Introduction
Chemoradiotherapy (CRT) prior to surgery has become
the preferred treatment approach for patients with locally-advanced
rectal cancer (LARC). In addition to improving local disease
control, pre-operative CRT leads to significant tumor regression
(downsizing) and a shift toward a lower stage (downstaging), in the
primary tumor and perirectal lymph nodes. In 10–30% of patients,
the specimens resected during radical surgery reveal no residual
cancer cells, i.e. a post-CRT pathological complete response (ypCR)
(1). For these who have markedly
radiosensitive tumors, the cancer cells are not present at the time
of surgery and thus patients may be overtreated and exposed
unnecessarily to the risks of major pelvic surgery, including
disorders of urinary, fecal and sexual functions, stoma formation
and even surgical mortality.
Therefore, patients with significant or complete
tumor regression following pre-operative CRT may undergo
alternative treatment strategies, including transanal local
excision, or even no immediate surgery with strict follow-up
(2). Habr-Gama et al
proposed the ‘wait-and-see’ strategy based on the observation of no
survival benefit in patients with a confirmed ypCR through radical
surgery over patients with a clinical complete response (ycCR) who
did not undergo any surgical management (3). Maas et al reproduced the
favorable results of this wait-and-see policy following a post-CRT
ycCR (4). Nevertheless, this
approach remains highly controversial and supporting evidence based
on long-term follow-up is lacking (5).
The present study reports the long-term outcomes of
five patients with LARC who were managed with pre-operative CRT
only, without any surgical resection, following a ycCR to
pre-operative CRT.
Materials and methods
Patients
A total of 577 patients with LARC received
pre-operative CRT between 2004 and 2008 at the National Cancer
Center (Goyang, Korea). Among them, five patients who had a ycCR
following pre-operative CRT and underwent no surgery were analyzed
retrospectively. All patients had biopsy-proven adenocarcinoma of
the middle or lower rectum and clinical T3 or T4 tumors on magnetic
resonance imaging (MRI). The wait-and-see approach was presented to
the patients as experimental and it was stressed that radical
surgery following pre-operative CRT is the standard oncological
treatment. Five patients selected this policy, largely due to the
possibility of avoiding major surgery or a permanent stoma. The
pre-treatment staging work-up included a digital rectal
examination, complete blood count, liver function tests, serum
carcinoembryonic antigen level measurements, video colonoscopy,
chest radiography, computed tomography (CT) of the abdomen and
pelvis and MRI with or without transrectal ultrasonography.
Clinically-positive lymph node involvement was defined as a lymph
node of ≥0.5 cm in the short-axis diameter observed on MRI or CT.
This study was performed in accordance with the guidelines of the
Institutional Review Board of the National Cancer Center (Goyang,
Korea) and informed consent was obtained for each patient.
Treatments
A dose of 45 Gy pre-operative radiotherapy was
delivered to the whole pelvis in 25 fractions, followed by a 5.4 Gy
boost in three fractions within 6 weeks. Each patient underwent CT
simulation for three-dimensional conformal radiotherapy planning
and a three-field treatment plan that consisted of a 6-MV photon
posterior-anterior field and 15-MV photon opposed lateral beams.
The gross tumor volume, mesorectum, presacral space, entire sacral
hollow and regional lymphatics, including perirectal, internal
iliac, presacral and distal common iliac lymphatics were
encompassed by the initial radiation field. The boost field
included the gross tumor volume and mesorectum with ≥2-cm margins
in all directions.
The pre-operative chemotherapy administered
concurrently with radiotherapy was one of the following three
regimens (Table I): i) Two cycles
of intravenous bolus injections of 400 mg/m2/day
5-fluorouracil and 20 mg/m2/day leucovorin for 3 days in
the first and fifth weeks of radiotherapy; ii) 825 mg/m2
oral capecitabine twice daily during radiotherapy without weekend
breaks; or iii) 825 mg/m2 oral capecitabine twice daily
during radiotherapy with weekend breaks and 40 mg/m2/day
intravenous irinotecan during each week of radiotherapy. Post-CRT
adjuvant chemotherapy was delivered in one patient; four 5-week
cycles with each cycle consisting of 400 mg/m2/day UFT-E
(tegafur-uracil) plus 90 mg/day leucovorin for 4 weeks followed by
a 1-week rest.
 | Table IPatient characteristics, treatments
and outcomes. |
Table I
Patient characteristics, treatments
and outcomes.
| Pt | Age, years | Gender | Pre-CRT CEA,
ng/ml | Diff | Location from AV,
cm | cStage | Conc CT | Post-CRT CEA,
ng/ml | Post-CRT Bx
(weeks) | Adj CT | Recurrence
(months) | Current status
(months) |
|---|
| 1 | 63 | M | 2.5 | M | 1.0 | T3N0M0 | X | 2.6 | - | - | - | NED (101) |
| 2 | 69 | M | 2.0 | W | 8.0 | T3N1M0 | IX | 2.6 | Y (12) | - | LR (59) | NED (101) |
| 3 | 64 | M | 1.8 | M | 5.5 | T3N1M0 | IX | 1.7 | Y (7) | - | - | NED (100) |
| 4 | 53 | M | 5.2 | M | 4.5 | T3N1M0 | IX | 4.2 | Y (8) | - | - | NED (93) |
| 5 | 52 | M | 2.2 | M | 9.0 | T4N2M0 | FL | 1.8 | Y (19) | UFT | DM (32) | NED (54) |
Evaluation and follow-up
The tumor response was assessed 7–12 weeks after the
completion of CRT using the same clinical, endoscopic and
radiological studies as for the initial work-up. The decision
criteria regarding a ycCR included: i) No palpable tumor or
stenosis on digital rectal examination; ii) no residual
intraluminal mass or ulceration at endoscopy; iii) no residual
mural tumor and suspicious lymph nodes on MRI or CT; and iv) a
negative biopsy. The primary rectal tumor site was biopsied
following CRT in four patients. In one patient, the biopsy was
conducted at 19 weeks following CRT, as the rectal tumor, located
within the peritoneal cavity, showed massive necrosis and there was
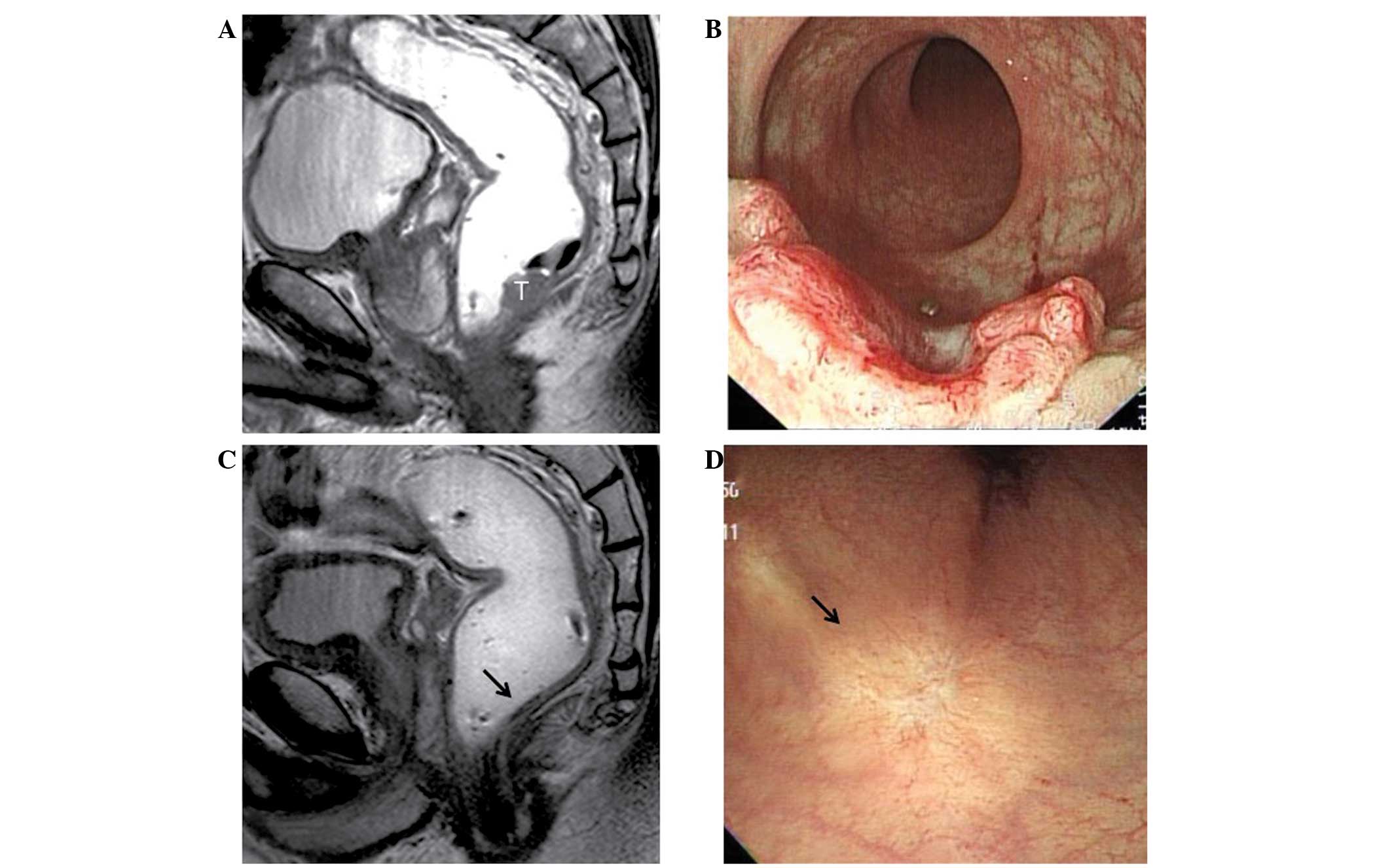
concern regarding bowel perforation upon biopsy. Fig. 1 shows an example of a pre-treatment
tumor and the post-CRT status of ycCR.
The patients were followed up every 3 months for the
first 2 years, every 6 months for the next 3 years and annually
thereafter. The follow-up evaluations consisted of a physical
examination, complete blood count, liver function tests and serum
carcinoembryonic antigen level measurement at each visit. Chest
radiography and CT scanning of the abdomen and pelvis were
conducted every 6 months for 5 years and annually thereafter. Video
colonoscopy or sigmoidoscopy was performed every year.
Results
Patient demographics
The patient demographics, treatments and outcomes
are presented in Table I. Patient
age ranged between 52 and 69 years old. All of the patients were
male. The distal end of the tumor was located 1–9 cm from the anal
verge. The clinical T classification was T3 in four patients and T4
in one (ureter invasion) patient.
Follow-up
The follow-up period ranged from 54–101 months.
Three patients had no tumor recurrence as of the last follow-up and
were alive with no evidence of disease at 101, 100 and 93 months,
respectively. Patient 2 developed local recurrence where the
primary tumor was located initially at 59 months. A low anterior
resection was performed, revealing a moderately-differentiated
adenocarcinoma that had invaded through the muscularis propria into
the perirectal tissue. Six retrieved lymph nodes were all negative
and the circumferential resection margin was clear (0.4 cm). The
patient refused the recommended post-operative chemotherapy.
Patient 5 developed lung metastasis at 32 months. This individual
received induction chemotherapy consisting of nine cycles of
5-fluorouracil, leucovorin and oxaliplatin (FOLFOX), then wedge
resection surgery and six post-operative cycles of FOLFOX. The
surgical specimen contained a 0.7-cm metastatic adenocarcinoma.
These two patients with disease recurrence remained disease-free 42
and 22 months after salvage pelvic and thoracic surgery,
respectively.
Discussion
The wait-and-see strategy of close observation
without surgery was proposed by Habr-Gama et al for selected
rectal cancer patients who achieve a ycCR following pre-operative
CRT (6). The authors compared
long-term outcomes between 71 patients who were managed with this
strategy and 22 patients who had a post-surgery ypCR. The clinical
assessment was conducted 8 weeks after the completion of CRT, and
only patients who sustained this ycCR status until 1 year were
selected. The 5-year overall and disease-free survival rates were
88 and 83%, respectively, in the resection group, and 100 and 92%,
respectively, in the observation group (3). The updated study by this group
analyzed 99 patients with a sustained ycCR, and the 5-year overall
and disease-free survival rates were 93 and 85%, respectively
(7). These favorable outcomes were
similar to those recorded by Yeo et al, who reported 5-year
overall and disease-free survival rates of 94.8 and 88.5%,
respectively, in 304 LARC patients with post-CRT ypT0N0 with
radical surgery (1). Recently, an
additional institution also reported no difference in outcomes
between 21 ycCR LARC patients treated with this approach and
patients with post-surgery ypCR. However, the follow-up period was
only 2 years (4). In a
meta-analysis of rectal cancer, late recurrence presenting >5
years after the initial therapy constituted 24% of all local
recurrences when pre-operative long-course CRT or radiotherapy was
performed (8). The favorable
outcomes following long-term follow-up in the present study,
although in a small series, may constitute additional supporting
evidence for this wait-and-see strategy. All five patients were
alive with no disease at the last follow-up, which was ~8 years
since CRT completion in four patients.
However, this approach remains highly controversial
and several limitations, which appear to be difficult to overcome,
have held back its widespread adoption. The major obstacle
preventing this policy from gaining popularity is the difficulty
distinguishing between residual cancer and CRT-induced fibrosis,
clinically or radiologically without using surgical pathology.
Habr-Gama et al defined a ycCR as the absence of a residual
mass or ulcer on digital examination and endoscopy, no signs of
residual tumor observed in radiological studies (CT and ultrasound)
and a negative biopsy. Since these methods are inevitably limited
in terms of objective and accurate identification of a ycCR, the
patients were followed up monthly for 1 year, at which time,
clinical complete responders were determined (3,7). In
addition to using modalities and criteria similar to those above,
MRI was used in the initial work-up and for assessing the tumor
response. Patients who maintained a ycCR for 1 year were not the
only individuals selected in the present study; however,
recurrences did not occur <2 years after CRT. Maas et al,
who first reproduced the results of this strategy, used more
sophisticated modalities, including MRI enhanced with novel
contrast agents or diffusion-weighted MRI (4). Restaging MRI, consisting of standard
T2-weighted MRI and diffusion-weighted MRI, significantly improved
the sensitivity for selecting complete responders, with a
specificity of >90%; i.e. the risk of underestimating the
residual tumor was <10% (9). In
the more recent ACOSOG Z6041 trial, which investigated the efficacy
of pre-operative CRT and local excision for treating cT2N0 rectal
cancer, a ycCR was concordant with a ypCR in 31 of 36 patients
(10). There has been an attempt to
standardize the clinical and endoscopic observations for defining a
post-CRT ycCR in rectal cancer (11), and the future development of
molecular and radiological tools may improve the accurate clinical
identification of such cases.
Two patients in the present series, who had disease
recurrence at 59 and 32 months, were salvaged successfully and were
alive with no disease 42 and 22 months following surgical resection
of a recurrent local and distant tumor, respectively. Earlier local
recurrence, including within an arbitrary 1 year in the study by
Habr-Gama et al, may be attributable to a misdiagnosis of a
ycCR and regrowth of the tumor. If the oncological outcome is
compromised by delaying surgery in these patients, the wait-and-see
strategy may have to be abandoned considering the current
restricted capability for accurate identification of a ycCR. In
this regard, Habr-Gama et al reported that patients who
eventually required surgery following a suspected, but not
sustained, ycCR for 1 year, did not have inferior oncological
outcomes compared with those who were considered to have had an
incomplete response and had undergone immediate surgery (12). The response to pre-operative CRT is
a significant predictor of the oncological outcome (1,13). It
has been hypothesized that prolonged intervals until surgery may
have been counterbalanced by favorable biological tumor behavior
leading to insignificant differences in oncological outcome
compared with patients with a less radiosensitive tumor, but
managed with immediate surgery (12). A comparison of the outcomes between
these suspected, but not sustained, ycCR patients with ycCR
patients undergoing immediate surgery is required in order to
reveal whether delayed surgery results in an oncological
compromise.
In an era of pursuing individualized, tailored
treatment strategies, the fact that a proportion of patients with
LARC develop a complete response is an advantage of a pre-operative
CRT approach. Non-operative management of LARC patients with a ycCR
following CRT may be feasible with strict selection criteria and
frequent follow-up. The results of ongoing prospective trials of
this wait-and-see policy are currently awaited (14). While sufficient evidence is
accumulated, this strategy may be of specific value for the elderly
and for patients with comorbidity, particularly if the planned
radical surgery involves a permanent colostomy.
Acknowledgements
This work was supported by the Soonchunhyang
University Research Fund.
References
|
1
|
Yeo SG, Kim DY, Kim TH, Chang HJ, Oh JH,
Park W, Choi DH, Nam H, Kim JS, Cho MJ, Kim JH, Park JH, Kang MK,
Koom WS, Kim JS, Nam TK, Chie EK, Kim JS and Lee KJ: Pathologic
complete response of primary tumor following preoperative
chemoradiotherapy for locally advanced rectal cancer: long-term
outcomes and prognostic significance of pathologic nodal status
(KROG 09-01). Ann Surg. 252:998–1004. 2010. View Article : Google Scholar
|
|
2
|
Kosinski L, Habr-Gama A, Ludwig K and
Perez R: Shifting concepts in rectal cancer management: a review of
contemporary primary rectal cancer treatment strategies. CA Cancer
J Clin. 62:173–202. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Habr-Gama A, Perez RO, Nadalin W, Sabbaga
J, Ribeiro U Jr, Silva e Sousa AH Jr, Campos FG, Kiss DR and
Gama-Rodrigues J: Operative versus nonoperative treatment for stage
0 distal rectal cancer following chemoradiation therapy: long-term
results. Ann Surg. 240:711–718. 2004.PubMed/NCBI
|
|
4
|
Maas M, Beets-Tan RG, Lambregts DM,
Lammering G, Nelemans PJ, Engelen SM, van Dam RM, Jansen RL, Sosef
M, Leijtens JW, Hulsewe KW, Buijsen J and Beets GL: Wait-and-see
policy for clinical complete responders after chemoradiation for
rectal cancer. J Clin Oncol. 29:4633–4640. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Glynne-Jones R and Hughes R: Critical
appraisal of the ‘wait and see’ approach in rectal cancer for
clinical complete responders after chemoradiation. Br J Surg.
99:897–909. 2012.
|
|
6
|
Habr-Gama A, Perez RO, São Julião GP,
Proscurshim I and Gama-Rodrigues J: Nonoperative approaches to
rectal cancer: a critical evaluation. Semin Radiat Oncol.
21:234–239. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Habr-Gama A, Perez RO, Proscurshim I,
Campos FG, Nadalin W, Kiss D and Gama-Rodrigues J: Patterns of
failure and survival for nonoperative treatment of stage c0 distal
rectal cancer following neoadjuvant chemoradiation therapy. J
Gastrointest Surg. 10:1319–1329. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Merkel S, Mansmann U, Hohenberger W and
Hermanek P: Time to locoregional recurrence after curative
resection of rectal carcinoma is prolonged after neoadjuvant
treatment: a systematic review and meta-analysis. Colorectal Dis.
13:123–131. 2011. View Article : Google Scholar
|
|
9
|
Lambregts DM, Vandecaveye V, Barbaro B,
Bakers FC, Lambrecht M, Maas M, Haustermans K, Valentini V, Beets
GL and Beets-Tan RG: Diffusion-weighted MRI for selection of
complete responders after chemoradiation for locally advanced
rectal cancer: a multicenter study. Ann Surg Oncol. 18:2224–2231.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Garcia-Aguilar J, Shi Q, Thomas CR Jr,
Chan E, Cataldo P, Marcet J, Medich D, Pigazzi A, Oommen S and
Posner M; ACOSOG. Pathologic complete response (pCR) to neoadjuvant
chemoradiation (CRT) of uT2uN0 rectal cancer (RC) treated by local
excision (LE): Results of the ACOSOG Z6041 trial. J Clin Oncol.
28(Suppl; abstr 3510): 15s2010.
|
|
11
|
Habr-Gama A, Perez RO, Wynn G, Marks J,
Kessler H and Gama-Rodrigues J: Complete clinical response after
neoadjuvant chemoradiation therapy for distal rectal cancer:
characterization of clinical and endoscopic findings for
standardization. Dis Colon Rectum. 53:1692–1698. 2010. View Article : Google Scholar
|
|
12
|
Habr-Gama A, Perez RO, Proscurshim I,
Nunes Dos Santos RM, Kiss D, Gama-Rodrigues J and Cecconello I:
Interval between surgery and neoadjuvant chemoradiation therapy for
distal rectal cancer: does delayed surgery have an impact on
outcome? Int J Radiat Oncol Biol Phys. 71:1181–1188. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yeo SG, Kim DY, Park JW, Oh JH, Kim SY,
Chang HJ, Kim TH, Kim BC, Sohn DK and Kim MJ: Tumor volume
reduction rate after preoperative chemoradiotherapy as a prognostic
factor in locally advanced rectal cancer. Int J Radiat Oncol Biol
Phys. 82:e193–e199. 2012.PubMed/NCBI
|
|
14
|
Yu SK, Brown G, Heald RJ, Chua S, Cook G,
Barbachano Y, Chau I, Wotherspoon A and Tait DM: Deferral of rectal
surgery following a continued response to preoperative
chemoradiotherapy (Watch and Wait) study: A phase II multicenter
study in the United Kingdom. J Clin Oncol. 29(suppl 4; abstr
489)2011.
|