Introduction
Epithelial ovarian cancer is the fourth most common
cause of cancer-related mortality in females worldwide (1). The current treatment algorithms for
newly diagnosed patients incorporate surgical cytoreduction and
platinum/taxane-based chemotherapy (2,3).
Despite initial response rates of ~80%, the disease recurs in 75%
of cases (2,3). The recurrent cases require additional
chemotherapy, but often terminate treatment due to the adverse
effects of repetitive chemotherapy. Therefore, new approaches to
patients with recurrent epithelial ovarian cancer are
imperative.
Signal transducer and activator of transcription 3
(STAT3) is a well-known signaling molecule that is associated with
cell proliferation, survival, angiogenesis and immunosuppression.
The activation of STAT3 is considered to be significant for cancer
progression (4). In numerous types
of malignant tumors, STAT3 activation in cancer cells is associated
with a poor clinical prognosis or higher grade histological
malignancies (5). Novel compounds
that inhibit STAT3 have been reported, a number of which are now in
clinical trials for patients with malignant tumors (6). As STAT3 activation in cancer cells is
known to cause resistance to chemotherapy and radiotherapy, STAT3
inhibition is considered to be effective for patients with advanced
malignant tumors (6–8).
Corosolic acid (CA), a natural compound derived from
apple pomace, is a potent STAT3 inhibitor and inhibits the
proliferation of glioblastoma and osteosarcoma cells (9,10).
Furthermore, the administration of CA has been shown to
significantly suppress subcutaneous tumor development and lung
metastasis in a model of osteosarcoma (10).
The present study examined whether CA has a
synergistic effect with chemotherapy on epithelial ovarian cancer
in vitro, in order to identify whether it may be beneficial
in the treatment of advanced epithelial ovarian cancer.
Materials and methods
Cell culture
The human ovarian carcinoma SKOV3, RMG-1, and ES-2
cell lines were purchased from American Type Culture Collection
(Manassas, VA, USA) and were maintained in RPMI-1640 supplemented
with 10% fetal bovine serum (FBS). Peripheral blood mononuclear
cells were obtained from healthy volunteer donors, who gave written
informed consent for participation in this study. The study was
approved by the ethics committee of Kumamoto University (Kumamoto,
Japan). CD14+ monocytes were purified from the
peripheral blood mononuclear cells by positive selection using
magnetic-activated cell sorting technology (Miltenyi Biotec.,
Bergisch Gladbach, Germany) as described previously (11). The monocytes were cultured in
Dulbecco’s modified Eagle’s medium supplemented with 10% FBS and 10
ng/ml granulocyte-macrophage colony-stimulating factor (Wako,
Tokyo, Japan) for five days, and stimulated with tumor cell
supernatant in order to differentiate the macrophages from the M2
phenotype.
Extraction and isolation of CA from apple
pomace
CA was isolated from the apple pomace as described
previously (9). Briefly, CA was
extracted with a mixed solution of MeOH and CHCl3 (1:1),
loaded onto a Diaion HP-20 column (Mitsubishi Chemical, Tokyo,
Japan) and eluted with H2O and MeOH. The MeOH eluate was
separated using a silica gel column (Kantochemical Co. Inc., Tokyo,
Japan) and eluted with a mixed solution of hexane and ethyl
acetate. The CA-containing fraction was further purified using a
silica gel column and eluted with a mixture of CHCl3 and
ethyl acetate to yield pure CA.
STAT3 activation assay
STAT3 activation was determined by measuring the
increased expression of phosphorylated STAT3 by western blot
analysis. The protein (10 μg) was run on a 10% sodium dodecyl
sulfate-polyacrylamide gel and transferred to polyvinylidine
fluoride transfer membranes (Millipore, Bedford, MA, USA). To
detect the phosphorylated (phospho)-STAT3, the membranes were
exposed to an anti-phospho-STAT3 antibody (D3A7, Cell Signaling,
Danvers, MA, USA) and visualized by horseradish
peroxidase-conjugated anti-rabbit IgG antibody (Santa Cruz
Biotechnology Inc., Santa Cruz, CA, USA) with an enhanced
chemiluminescence western blotting detection reagent (GE
Healthcare, Tokyo, Japan). The molecular size of phospho-STAT3 that
was detected by the immunoblotting procedure was ~80 kDa. To detect
the STAT3 protein, the membranes were exposed to an anti-STAT3
antibody (sc-8019; Santa Cruz Biotech, Dallas, TX, USA) and
visualized by horseradish peroxidase-conjugated anti-mouse IgG
antibody with an ECL western blotting detection reagent. The
molecular size of STAT3 that was detected by the immunoblotting
procedure was ~80 kDa. These membranes were re-blotted with an
anti-β-actin antibody as an internal calibration control.
Cell proliferation and cytotoxic
assay
Briefly, 1×104 SKOV3, RMG-1 or ES-2 cells
were cultured in 96-well plates in quadruplicate as previously
described. Anticancer drugs, including CA, paclitaxel (PTX),
cisplatin (CDDP) or doxorubicin (DOX) (Wako), were then added to
the cells. The cell viability was determined using a WST assay
(WST-8 cell counting kit; Dojin Chemical, Kumamoto, Japan)
according to the manufacturer’s instructions. In order to analyze
the cytotoxic activity, the amount of lactate dehydrogenase (LDH)
that was released into the culture supernatants was calculated
using an LDH release assay (LDH-cytotoxic test kit; Wako).
Assessment of apoptosis
The apoptotic cells in the sections were detected by
the terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick
end-labeling (TUNEL) technique using an ApopTag Peroxidase In Situ
Apoptosis Detection kit (Intergen Co., Purchase, NY, USA). To
visualize the reaction, anti-digoxigenin-peroxidase was applied for
30 min at room temperature. For the negative controls, distilled
water was used instead of the TdT enzyme.
Immunohistochemistry
The co-culture cells were fixed in 10% neutral
buffered formalin and embedded in paraffin wax. Deparaffinized
sections were immersed in 0.3% hydrogen peroxide solution and
treated with anti-BrdU (Abbiotec, San Diego, CA, USA) and
anti-pSTAT3 (Cell Signaling Technology, Tokyo, Japan) antibodies.
The sections were subsequently treated with a HRP-conjugated
secondary antibody (Nichieri Bioscience, Tokyo, Japan). Reactions
were visualized with diaminobenzidine. The number of BrdU-positive
cells were counted among 200 randomly selected tumor cells under a
microscope.
Statistical analysis
All data are representative of two or three
independent experiments that were performed in quadruplicate. The
data are expressed as the mean ± standard deviation. The
Mann-Whitney U test was used for the two-group comparison.
P<0.05 was considered to indicate a statistically significant
difference.
Results
CA inhibits epithelial ovarian cancer
cell proliferation by suppressing STAT3 activation
The effect of CA on the proliferation of epithelial
ovarian cancer cells was measured. CA was observed to inhibit the
proliferation of the SKOV3, RMG-1 and ES-2 cells at a concentration
of at least 30 μM (Fig. 1A and B).
Following this, it was investigated whether CA caused cancer cell
apoptosis using a TUNEL assay. CA induced cell apoptosis in the
SKOV3, RMG-1 and ES-2 cells in the preliminary examination. As
shown in Fig. 1C, CA was clearly
observed to induce apoptosis in the ES-2 cells in the main
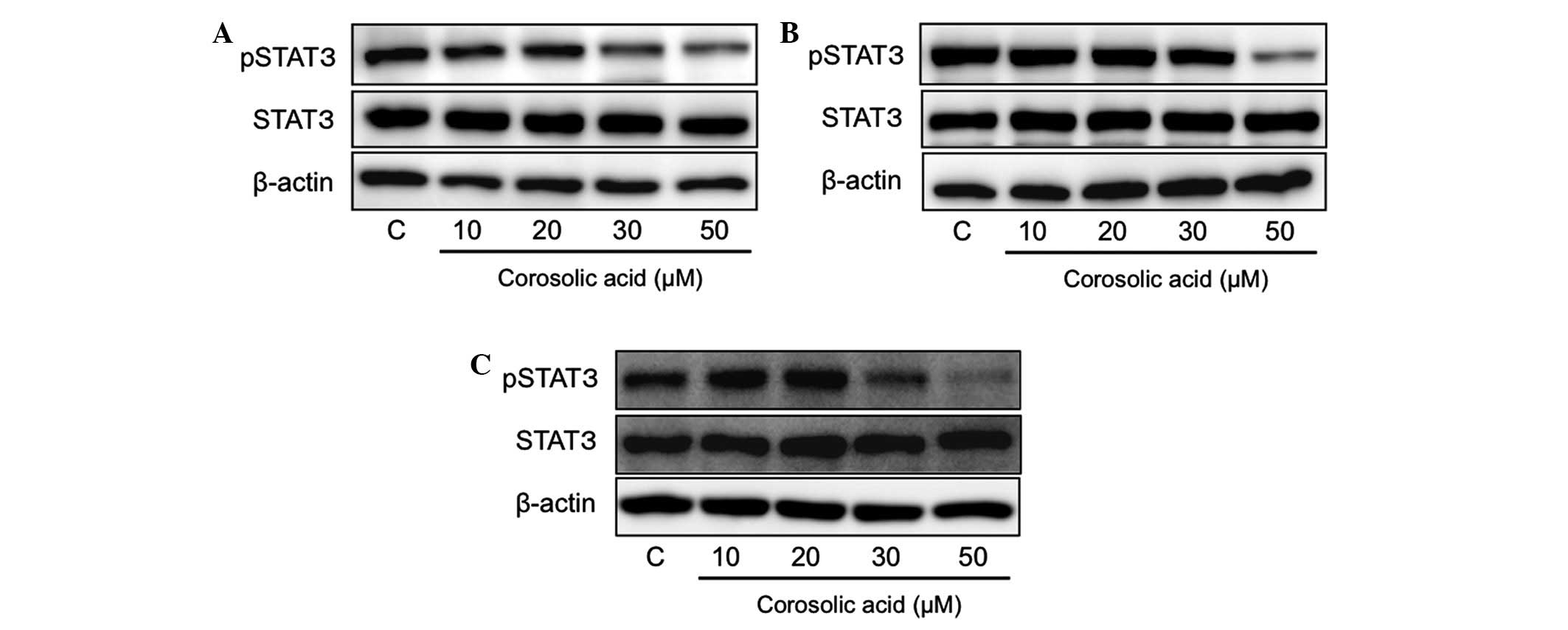
examination. The effect of CA on STAT3 activation was examined and
it was demonstrated that CA inhibited STAT3 activation at a
concentration of at least 30 μM in the epithelial ovarian cancer
cells (Fig. 2).
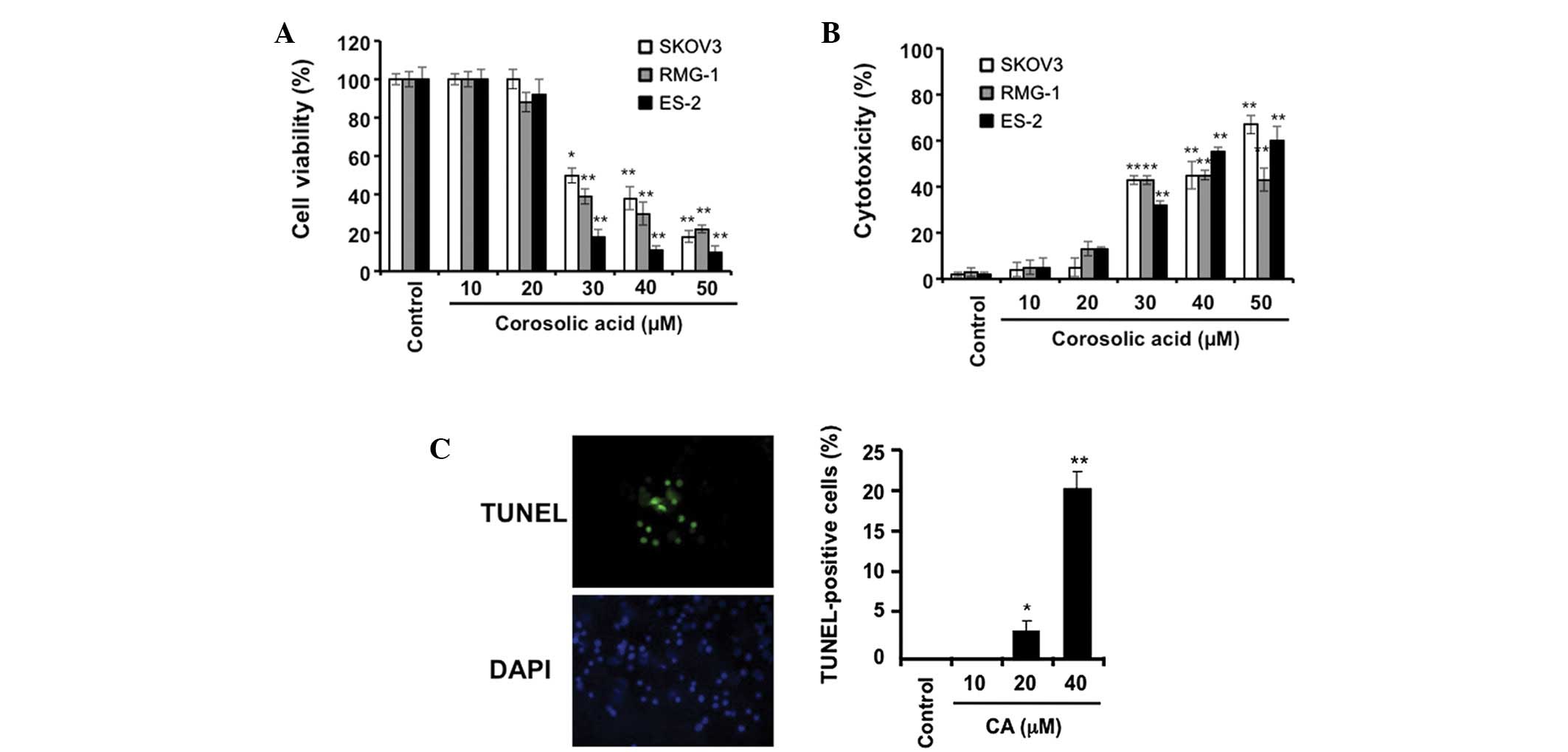 | Figure 1Effect of CA on the proliferation of
ovarian carcinoma cells. The ovarian carcinoma cells (SKOV3, RMG-1,
and ES-2) were incubated with the indicated concentrations of CA
for 48 h, followed by (A) determination of cell viability and (B)
cell cytotoxicity, by WST-8 assay and LDH assay, respectively (as
described in Materials and methods). (C) The ES-2 cells were
incubated with the indicated concentrations of CA for 5 h, followed
by the determination of cell apoptosis by TUNEL staining (as
described in Materials and methods). Data are presented as the mean
± SD. *P<0.01 and **P<0.001 vs. the
control. CA, corosolic acid; DAPI, 4′,6-diamidino-2-phenylindole;
TUNEL, terminal deoxynucleotidyl transferase-mediated dUTP nick
end-labeling; LDH, lactate dehydrogenase. |
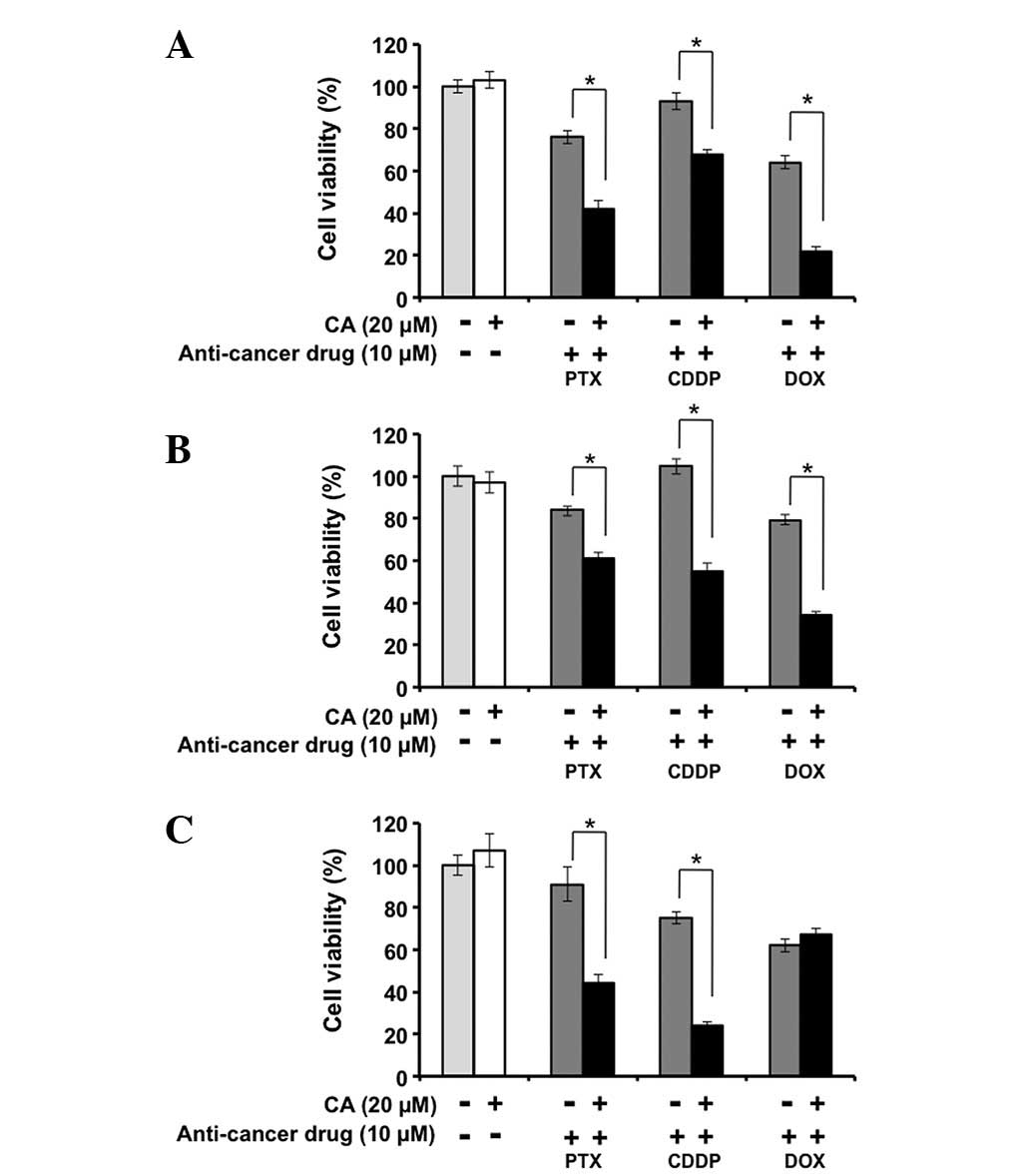
CA increases the sensitivity of
epithelial ovarian cancer cells to anticancer drugs
To elucidate whether CA enhances the anticancer
activity of the anticancer drugs in the epithelial ovarian cancer
cells, the combinational effects of CA and the anticancer drugs,
including PTX, CDDP and DOX, was examined. As CA demonstrated no
anticancer effects at a concentration of 20 μM, three epithelial
ovarian cancer cell lines (SKOV3, RMG-1 and ES-2) were incubated
with 20 μM CA for 24 h, concurrently with an incubation of 10 μM
anticancer drugs. As shown in Fig.
3A, the cell viability was not changed by stimulation with 20
μM CA alone in the SKOV3 cells. By contrast, 20 μM CA enhanced the
inhibitory effect of the anticancer drugs on the proliferation of
the SKOV3 cells. Similar results were observed in the RMG-1 and
ES-2 cells (Fig. 3B and 3C). These
results demonstrate that CA enhances the anticancer activity of
anticancer drugs in epithelial ovarian carcinoma cells. Notably,
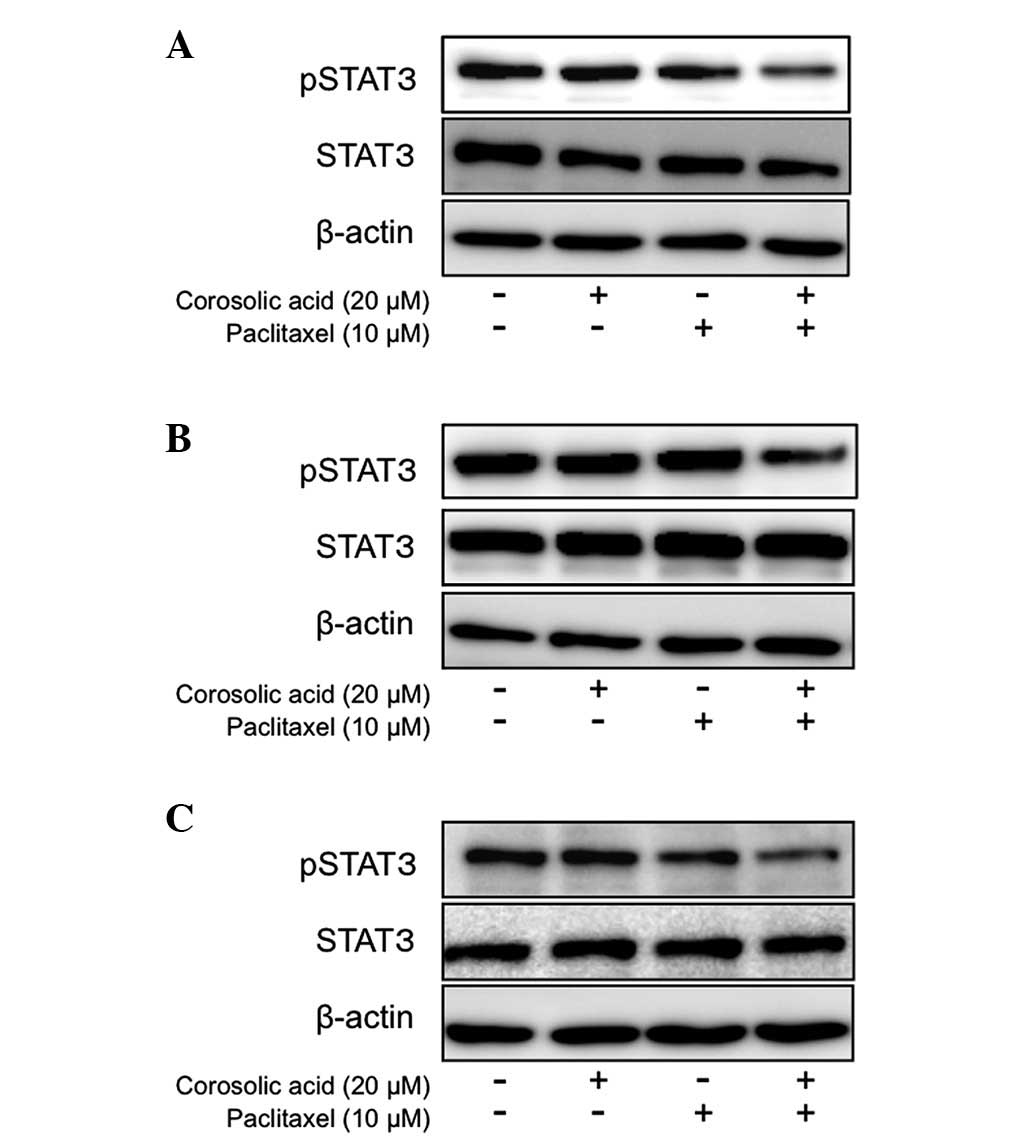
the combination of 20 μM CA and PTX inhibited STAT3 activity in the
epithelial ovarian cancer cells (Fig.
4), though CA alone or PTX alone had lesser effects on the
STAT3 activity (Fig. 4). These
findings suggest that CA enhances the inhibitory effects of
anticancer drugs by STAT3 inhibition.
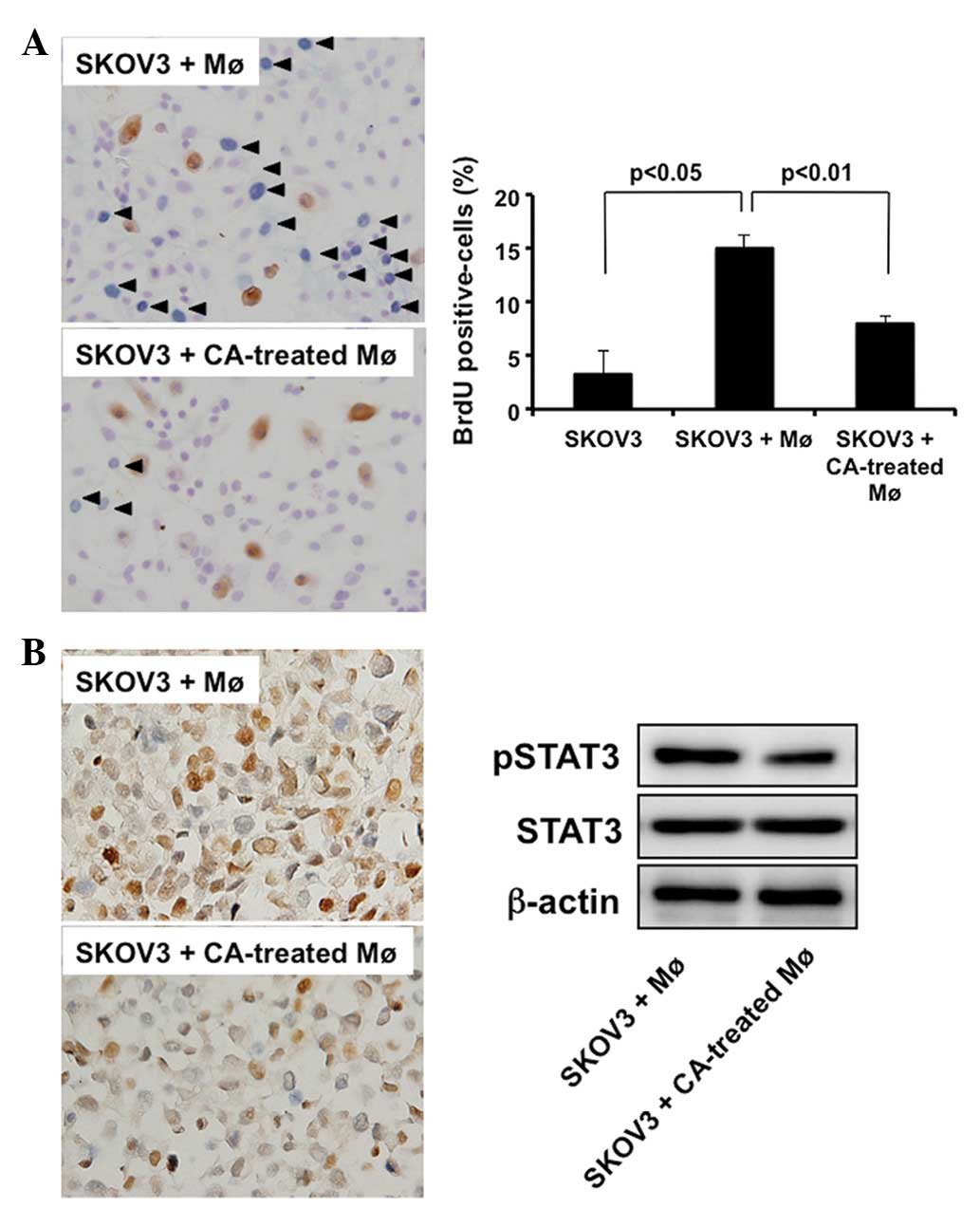
CA inhibits macrophage polarization into
the M2 phenotype, which induces cancer cell proliferation
Direct cell-cell interactions between M2 macrophages
and epithelial ovarian cancer cells have previously been reported
to induce STAT3 activation and a tumorigenic microenvironment in
the ascites fluid of advanced epithelial ovarian cancer patients
(12,13). Furthermore, CA has previously been
observed to significantly inhibit M2 polarization of macrophages
(9). Therefore, the present study
examined the effect of CA-treated macrophages on STAT3 activation
and cell proliferation in the SKOV3 cells. As shown in Fig. 5A, BrdU incorporation in the SKOV3
cells was strongly increased by coculture with the M2 macrophages,
whereas BrdU incorporation did not differ following coculture with
the CA-treated macrophages. STAT3 activation was also lower in the
SKOV3 cells that were cocultured with the CA-treated macrophages
than in the SKOV3 cells that were cocultured with the M2
macrophages (Fig. 5B).
Discussion
The present study demonstrated that the anticancer
effects of CA on epithelial ovarian cancer cells are due to its
suppressive effect on STAT3. Furthermore, CA enhanced the
anticancer effect of chemotherapeutic agents. STAT3 activation is a
well-known signal that is associated with cell survival or
resistance to apoptosis and this effect is considered to be induced
by the upregulation of anti-apoptotic genes, including Bcl-X, MCL-1
and survivin (6). CA is suggested
to downregulate these anti-apoptotic genes in epithelial ovarian
cancer cells (6).
Numerous tumor-associated macrophages (TAMs) are
detected in the cancer tissues (14–16)
and ascite fluid of patients with advanced epithelial ovarian
cancer. Almost all TAMs in epithelial ovarian cancer and ascites
have shown to be polarized to the M2 anti-inflammatory phenotype
(12,13). In vitro coculture experiments
have demonstrated that STAT3 activation in epithelial ovarian
cancer cells was strongly induced by coculturing with M2
macrophages and was only slightly induced by coculturing with M1
macrophages (12). A similar
phenomenon has been observed in glioma and lymphoma cells (17,18).
In the present study, coculture experiments were performed, which
demonstrated that CA suppressed STAT3 activation and BrdU
incorporation in epithelial ovarian cancer cells by abrogating
macrophage differentiation into the M2 phenotype.
In conclusion, the in vitro efficacy of CA
for the treatment of advanced epithelial ovarian cancer was
demonstrated in this study. CA inhibited cancer cell proliferation
and enhanced chemosensitivity by suppressing STAT3 activation. CA
also abrogated macrophage differentiation into the M2 phenotype and
cancer cell activation due to cell-cell interaction with
macrophages. As STAT3 activation leads to cancer progression in
other types of malignant tumors, including glioma and kidney
cancer, and as M2 TAMs are also associated with cancer development
in numerous kinds of malignant tumors, CA may also be useful as an
adjunctive treatment for patients with advanced malignant tumors
other than epithelial ovarian cancer.
Acknowledgements
The authors would like to thank Mr. Takenobu
Nakagawa, Mrs. Emi Kiyota and Miss. Yui Hayashida for their
technical assistance. This study was supported in part by the
Kanazawa Medical Research Foundation and Grants-in-Aid for
Scientific Research (nos. 23790407, 23790747 and 20390113) from the
Ministry of Education, Culture, Sports, Science and Technology of
Japan.
References
|
1
|
Modugno F and Edwards RP: Ovarian cancer:
prevention, detection, and treatment of the disease and its
recurrence. Molecular mechanisms and personalized medicine meeting
report. Int J Gynecol Cancer. 22:S45–S57. 2012. View Article : Google Scholar
|
|
2
|
Ali AY, Farrand L, Kim JY, et al:
Molecular determinants of ovarian cancer chemoresistance: new
insights into an old conundrum. Ann N Y Acad Sci. 1271:58–67. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Okamura H and Katabuchi H:
Pathophysiological dynamics of human ovarian surface epithelial
cells in epithelial ovarian carcinogenesis. Int Rev Cytol.
242:1–54. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yu H, Kortylewski M and Pardoll D:
Crosstalk between cancer and immune cells: role of STAT3 in the
tumour microenvironment. Nat Rev Immunol. 7:41–51. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yu H, Pardoll D and Jove R: STATs in
cancer inflammation and immunity: a leading role for STAT3. Nat Rev
Cancer. 9:798–809. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Page BD, Ball DP and Gunning PT: Signal
transducer and activator of transcription 3 inhibitors: a patent
review. Expert Opin Ther Pat. 21:65–83. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tseng LM, Huang PI, Chen YR, et al:
Targeting signal transducer and activator of transcription 3
pathway by cucurbitacin I diminishes self-renewing and
radiochemoresistant abilities in thyroid cancer-derived CD133+
cells. J Pharmacol Exp Ther. 341:410–423. 2012.PubMed/NCBI
|
|
8
|
Zhang X, Liu P, Zhang B, Wang A and Yang
M: Role of STAT3 decoy oligodeoxynucleotides on cell invasion and
chemosensitivity in human epithelial ovarian cancer cells. Cancer
Genet Cytogenet. 197:46–53. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fujiwara Y, Komohara Y, Ikeda T and Takeya
M: Corosolic acid inhibits glioblastoma cell proliferation by
suppressing the activation of signal transducer and activator of
transcription-3 and nuclear factor-kappa B in tumor cells and
tumor-associated macrophages. Cancer Sci. 102:206–211. 2011.
View Article : Google Scholar
|
|
10
|
Horlad H, Fujiwara Y, Takemura K, et al:
Corosolic acid impairs tumor development and lung metastasis by
inhibiting the immunosuppressive activity of myeloid-derived
suppressor cells. Mol Nutr Food Res. 57:1046–1054. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Komohara Y, Ohnishi K, Kuratsu J and
Takeya M: Possible involvement of the M2 anti-inflammatory
macrophage phenotype in growth of human gliomas. J Pathol.
216:15–24. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Takaishi K, Komohara Y, Tashiro H, et al:
Involvement of M2-polarized macrophages in the ascites from
advanced epithelial ovarian carcinoma in tumor progression via
Stat3 activation. Cancer Sci. 101:2128–2136. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kawamura K, Komohara Y, Takaishi K,
Katabuchi H and Takeya M: Detection of M2 macrophages and
colony-stimulating factor 1 expression in serous and mucinous
ovarian epithelial tumors. Pathol Int. 59:300–305. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lewis CE and Pollard JW: Distinct role of
macrophages in different tumor microenvironments. Cancer Res.
66:605–612. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444. 2008.
View Article : Google Scholar
|
|
16
|
Sica A, Larghi P, Mancino A, et al:
Macrophage polarization in tumour progression. Semin Cancer Biol.
18:349–355. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Komohara Y, Horlad H, Ohnishi K, et al: M2
macrophage/microglial cells induce activation of Stat3 in primary
central nervous system lymphoma. J Clin Exp Hematop. 51:93–99.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Komohara Y, Horlad H, Ohnishi K, et al:
Importance of direct macrophage-tumor cell interaction on
progression of human glioma. Cancer Sci. 103:2465–2172. 2012.
View Article : Google Scholar : PubMed/NCBI
|