Introduction
Approximately 90% of patients with pancreatic cancer
present with a metastatic as opposed to localized form of the
disease at the time of diagnosis (1). With the incidence of pancreatic cancer
increasing over the last few decades, the treatment of metastases
has become a challenge for oncologists. Pancreatic cancer cells
metastasize mainly via the lymph nodes and by direct invasion,
followed by hematogenous spread and extension through the
neurilemma. Liver metastases are observed in half of patients in
whom the cancer has metastasized and the prognosis for this group
is extremely poor (1).
The midkine (MK) gene was first identified in
1998 by Takada et al(2) from
the cDNA library for the retinoic acid-induced mouse testicular
teratocarcinoma HM-1 cell line. Since then, the gene has been
identified in several animal species and in humans. MK and the
associated protein, pleiotrophin, are members of the
heparin-binding factor family (3).
MK is overexpressed in numerous cancers, including esophageal
cancer, gastric cancer, colon cancer, pancreatic cancer,
hepatocellular carcinoma and lung cancer, but is expressed at low
concentrations or is absent in normal tissues (2). MK therefore possesses the potential to
serve as a biomarker in the diagnosis, prognosis and treatment of
patients with cancer. MK expression has been reported to correlate
with the extent of metastasis of pancreatic cancer to the liver
(4). However, further investigation
is required to ascertain whether MK is essential to the mechanisms
through which pancreatic cancer cells metastasize to the liver and
other organs.
In the present study, MK-targeting small
interfering RNA (siRNA) was employed to silence the expression of
MK in human pancreatic cancer AsPC-1 cells. The migration and
invasion of these cancer cells in vitro and in vivo
was observed to be reduced as MK expression decreased. Experiments
were also performed to investigate the involvement of vascular
endothelial growth factor (VEGF) in the mechanism(s) through which
the suppression of MK expression constrains the migratory and
invasive capacities of these cells.
Materials and methods
Materials
Human pancreatic cancer AsPC-1 cells were acquired
from the Tissue and Cell Bank of Jiangsu Cancer Hospital (Nanjing,
China). The sense sequence of MK siRNA was
5′-AGGACUAGACGCCAAGCCUTT-3′ (Dharmacon, Inc., Lafayette, CO, USA).
A monoclonal antibody to MK was obtained from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA) and TRIzol, RNase
inhibitor, reverse transcriptase and Taq polymerase were
purchased from Qiagen (Hilden, Germany). Oligofectamine 2000 was
purchased from Invitrogen Life Technologies (Carlsbad, CA, USA).
Male Balb/c nude mice (n=24) aged 4 weeks and weighing 14–18 g were
purchased from the Shanghai Institute of Biochemistry of the
Chinese Academy of Sciences (Shanghai, China) and housed in a
specific pathogen-free (SPF) environment. Approval for this study
was obtained from the Committee on Medical Ethics of the Affiliated
Hospital of Jiangsu University (no. 2010035; Zhenjiang, Jiangsu,
China).
Cell culture and transfection
procedures
The AsPC-1 cells were cultured at 37°C in RPMI-1640
medium containing 10% fetal bovine serum (FBS) in humidified air
with 5% CO2. One day prior to the transfection, the
logarithmically growing cells (1.0×105) were seeded in
24-well plates (1 ml/well) and incubated overnight. The
transfections were performed using Oligofectamine 2000 according to
the manufacturer’s instructions. The cells were divided into the
following groups: i) untreated control; ii) vector control (cells
transfected with liposomes only) and iii) MK siRNA transfection.
For the transfections, the cells were incubated in RPMI-1640
containing 10% FBS and liposomes alone or MK siRNA (embedded in
liposomes) at varying concentrations. The cells were harvested for
experiments by trypsinization.
Measurement of MK expression
mRNA expression for MK was determined using qPCR.
Total RNA was extracted from the harvested cells using TRIzol. The
total RNA (1 μg) was used for the synthesis of the first cDNA
strand using oligo dT (15-mer) as a primer and 2 μl cDNA was
applied for PCR. The expression of GAPDH mRNA served as the
internal reference. The PCR amplification was performed as
previously described (5). The
primers were forward, 5′-GCGCGCTACAATGCTCAGT-3′ and reverse,
5′-CCCTTCCCTTTCTTGGCTTT-3′. The FAM-labeled TaqMan probe was
5′-CATGGGTG CCCCGACGTTGC-3′-TAMRA. The PCR conditions included a
pre-denaturation step at 95°C for 3 min followed by 35 cycles of
denaturation at 95°C for 30 sec, annealing at 52°C for 45 sec and
extension at 72°C for 45 sec, followed by a final extension at 72°C
for 7 min.
MK protein expression was measured using western
blotting, which was performed as described previously (6). The expression of β-actin
(Sigma-Aldrich, St Louis, MO, USA) was used as a normalization
control for protein loading.
Measurement of extracellular VEGF
concentration
Following the transfections or control incubations
for 24 h, the media were collected and the cell numbers were
determined. Subsequent to the clarification of the media by
centrifugation at 4°C for 3 min at 800 × g, the VEGF concentration
was determined using an enzyme-linked immunosorbent assay (ELISA)
kit (R&D Systems, Minneapolis, MN, USA).
Cell migration assay
A Transwell system (BD Biosciences, Franklin Lakes,
NJ, USA) was employed to determine cell migration. This system was
comprised of a polycarbonate microporous membrane (filter, 8-μm
pore size) situated between an upper and a lower chamber that were
coated with Matrigel™ (BD Biosciences). The system was placed in
the wells of a 24-well plate. The cells were collected, washed
three times with serum-free Dulbecco’s modified Eagle’s medium
(DMEM), resuspended in serum-free DMEM and adjusted to
5×104 cells/ml. DMEM containing 10% FBS (500 μl) was
added to the lower chamber and the cell suspension (200 μl) was
added to the upper chamber. The cells were then incubated in
humidified air with 5% CO2 at 37°C for 24–48 h. The
cells that remained on the upper filter were scraped off gently
using a cotton swab and the inserts were washed gently with
phosphate buffered saline (PBS). The cells that migrated to the
lower chamber were fixed in 4% paraformaldehyde for 30 min, washed
twice for 2–5 min, stained with 0.5% crystal violet for 30 min and
washed three times with PBS for 3–5 min. Filters were placed onto
the slides, which were then secured with a coverslip. Five fields
were randomly selected for viewing at a magnification of ×200 and
the cell number was determined. Cell migration (%) was expressed as
the number of migrating cells divided by the total number of cells.
Each experiment was performed three times and the results were
averaged for the statistical analysis.
Cell invasion assay
The Transwell system that was described previously
was also used to measure cell invasion. The wraps were removed and
the system was held at room temperature. Serum-free medium (0.5 ml)
was added to the upper and lower chambers and incubated at 37°C for
2 h, following which, the medium was removed carefully. A
2.5×104/ml single-cell suspension was prepared in
serum-free medium and 500 μl of this suspension was placed in the
upper chamber. An additional 500 μl medium containing 10% FBS was
added to the lower chamber to serve as a chemoattractant. The
invasion chambers were placed in bubble-free wells of plates and
incubated at 37°C for 48 h in humidified air with 5%
CO2. All the steps subsequent to the incubation were
identical to those described previously for the measurement of cell
migration. Each experiment was performed in triplicate and the
results were averaged for the statistical analysis.
Determination of metastasis to the liver
Preparation of cells for treatments
The cells were divided into three groups, untreated
control, vector control (liposomes only) and MK siRNA-treated. The
transfections were performed as described previously, with the
exception that liposome-embedded siRNA was used at a concentration
of 12.5 nmol/l. Following two days of transfection, the cells were
digested with 0.25% trypsin and 0.02% EDTA and the suspensions were
subjected to centrifugation at 1,500 × g for 5 min. The supernatant
fluid was removed and the cells were resuspended in normal saline
to 2×108/ml. The percentage of viable cells was
calculated following trypan blue staining and the viability was
routinely ≥95%.
Mouse model of pancreatic cancer
metastasis to the liver
A mouse model of pancreatic cancer metastasis to the
liver was established using the splenectomy method (5). In brief, the nude mice were
intraperitoneally anesthetized with 1% sodium pentobarbital (35
mg/kg) and fixed to a table. Following sterilization, a 1-cm
longitudinal incision was made in the left upper quadrant. The
gastrosplenic ligament and short gastric vessels were disconnected
and the spleen was exposed. The cell suspension (0.1 ml) was
injected into the upper region of the spleen over a period of 1–2
min, the syringe needle was slowly removed and this was followed by
kneading. The splenic pedicle was ligated, the spleen was removed
and the wound was closed. A total of five nude mice were used for
each treatment group. Following the surgery, the mice were housed
in an SPF environment and the spirit, food intake and body weight
of the animals were monitored daily. The mice were sacrificed by
cervical dislocation at 28 days post-surgery and metastasis of the
pancreatic cancer cells to the liver was observed. The gross tumor
nodules were counted and liver cross-sections were then used to
count the tumor nodules under a light microscope. The liver was
then obtained, fixed in 10% neutral formaldehyde, embedded in
paraffin and cut into 4-μm sections. Five sections were selected,
with 2 mm serving as the distance between the two adjacent
sections. The coronal section with the maximal area was set as the
center and the number of tumor nodules was then determined under a
microscope. The same nodule appearing in various sections was
considered a single nodule. The absence of gross or microscopic
tumor nodules was regarded as the absence of metastasis. The total
number of tumor nodules was determined as the sum of the gross and
microscopic tumor nodules.
Measurement of intratumoral microvessel
density
The microvessel densities were determined as
described previously by Rydén et al(6). Brown endothelial cells or endothelial
cell clusters with clear boundaries with adjacent microvessels,
cancer cells and other tissues were counted as a vessel, but
lumen-like structures or red blood cells were not considered in
this measurement. Subsequent to performing immunohistochemistry to
confirm the presence of CD34, three areas that were rich in
microvessels were selected under a microscope at low magnifications
(×40 and ×100) and the number of vessels was determined at a
magnification of ×400. The results were averaged for the
statistical analysis.
Statistical analyses
The results are presented as the mean ± standard
deviation. The statistical analysis was performed using the SPSS
version 11.5 statistical software package (SPSS, Inc., Chicago, IL,
USA). The comparisons between the groups were performed using
one-way analysis of variance. Analysis of variance was used to
evaluate the findings from the animal model studies. P<0.05 was
considered to indicate a statistically significant difference.
Results
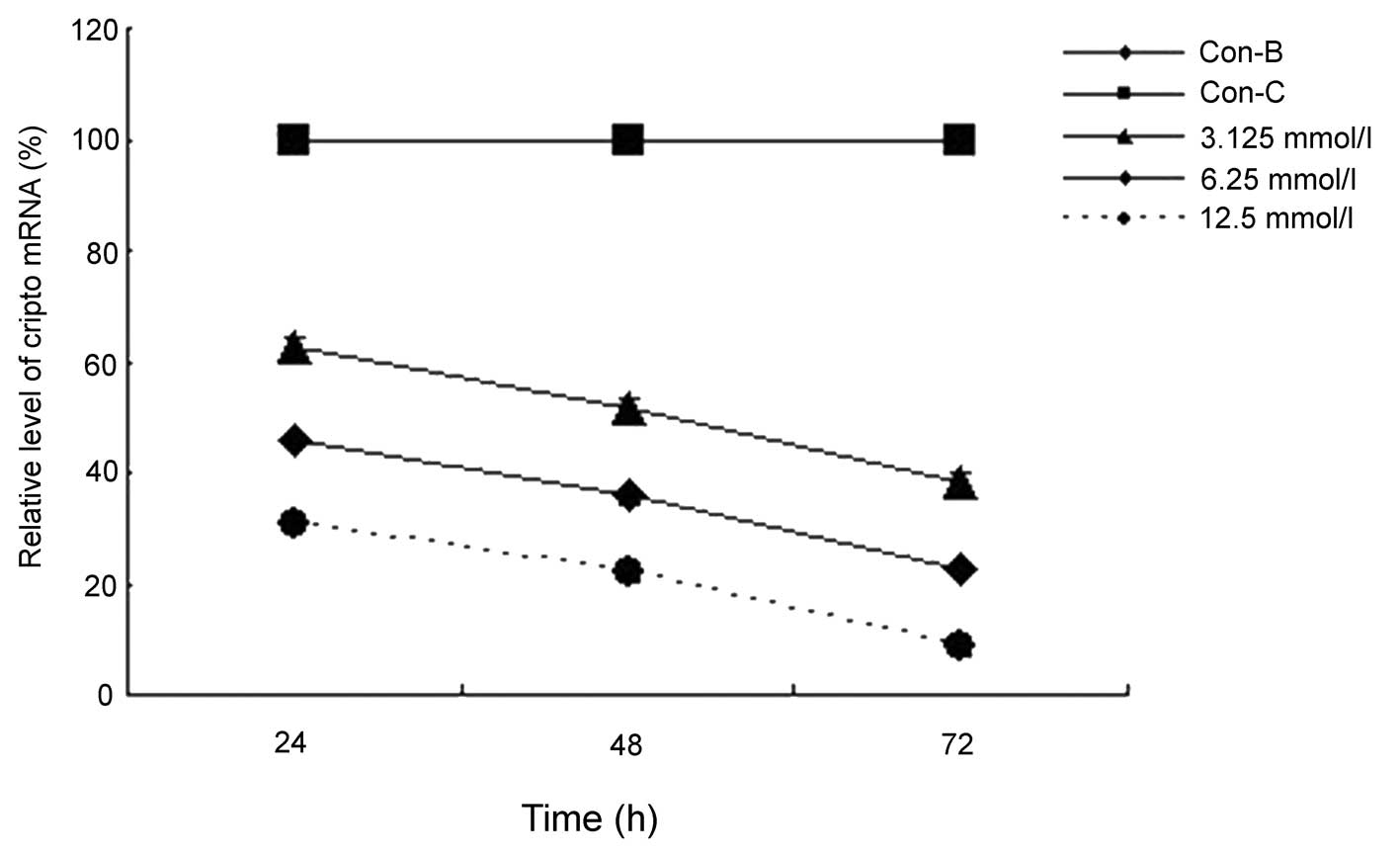
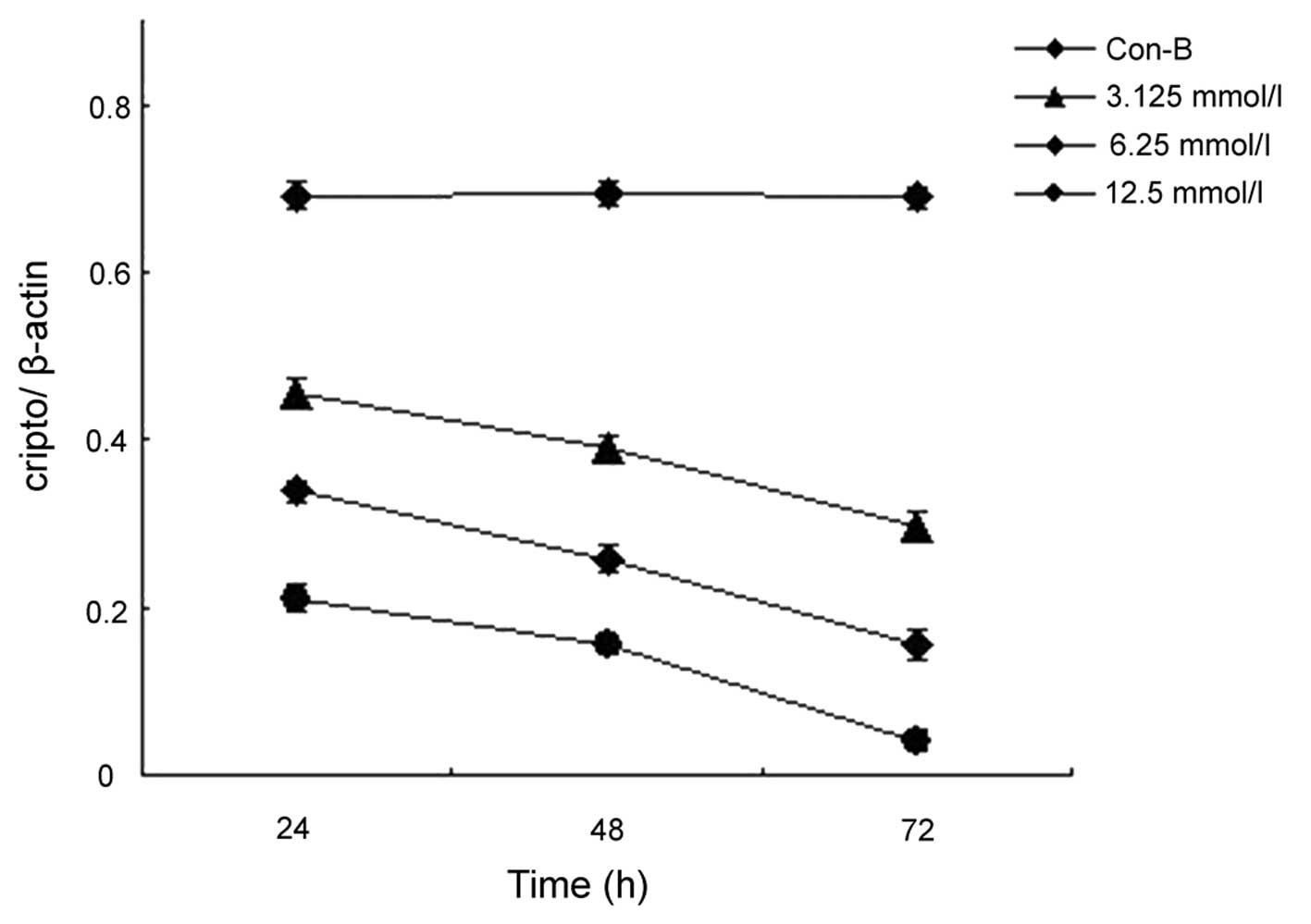
Expression of MK mRNA and protein as a
function of transfection with MK siRNA
When compared with the AsPC-1 cells that were
treated with the vector only (Con-B), the cells that were
transfected with MK siRNA exhibited significantly decreased levels
of MK mRNA (Fig. 1) and protein
(Fig. 2) expression. Statistically
significant decreases were observed at 24, 48 and 72 h following
the transfection procedure, and these decreases were concentration-
and time-dependent (P<0.0001 and P<0.0001, respectively).
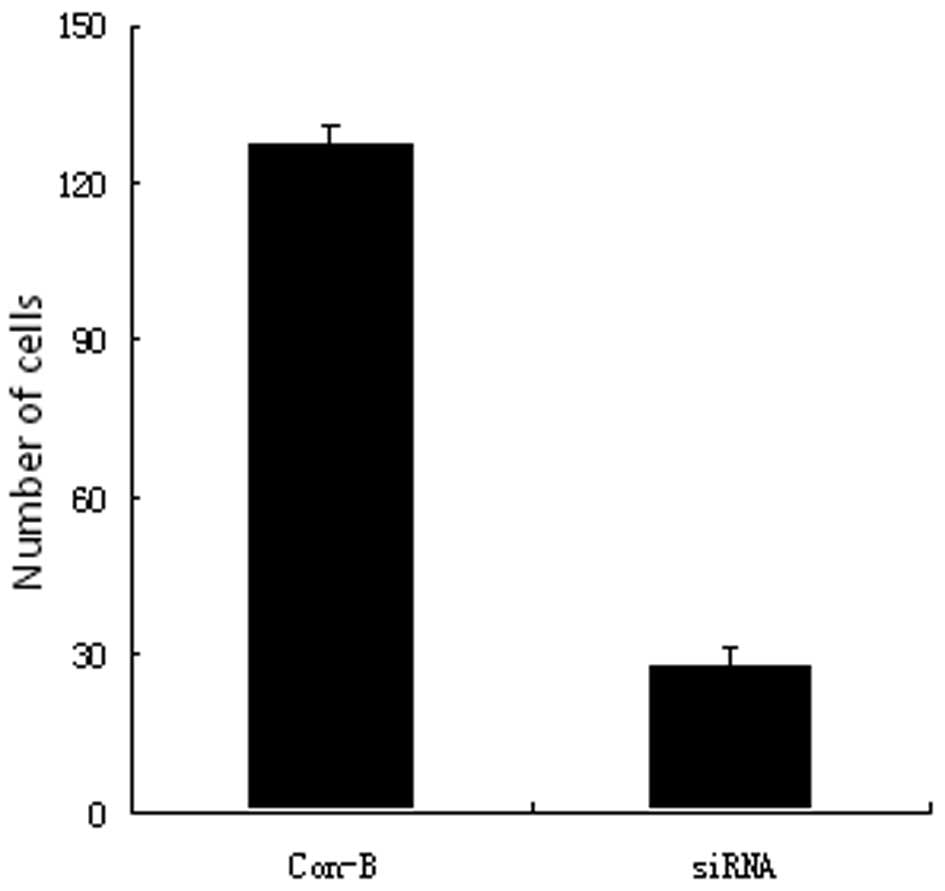
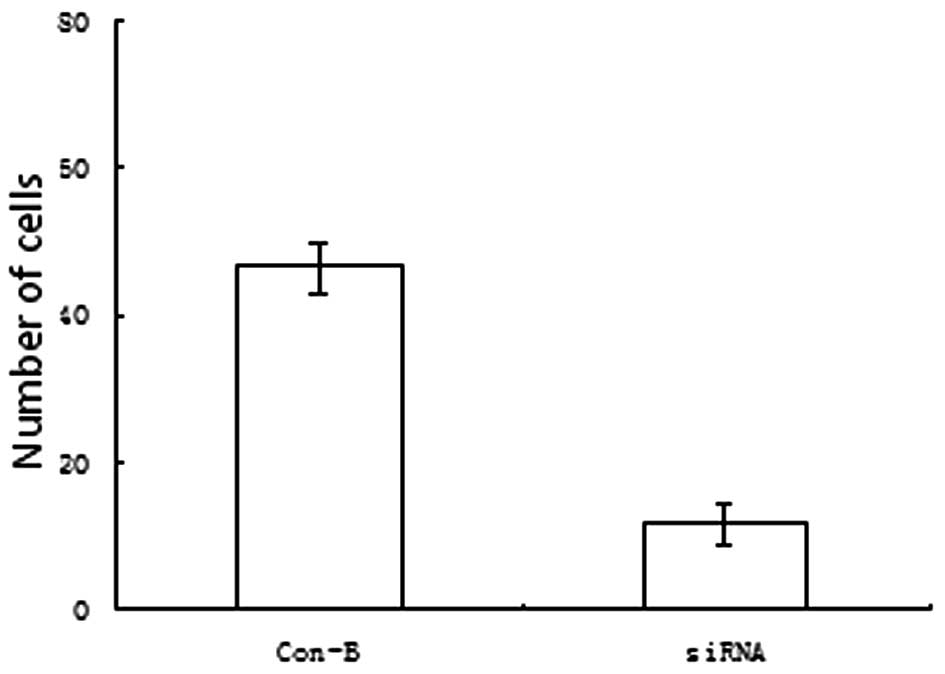
Pancreatic cancer cell migration and
invasion as a function of transfection with MK siRNA
The AsPC-1 cells were harvested at 48 h following
treatment with the vector only or with the vector containing MK
siRNA, and a Transwell system was employed for obtaining the
measurements of migration and invasion. The numbers of migrating
(Fig. 3) and tissue-penetrating
(Fig. 4) cells were observed to be
markedly lower for the cells that were transfected with MK siRNA
compared with the vector-only controls (P<0.005 and P<0.005,
respectively).
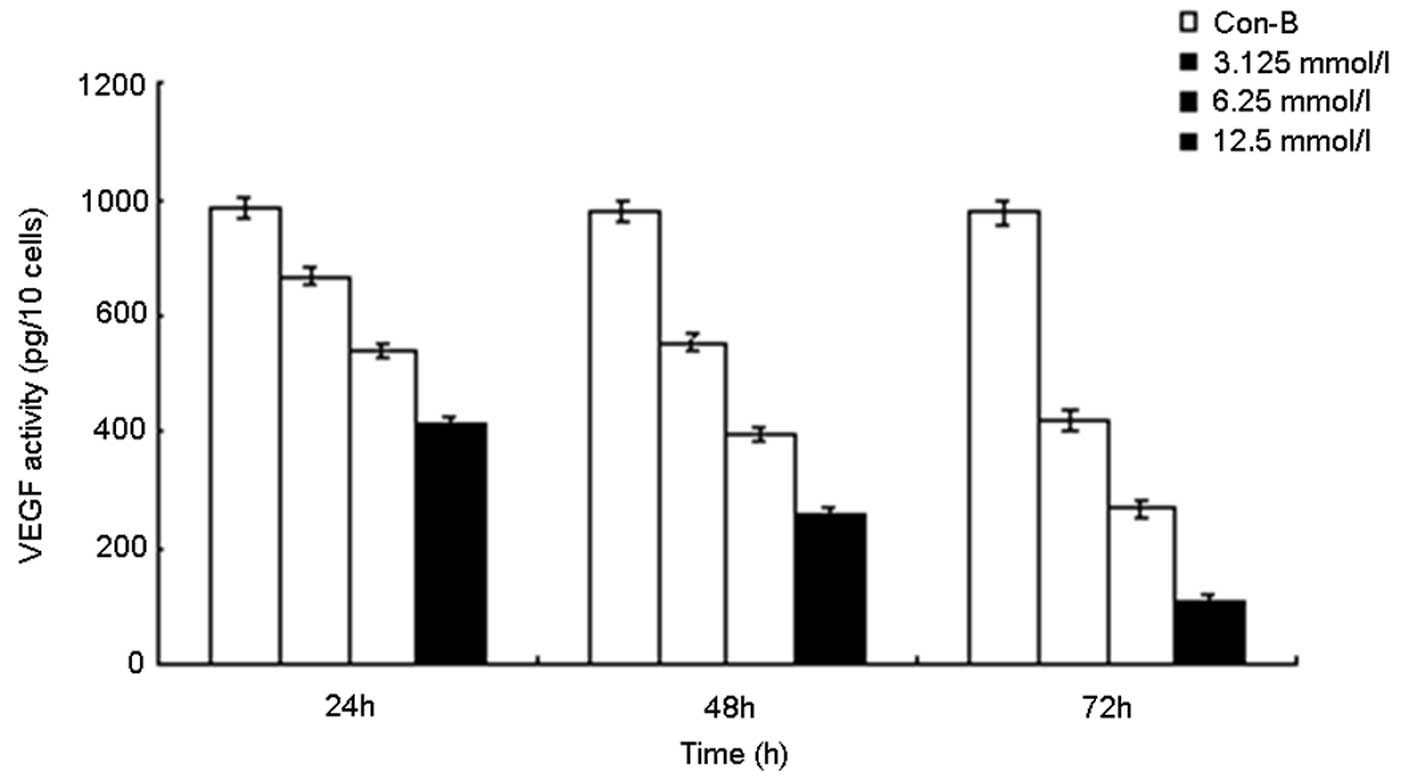
Extracellular VEGF concentrations for
mock-transfected and MK siRNA-transfected AsPC-1 cells
The cells were treated with the vector only or were
transfected with liposomes containing various concentrations of MK
siRNA. Following 0, 24, 48 and 72 h of incubation, the medium was
collected for measurement of VEGF by ELISA (Fig. 5). The VEGF concentration in the
medium from the MK siRNA-transfected group was observed to decline
in a manner that was dependent on the MK siRNA concentration and
the time of incubation (r=0.928). By contrast, the VEGF
concentration in the medium from the vector-only control group was
stable throughout the 72-h incubation period.
Effect of transfection of AsPC-1 cells
with MK siRNA on liver tumor nodule number and rate of liver
metastasis in vivo
A splenectomy method was employed to establish a
nude mouse model of liver metastasis of pancreatic cancer cells.
The livers of these animals were bright red, soft and lacked gross
and microscopic tumor nodules prior to being injected with the
AsPC-1 cells that had been transfected with MK siRNA
(siRNA-transfected group), mock-transfected (vector only group) or
left untreated (non-treated control group). However, in the mice
harboring the AsPC-1 cells that had metastasized to the liver, the
liver volume was decreased and the organ was hard and fragile.
Furthermore, multiple gray nodules were present in the livers of
these animals. The metastasis rates were 22.8±1.8, 82.6±1.6 and
81.9±1.7% for the siRNA-transfected, vector only and non-treated
control groups, respectively. The tumor nodule numbers were
3.6±0.8, 18.6±1.6 and 19.1±1.5 for the siRNA-transfected, vector
only and non-treated control groups, respectively. The statistical
analysis revealed that the metastasis rate and the tumor nodule
number for the siRNA-transfected group were significantly lower
than those for the other two groups (P<0.05 and P<0.05,
respectively).
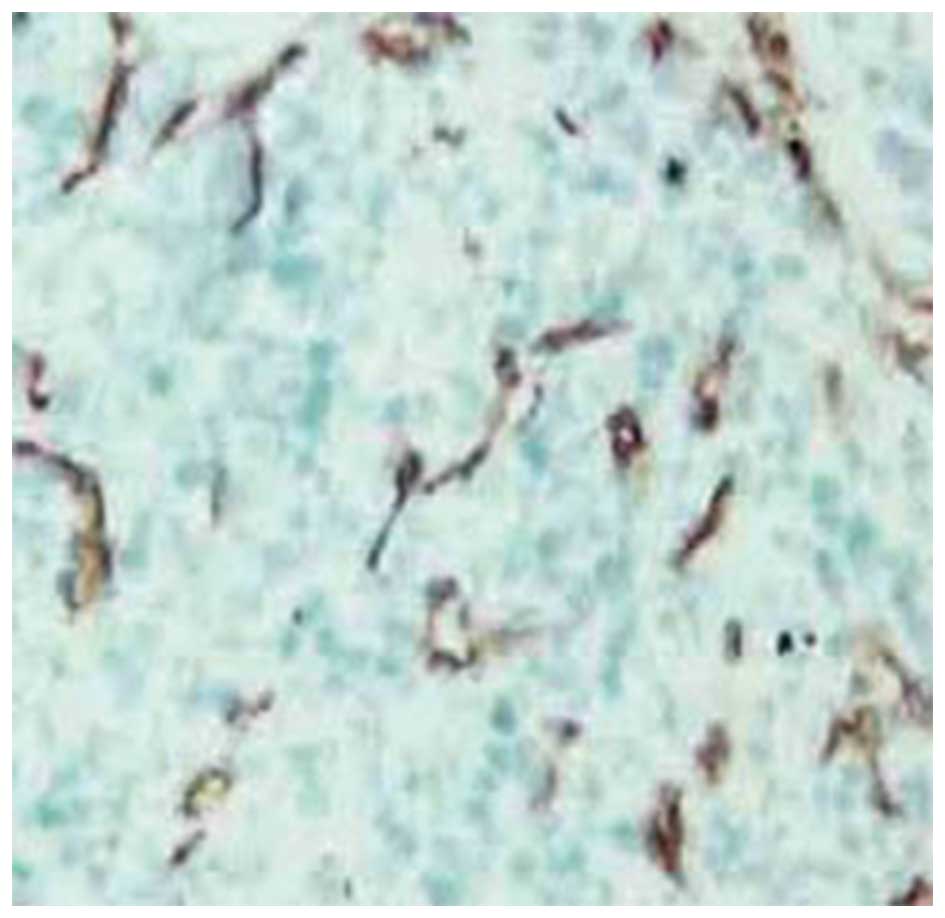
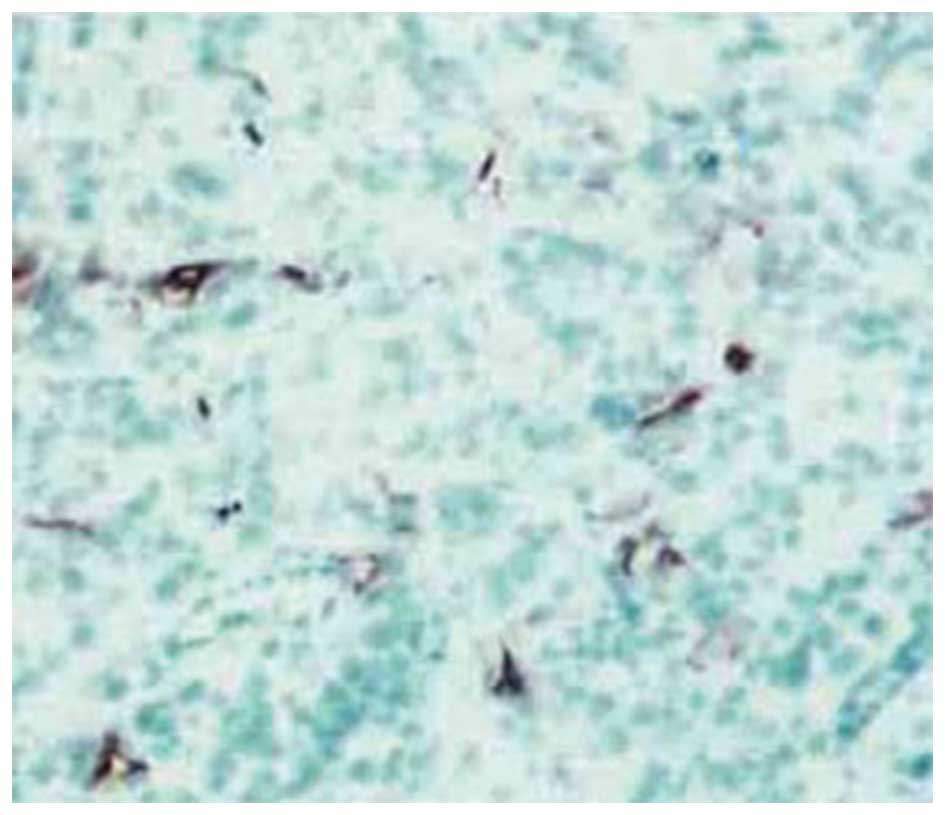
Effect of transfection of AsPC-1 cells
with MK siRNA on liver tumor microvessel density in vivo
The microvessel density values were determined using
the nude mouse model described previously. The value for the
siRNA-transfected group was 7.56±1.68, which was significantly
lower than that of the vector-only group (15.69±2.51) and for the
untreated control group (16.35±2.08; P<0.05). The difference in
the values between the vector-only and untreated control groups was
not significant (P>0.05). The microvessel-rich areas of the
liver tumors from the animals harboring the AsPC-1 cells that were
treated with the vector only (Fig.
6) or transfected with MK siRNA (Fig. 7) are presented for comparison.
Discussion
The present study revealed that the transfection of
AsPC-1 cells with siRNA directed against MK is highly effective in
reducing the expression of MK and its mRNA by pancreatic cancer
cells. The expression was decreased in a time- and
concentration-dependent manner, supporting the conclusion that RNA
interference through the use of siRNA against MK is a
suitable approach for examining the significance of MK expression
in the ability of these cells to metastasize to the liver.
Accordingly, transfection with MK siRNA was observed to decrease
the capacities for migration and tissue penetration. Furthermore,
the liver transmission rate and the number of liver tumor nodules
for animals harboring the siRNA-transfected cells were reduced
compared with those of the animals that harbored the
non-siRNA-transfected cells. The microvessel densities of the
livers from the mice that were transplanted with the
siRNA-transfected cells were also significantly lower than those
from the mice that were transplanted with the non-siRNA-transfected
cells. Collectively, these results strongly support the conclusion
that metastasis of pancreatic cancer cells to the liver requires
the expression of MK by these cells.
The Transwell system has been a useful tool for
studies of cellular migration and invasion in vitro(7,8).
Cancer cells bind to a specific matrix material (Matrigel) in this
system in a manner that mimics their binding to laminin,
fibronectin or type IV collagen that is present in the basement
membrane in vivo. The secretion of proteases or the
activation of zymogen in the matrix by the bound cancer cells
results in the degradation of the matrix. Subsequent migration of
the cancer cells results in the matrix gap being filled. In
vivo, these processes are repeated, leading to deep invasion
and distant metastasis. Using the Transwell system, transfection of
the AsPC-1 cells with MK siRNA was observed to decrease the number
of migrating and invading cells in a manner that was dependent on
the siRNA concentration, supporting a requirement for MK expression
in the migratory and invasive capacities of pancreatic cancer
cells.
The present study employed the splenectomy method to
establish a mouse model of liver metastasis of pancreatic cancer.
Although this model did not involve a splenic tumor, liver
metastasis was observed. The process of metastasis using this model
is similar to that observed in clinical practice, involving the
spread of the cancer cells through the portal vein following
pancreatic cancer resection. For the animals that were transplanted
with the AsPC-1 cells that were transfected with MK siRNA, the
number of metastatic liver nodules and the rate of metastasis to
the liver were markedly lower compared with those in the animals
that were transplanted with the control AsPC-1 cells. These
observations strongly support a requirement for MK in the process
through which pancreatic cancer cells spread and invade the
liver.
Liver metastasis is a complex process involving
multiple factors. VEGF is considered to be involved in the
mechanism through which gastrointestinal cancer cells metastasize
to the liver (9–11). The downregulation of VEGF is
reported to suppress metastasis to the liver and the invasion of
this organ by various cancers (11–14).
Seo et al reported that VEGF is closely associated with
metastasis of pancreatic cancer to the liver (11). In the present study, VEGF expression
by the AsPC-1 cells was significantly decreased following their
transfection with MK siRNA. Furthermore, the microvessel density of
the liver tumors from the mice that were transplanted with MK
siRNA-transfected AsPC-1 cells was significantly lower than that of
the tumors from the mice that were transplanted with the
non-siRNA-transfected AsPC-1 control cells. These findings are
consistent with the hypothesis that downregulation of VEGF
expression mediates the suppression of liver metastasis of
pancreatic cancer cells due to repressed MK expression.
In conclusion, the expression of MK by pancreatic
cancer cells is required for the metastasis of these cells to the
liver. Silencing of MK expression by transfection with MK siRNA
markedly decreases the capacities of cloned pancreatic cancer cells
for migration and tissue penetration in vitro and their
capacity to invade the liver in vivo. The downregulation of
VEGF expression is likely to be involved in the mechanisms through
which the reduction of MK limits the migratory and invasive
properties of pancreatic cancer cells.
Acknowledgements
This study was supported by grants from the
Zhenjiang Key Laboratory of Molecular Endocrinology (no.
SS2009012).
References
|
1
|
Michalski CW, Erkan M, Hüser N, et al:
Resection of primary pancreatic cancer and liver metastasis: a
systematic review. Dig Surg. 25:473–480. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Takada T, Toriyama K, Muramatsu H, Song
XJ, Torii S and Muramatsu T: Midkine, a retinoic acid-inducible
heparin-binding cytokine in inflammatory responses: chemotactic
activity to neutrophils and association with inflammatory
synovitis. J Biochem. 122:453–458. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Aridome K, Tsutsui J, Takao S, et al:
Increased midkine gene expression in human gastrointestinal
cancers. Jpn J Cancer Res. 86:655–661. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Maeda S, Shinchi H, Kurahara H, et al:
Clinical significance of midkine expression in pancreatic head
carcinoma. Br J Cancer. 97:405–411. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fan Y, Zheng S and Ding JY: Inhibition of
telomerase activity in colon cancer LS- 174T cells with
liposome-mediated cripto antisense oligodeoxynucleotide. Chin J
Pathophysiol. 22:762–765. 2006.
|
|
6
|
Rydén L, Boiesen P and Jönsson PE:
Assessment of microvessel density in core needle biopsy specimen in
breast cancer. Anticancer Res. 24:371–375. 2004.PubMed/NCBI
|
|
7
|
Fan Y, Zhang YL, Wu Y, et al: Inhibition
of signal transducer and activator of transcription 3 expression by
RNA interference suppresses invasion through inducing anoikis in
human colon cancer cells. World J Gastroenterol. 14:428–434. 2008.
View Article : Google Scholar
|
|
8
|
Woo MM, Salamanca CM, Minor A and
Auersperg N: An improved assay to quantitate the invasiveness of
cells in modified Boyden chambers. In Vitro Cell Dev Biol Anim.
43:7–9. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Takeda A, Stoeltzing O, Ahmad SA, et al:
Role of angiogenesis in the development and growth of liver
metastasis. Ann Surg Oncol. 9:610–616. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Stoeltzing O, Liu W, Reinmuth N, et al:
Angiogenesis and antiangiogenic therapy of colon cancer liver
metastasis. Ann Surg Oncol. 10:722–733. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Seo Y, Baba H, Fukuda T, Takashima M and
Sugimachi K: High expression of vascular endothelial growth factor
is associated with liver metastasis and a poor prognosis for
patients with ductal pancreatic adenocarcinoma. Cancer.
88:2239–2245. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Solorzano CC, Baker CH, Tsan R, et al:
Optimization for the blockade of epidermal growth factor receptor
signaling for therapy of human pancreatic carcinoma. Clin Cancer
Res. 7:2563–2572. 2001.PubMed/NCBI
|
|
13
|
Rubbia-Brandt L, Terris B, Giostra E,
Dousset B, Morel P and Pepper MS: Lymphatic vessel density and
vascular endothelial growth factor-C expression correlate with
malignant behavior in human pancreatic endocrine tumors. Clin
Cancer Res. 10:6919–6928. 2004. View Article : Google Scholar
|
|
14
|
Fukuhara M, Uchida E, Tajiri T, Aimoto T,
Naito Z and Ishiwata T: Re-expression of reduced VEGF activity in
liver metastases of experimental pancreatic cancer. J Nippon Med
Sch. 72:155–164. 2005. View Article : Google Scholar : PubMed/NCBI
|