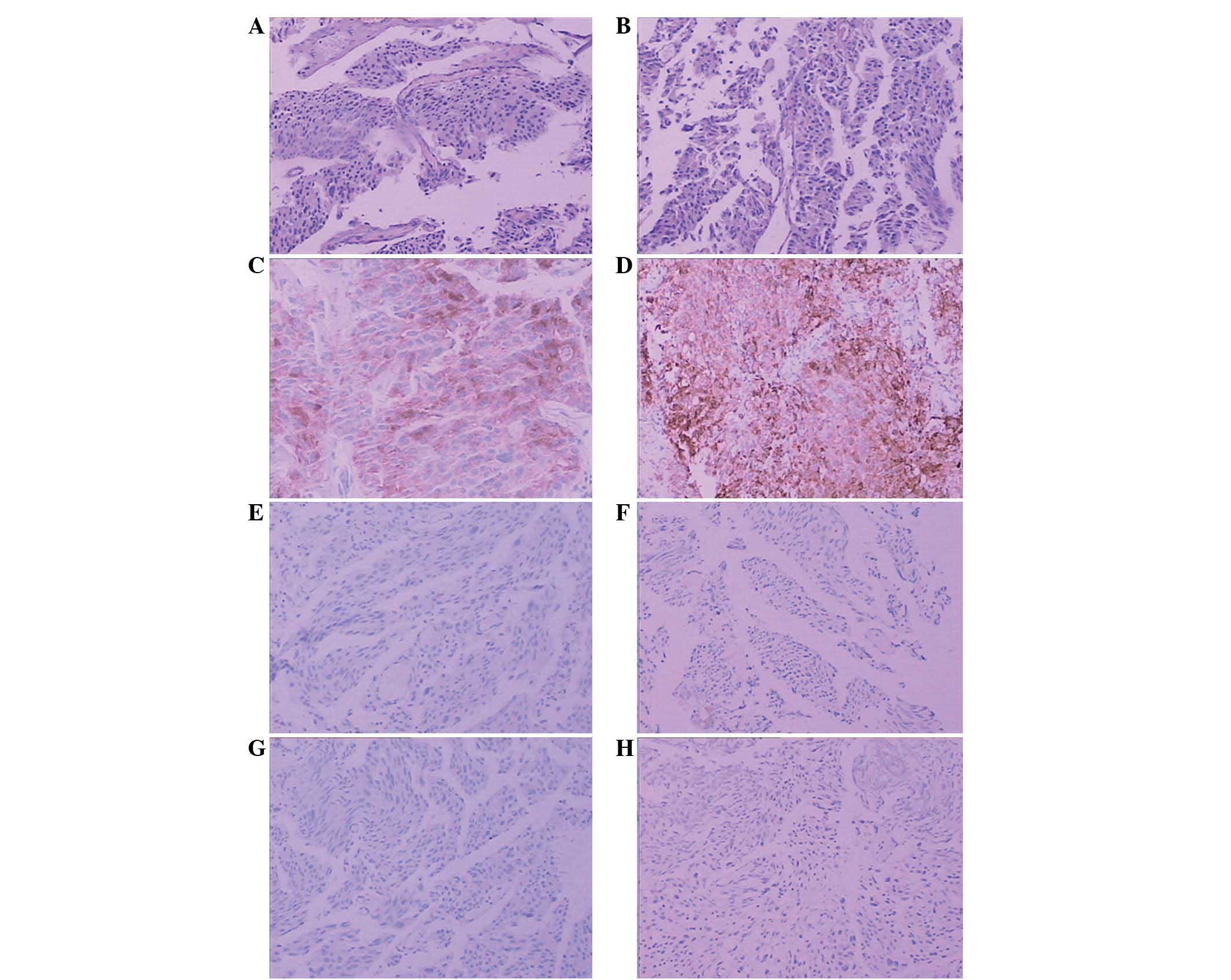 | Figure 2(A and B) UC-like structures with
tumor cells that are lamellar in growth. Gradually maturing pattern
of differentiation from the bottom to the surface, reflecting the
hierarchical structure of the transitional epithelium. The nuclei
display a polarity (HE). Scattered cancer cells strongly positive
for (C) PSA and (D) P504s, and negative for (E) 34βE12, (F) CK7,
(G) CK20 and (H) p63. UC, urothelial carcinoma; HE, hematoxylin and
eosin; PSA, prostate-specific antigen; CK, cytokeratin.
Magnification (C and D), ×200; (A, B, E, F and G), ×100. |
The tissue specimens were fixed in 10% buffered
formalin and the paraffin sections were stained with hematoxylin
and eosin (HE) for conventional histology. The immunohistochemical
staining was performed on representative sections of 5 μm in
thickness using the labeled streptavidin biotin technique for the
following antibodies: PSA (1:100 dilution; ZSGB-BIO, Beijing,
China); α-methylacyl coenzyme A racemase (1:80 dilution; ZSGB-BIO);
CK7 (1:50 dilution; ZSGB-BIO), CK20 (1:50 dilution; ZSGB-BIO); and
HMWCK (34βE12; 1:100 dilution; DAKO, Carpinteria, CA, USA).
Antibody detection was performed using the DAKO EnVision-System,
with 3,3′-diaminobenzidine as a chromogen. Adequate positive and
negative tissue controls were used throughout. The intensity of the
immunohistochemical staining was evaluated semi-quantitatively
using the following system: +++, strong, diffuse staining; ++,
moderate staining; +, weak and focal staining; and −, no
staining.
The histopathological analysis revealed UC-like
structures with tumor cells that were lamellar in growth. Gradually
maturing patterns of differentiation were observed from the bottom
to the surface of the sections, which reflected the hierarchical
structure of the transitional epithelium. The nuclei displayed a
polarity, as observed by the HE staining. The immunohistochemical
findings are summarized in Table I.
The carcinoma component was positive for PSA and P504S, but
negative for 34βE12, CK7, CK20 and p63 (Fig. 2). A diagnosis of a primary PAC
(solid carcinoma), Gleason 8 grade (4A + 4A) with a prostatic
origin, was made based on the clinical features, serum PSA level
and immunohistochemical findings. The patient was treated with
maximal androgen blockade, including luteinizing hormone
releasing-hormone analogue (3.65 mg/28 days) and bicalutamide (50
mg qd). At three years post-diagnosis, the patient underwent a
transurethral resection of the prostate (TURP) to relieve the
obstructive symptoms. A pathological examination of the resected
specimens revealed UC, and the immunohistochemistry of PSA, 34βE12,
P504S, CK7, CK20 and p63 also yielded the same results.
 | Table IImmunohistochemical observations. |
Table I
Immunohistochemical observations.
| Antibody | PSA | P504S | 34βE12 | CK7 | CK20 | p63 |
|---|
| Area of cancer | +++ | +++ | − | − | − | − |
Discussion
Since prostate cancer or UC may occur in elderly men
synchronously or metachronously, the differential diagnosis of a
poorly-differentiated carcinoma of the bladder or prostate includes
urothelial and prostate carcinoma (2). An accurate distinction between the two
has significant therapeutic and prognostic ramifications. A PAC may
respond to hormonal therapy and should not be treated by a
cystoprostatectomy. An appropriate diagnosis also determines the
stage for prognostication. In problematic cases,
immunohistochemistry may be used to aid in the identification of
the origin of the tumor cell when the morphological features on HE
stained sections are unclear. The present study analyzed patients
with UC and primary transitional cell carcinoma (TCC) components in
prostatic tissue sections using studies obtained from the
literature (Table II). The tumors
were primary PACs with coexisting UC, which originated from the
urothelium. PAC or pure UC/TCC were shown on HE sections. The
present case varied from those cases that were reported in the
literature, as it was pure UC, as shown by HE sections, originating
in the prostatic glandular epithelium. UC is distinguished from
poorly-differentiated PAC by its histopathological characteristics,
including the presence of solid nests of cells associated with
dense or abundant cytoplasm and striking nuclear pleomorphism, with
the absence or rarity of glandular lumina. The serum free PSA level
is a main marker for prostate cancer screening. However, in the
present case, the serum PSA level was high (1,130 ng/ml),
indicating prostate cancer. UC was detected by transurethral
biopsy. A bone ECT revealed multiple bone metastases. Due to these
factors, achieving a definite diagnosis was difficult. In order to
identify the origin and character of the prostatic cancer, a panel
of immunohistochemistry was selected.
 | Table IIPatients with UC involving the
prostate. |
Table II
Patients with UC involving the
prostate.
| First author, year
(ref.) | n | Age, years | Site | Histopathological
feature | Immunohistochemistry
of UC | Origin | Diagnosis |
|---|
|
|---|
| PSA | PAP | CK7 | CK20 | 34βE12 | CEA | p63 |
|---|
| Hashimoto et
al, 1989 (6) | 1 | 58 | Prostate | TCC | − | | | | | | | Prostate | TCC-P |
| Mottola et al,
1991 (7) | 3 | - | Prostate | TCC | | | | | | | | Prostate | TCC-P |
| Mai et al,
2002 (8) | 6 | - | Prostate | UC and AC | −/+ | +/++ | −/+ | − | | −/+ | | Prostate/urinary | PAC |
| Morikawa et
al, 2003 (9) | 1 | 77 | Prostate | TCC | − | | | | | | | Prostate | TCC-P |
| Ushida et al,
2004 (10) | 1 | 77 | Prostate | PAC and TCC | +/− | | | | | | | | PDC |
| Huang et al,
2004 (11) | 1 | 83 | Bladder | PAC and UC | − | − | + | + | + | | | Prostate | PAC |
| Curtis et al,
2005 (12) | 1 | 89 | Prostate | AC and UC | − | − | ++ | ++ | + | ++ | | Prostatic
urethra | UTA |
| Martínez et
al, 2007 (13) | 1 | - | Prostate | PAC and UC | − | − | ++ | ++ | +++ | | +++ | Urinary bladder | PAC |
PSA is a 33-kDa serine protease that is secreted by
the prostatic epithelium. The sensitivity and specificity of PSA is
high in prostate cancer, at 100% sensitivity. In
poorly-differentiated prostate cancer and PAC, the expression
levels of PSA may reach 85–95%. PSA is the oldest and most commonly
used immunohistochemical marker to identify cancers of prostatic
origin (3). The basal cell marker,
34βE12, is a protein cloned by HMWCK, and is useful for the
observation of basal cells, as their presence contradicts a
diagnosis of prostatic carcinoma (4).
p63 is expressed in the majority of UCs, but is not
present in the majority of PACs. The protein may be used as a
reliable marker to distinguish PACs from UCs in difficult cases in
conjunction with other markers, such as PSA (5). In order to obtain an optimal
immunohistochemical panel to distinguish poorly-differentiated PAC
from UC, Kunju et al(1)
analyzed a panel consisting of PSA, prostatic acid phosphatase
(PAP), 34βE12, CK7, CK20, p63 and P504S. The results indicated that
PSA stained 95% of prostate cancer vs. 0% of UC cases. 34βE12 and
p63 stained 97% and 92% of UC vs. 2% and 0% of PAC cases,
respectively. A panel of PSA, 34βE12 and p63 was optimal for
separating 95% PAC (PSA+/34βE12 and/or p63−)
vs. 97% UC (PSA−/34βE12 and/or p63+)
(1). P504S is a metabolic enzyme
whose overexpression has been shown to be a diagnostic indicator of
PAC and other solid tumors. The prostate and basal cell biomarker,
P504S, has been used together with the morphology to assist in the
formation of a diagnosis in diagnostically suspicious cases, with a
very high sensitivity and specificity. This has increased the
diagnostic accuracy of prostate cancer worldwide. A binding of
34βE12 and P504S is of great value in diagnosing morphologically
suspicious cases and significantly increasing the 34% diagnostic
accuracy in prostate cancer (14).
In the present case, basal cell staining recorded positive results
for PSA and P504S, supporting the definitive diagnosis of malignant
PAC. 34βE12 and p63 were negative in this case, discouraging a
diagnosis of UC.
CK7 and CK20 are also useful markers to distinguish
PAC from UC. Bassily et al studied the expression of CK7 and
CK20 in PAC and UC, and estimated their usefulness for
distinguishing between the two tumors. In the prostatic and
metastatic tumors, neither were positive for the markers. However,
61% of the UC cases were positive for CK7 and CK20 (15). The present case was also negative
for CK7 and CK20.
The common subtype of PAC is acinar adenocarcinoma,
however, several rare subtypes exist, including atrophic,
pseudohyperplastic variant, foamy, colloid, signet-ring, oncocytic,
lymphoepithelioma-like and sarcomatoid carcinoma. Due to the
observations in this novel case of PAC, in which histopathological
study revealed pure UC, with, however, a prostatic glandular
epithelial origin, a new subtype of adenocarcinoma of the prostate,
termed the UC-like subtype, was identified. The distinction between
the UC-like subtype of PAC and UC is significant, since the latter
is associated with multifocal lesions in the urinary tract and
requires a alternative form of therapy.
Acknowledgements
This study was supported by the Department of
Pathology, Tianjin Institute of Urology and the Department of
Urology, Second Hospital of Tianjin Medical University, Tianjin,
China.
References
|
1
|
Kunju LP, Mehra R, Snyder M and Shah RB:
Prostate-specific antigen, high-molecular-weight cytokeratin (clone
34betaE12), and/or p63: an optimal immunohistochemical panel to
distinguish poorly differentiated prostate adenocarcinoma from
urothelial carcinoma. Am J Clin Pathol. 125:675–681. 2006.
View Article : Google Scholar
|
|
2
|
Varma M, Morgan M, Amin MB, et al: High
molecular weight cytokeratin antibody (clone 34betaE12): a
sensitive marker for differentiation of high-grade invasive
urothelial carcinoma from prostate cancer. Histopathology.
42:167–172. 2003. View Article : Google Scholar
|
|
3
|
Epstein JI: PSA and PAP as
immunohistochemical markers in prostate cancer. Urol Clin North Am.
20:757–770. 1993.PubMed/NCBI
|
|
4
|
Shah RB, Zhou M, LeBlanc M, Snyder M and
Rubin MA: Comparison of the basal cell-specific markers, 34betaE12
and p63, in the diagnosis of prostate cancer. Am J Surg Pathol.
26:1161–1168. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ud Din N, Qureshi A and Mansoor S: Utility
of p63 immunohistochemical stain in differentiating urothelial
carcinomas from adenocarcinomas of prostate. Indian J Pathol
Microbiol. 54:59–62. 2011.PubMed/NCBI
|
|
6
|
Hashimoto H, Watanabe Y, Mizunaga M, et
al: A case of primary transitional cell carcinoma of the prostate.
Hinyokika Kiyo. 35:1235–1238. 1989.(In Japanese).
|
|
7
|
Mottola A, Di Cello V, Lunghi F, et al:
Diagnostic, clinical and therapeutic aspects of primary
transitional cell carcinoma of the prostate. Minerva Urol Nefrol.
43:37–39. 1991.(In Italian).
|
|
8
|
Mai KT, Collins JP and Veinot JP:
Prostatic adenocarcinoma with urothelial (transitional cell)
carcinoma features. Appl Immunohistochem Mol Morphol. 10:231–236.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Morikawa H, Cho M, Takada S, et al: A case
of primary transitional cell carcinoma of the prostate. Hinyokika
Kiyo. 49:357–360. 2003.(In Japanese).
|
|
10
|
Ushida H, Koizumi S and Okada Y: A
prostatic duct carcinoma difficult to distinguish from transitional
cell carcinoma: a case report. Hinyokika Kiyo. 50:535–538. 2004.(In
Japanese).
|
|
11
|
Huang Q, Chu PG, Lau SK and Weiss LM:
Urothelial carcinoma of the urinary bladder with a component of
acinar/tubular type differentiation simulating prostatic
adenocarcinoma. Hum Pathol. 35:769–773. 2004. View Article : Google Scholar
|
|
12
|
Curtis MW, Evans AJ and Srigley JR:
Mucin-producing urothelial-type adenocarcinoma of prostate: report
of two cases of a rare and diagnostically challenging entity. Mod
Pathol. 18:585–590. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Martínez-Rodríguez M, Ramos D, et al:
Poorly differentiated adenocarcinomas of prostate versus high-grade
urothelial carcinoma of the bladder: a diagnostic dilemma with
immunohistochemical evaluation of 2 cases. Int J Surg Pathol.
15:213–218. 2007.
|
|
14
|
Kumaresan K, Kakkar N, Verma A, et al:
Diagnostic utility of α-methylacyl CoA racemase (P504S) & HMWCK
in morphologically difficult prostate cancer. Diagn Pathol.
5:832010.
|
|
15
|
Bassily NH, Vallorosi CJ, Akdas G, Montie
JE and Rubin MA: Coordinate expression of cytokeratins 7 and 20 in
prostate adenocarcinoma and bladder urothelial carcinoma. Am J Clin
Pathol. 113:383–388. 2000. View Article : Google Scholar : PubMed/NCBI
|