Introduction
Among a variety of targeted anti-angiogenic drugs,
the angiogenesis inhibitor, bevacizumab (Avastin), is the first to
be confirmed to lead to a survival advantage in patients with
advanced non-small cell lung cancer (NSCLC). The combination of
chemotherapy and bevacizumab has become the new choice for advanced
non-squamous NSCLC treatments (1).
The safety of Avastin in lung cancer (SAiL) study (2) is a multi-center, open-source,
stand-alone study. Patients with untreated, locally advanced,
metastatic or recurrent non-squamous NSCLC were administered up to
six cycles of chemotherapy combined with humanized monoclonal
vascular endothelial growth factor A (VEGF-A) antibody
(bevacizumab/Avastin) administration, followed by bevacizumab
maintenance therapy until further progression of the disease. From
August, 2006 to July, 2008, there were a total of 2,172 patients
enrolled in the study, with a median progression-free survival
(PFS) time of 7.8 months and an overall survival (OS) time of 14.6
months. The present study describes the case of one specific
patient who participated in the SAiL research program. The patient
presented with right lung adenocarcinoma and subcutaneous
metastasis and had a PFS time of nearly five years while undergoing
bevacizumab maintenance therapy following chemotherapy combined
with bevacizumab.
Case report
Patient
A 54-year-old male patient was admitted to the
Zhejiang Cancer Hospital on October 16, 2007, due to a phyma at the
left frontal brow. The phyma was 1.5 × 1.0 cm in size, with mild
tenderness and no local redness. The patient presented with no
abnormal coughing, sputum or fever. A surgical resection was
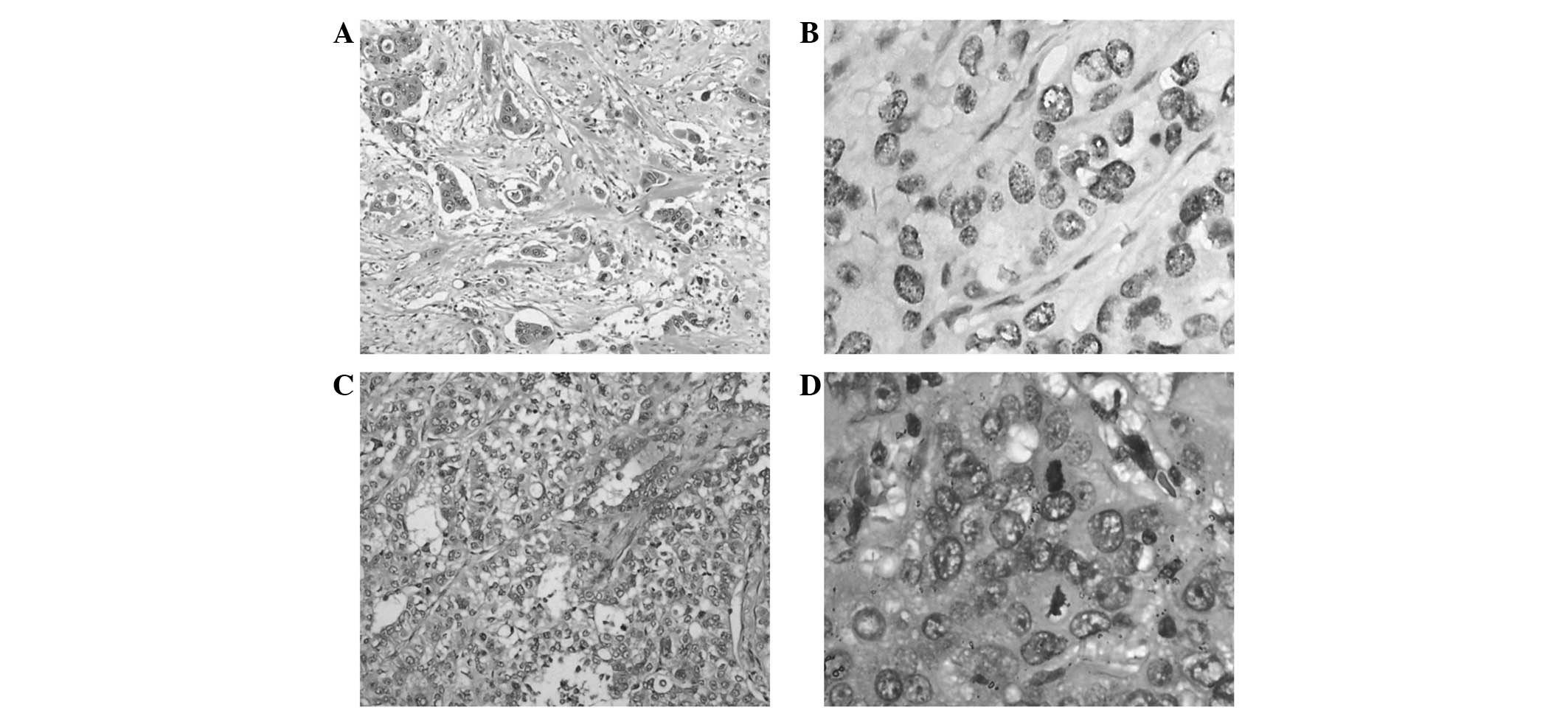
performed on October 26, 2007, and the phyma was identified as a
left frontal metastatic poorly-differentiated adenocarcinoma by
pathology (Fig. 1A and B). Further
examinations were performed in order to identify the primary tumor,
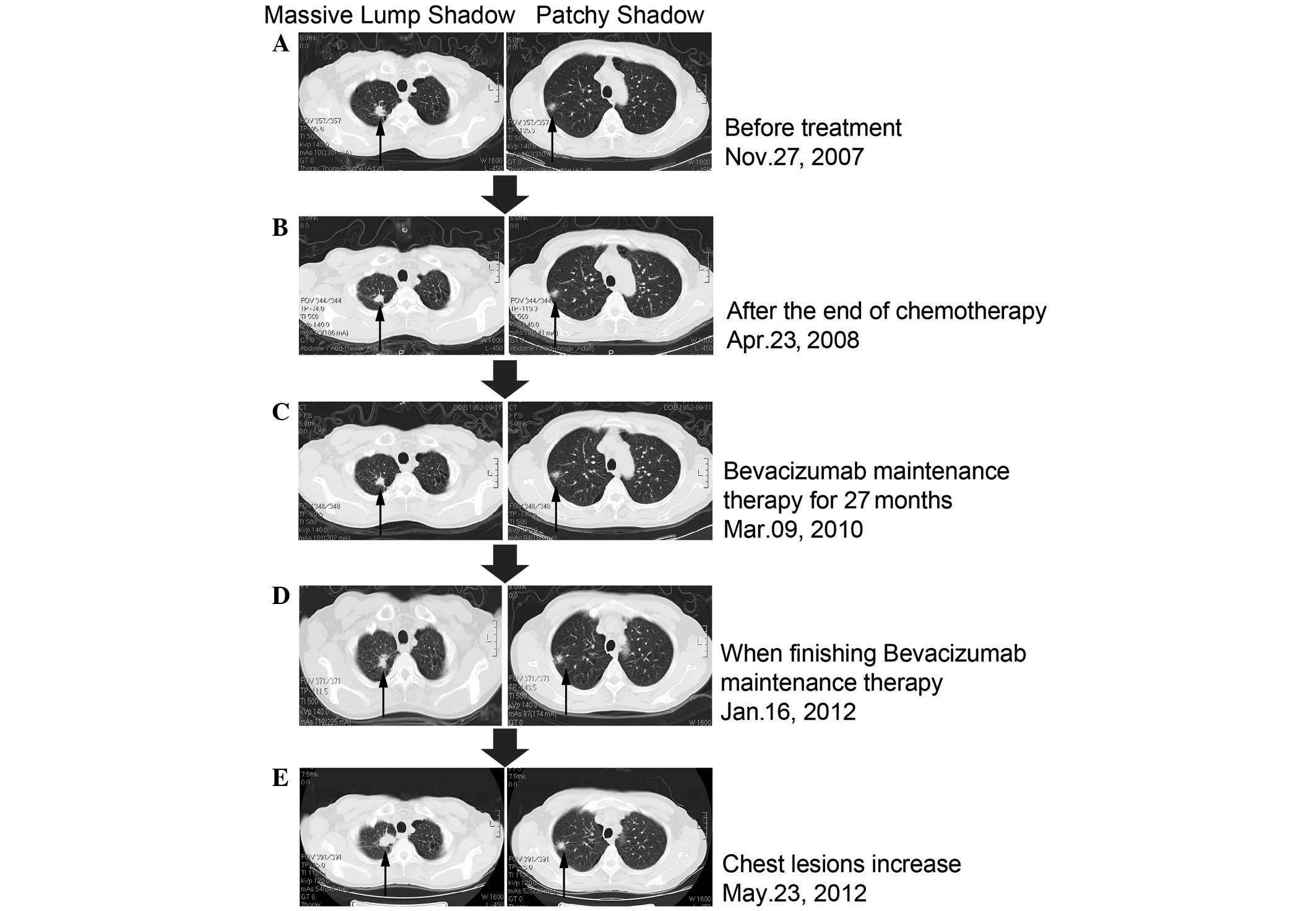
and a chest computed tomography (CT) scan disclosed the presence of
two lesions. One was a lump shadow with a small burr of ~2.0 cm in
size in the upper right lung, while the other was also in the upper
right lung, but was a patchy shadow with an unclear edge, thereby
indicating a high possibility of lung cancer (Fig. 2A). Using emission computed
tomography (ECT), several lightly visible restricted radioactivity
concentration sites were detected at the left temporal bone, the
7th and 10th thoracic vertebra and the right ileum, while no
evident abnormalities were observed in the brain. There were also
no evident abnormalities in the pancreas, spleen, kidney or
double-adrenal gland, which were examined by an ultra-B scan. The
gastroscopic results and blood carcinoembryonic antigen (CEA)
levels were normal and the patient had no history of smoking or
high blood pressure.
Physical examination
The general health of the patient was fine and the
performance status (PS) was scored as 0. A surgical scar was
observed on the left frontal area of the face. There was no
enlargement of the two supraclavicular lymph nodes and heart and
lung auscultation heralded negative results. No abnormality was
observed by abdominal examination, routine blood tests, blood
biochemical examination or routine urine tests. The patient was
diagnosed with lung cancer combined with subcutaneous T3N0M1
metastasis.
Treatment procedure
The patient was selected for the SAiL clinical
trial. According to the SAiL scheme, the patient was initially
administered 15 mg/kg (900 mg)d1 bevacizumab monoclonal antibody
(mAb), 175 mg/m2 (300 mg)d1 paclitaxel and the area
under the concentration time curve 6.0 (770 mg)d1 carboplatin.
Grade I bone marrow suppression and peripheral nerve
toxicity and grade III hair loss and epistaxis developed following
the chemotherapy. Following two chemotherapy cycles, the tumor in
the upper right lung was observed to be slightly shrunken by chest
CT, with the therapeutic evaluation of a stable disease (SD;
Fig. 2). On January 11, February 1,
February 28 and March 25, four chemotherapy cycles were applied as
planned, resulting in six cycles in total. Compared with the
pre-treatment evaluation, the therapeutic evaluation was of SD
(Fig. 2B). On April 23, 2008, the
patient was initially administered 15 mg/kg (900 mg/d1) bevacizumab
mAb and then maintenance treatment every 3 weeks (Fig. 2B). The final bevacizumab mAb
treatment was administered on January 16, 2012. The total
bevacizumab mAb treatment time lasted 50 months, with a cumulative
dose of 54 g. During the bevacizumab maintenance treatment, the
patient was examined by chest CT every three months and by brain
magnetic resonance imaging (MRI) every six months for the
therapeutic evaluation. On January 16, 2012, the efficacy
evaluation review using CT scans revealed the stable status of the
tumor (Fig. 2D). The patient
required to be withdrawn from the SAiL clinical trial due to
long-lasting 3+ proteinuria. On May 23, 2012, a chest CT indicated
that the upper right lung lesions were significantly increased
compared with the previous data (Fig.
2E). The patient did not cough blood and had no fever, sputum,
chest pain, hemoptysis, chest tightness, shortness of breath or
headache. No abnormalities were identified from the brain MRI, bone
ECT or abdominal CT, and the blood CEA was 33.2 ng/ml. A lung tumor
biopsy was performed and cancer cells were identified. Considering
the progress of the disease, the PFS was 54 months. On May 31,
2012, one chemotherapy cycle of gemcitabine (1250 mg/m2)
combined with cisplatin (75 mg/m2) was administered and
the patient developed grade III neutropenia and grade IV platelet
decline. On July 17, 2012, a palliative treatment was performed on
the right-sided lung cancer. Pathological lung nodules with
poorly-differentiated adenocarcinoma cells combined with
disintegration and necrosis were discovered forming two tumors, one
small, sized 2 × 2 × 1.5 cm, and one large, sized 3.5 × 3 × 2.8 cm.
Tumor invasion of the visceral pleura, infiltration and metastasis
statuses were: bronchial roots lymph node 1/6, intrapulmonary
bronchial lymph node 1/2, the 7th groups of lymph nodes 0/2, the
9th groups of lymph nodes 0/1 and the 10th lymph nodes 1/4
(Fig. 1C and D). G/C chemotherapies
were performed on August 15, and September 6, 2012. The current
Eastern Cooperative Oncology Group (ECOG) PS score was 1. Approval
for the study was obtained from the ethical committee of Zhejiang
Cancer Hospital and informed consent was obtained from all the
participants.
Adverse reactions to bevacizumab
During the bevacizumab treatment there was no sign
of hemoptysis. During the 1–2 cycles of chemotherapy, grade I
epistaxis was observed, but no high blood pressure. During cycles
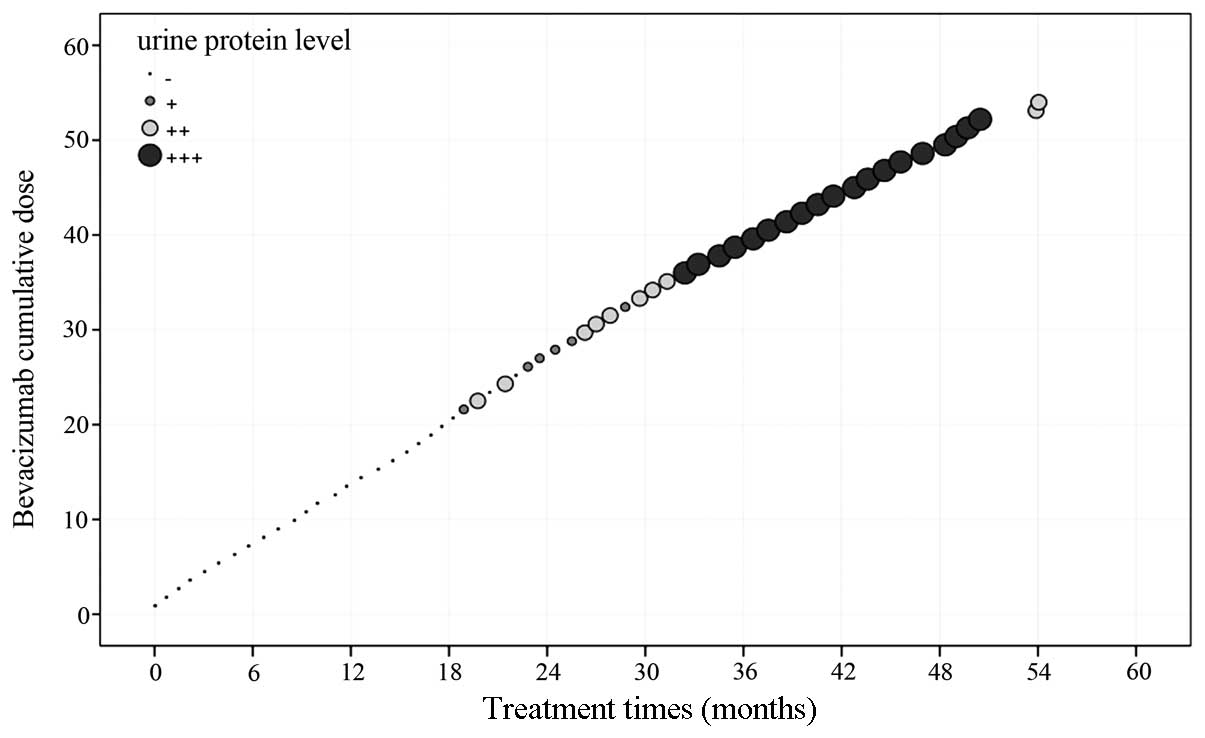
1–17 of bevacizumab treatment, there was no significant
proteinuria. Weak positive proteinuria was diagnosed from the start
of the 18th cycle, but from cycles 33–39, the proteinuria was
1.0–2.0 g/l (++). Following several 24-h interval examinations, the
total urine protein was shown to peak at 1.38 g/24 h. During cycles
40–57, the proteinuria was 2.0–4.0 g/l (+++). The total urine
protein measurement at the 57th cycle was 2.284g/24 h on January
17, 2012. The urinary protein level in the patient demonstrated a
positive correlation with the cumulative dose of bevacizumab
(P=0.000; Fig. 3). Subsequent to
withdrawing the medication and pausing for more than a month,
proteinuria was diagnosed [<0.15 g/l (−)] on May 25, 2012, and
was kept at a constant negative from then on. In this patient with
advanced non-small cell adenocarcinoma and a PS score of 0,
bevacizumab combined with paclitaxel and carboplatin was able to
stabilize the tumor condition. The bevacizumab maintenance therapy
lasted for 50 months. At three months after treatment withdrawal
the tumor progression restarted. Overall, the bevacizumab therapy
demonstrated a good long-term effect with the major adverse
reaction of proteinuria correlating with the drug cumulative dose,
which may be completely restored following withdrawal.
Discussion
There are several studies with regard to bevacizumab
therapy. Sandler et al reported that when comparing
bevacizumab-based monoclonal antibody therapies combined with C/P
with solely C/P chemotherapies, the former was able to
significantly increase the objective response rate (35% vs. 15%;
P<0.0001) and PFS time (6.2 months vs. 4.5 months; P<0.001).
The treatment may also significantly increase the survival length,
with a mean of 12.3 months compared with 10.3 months (P=0.001)
(3,4). The present study is the first advanced
NSCLC treatment study, which extended the survival time to more
than a year. Based on the results of phase III clinical studies,
the U.S. Food and Drug Administration (FDA) has approved the
combination of bevacizumab with C/P as the first-line therapy for
advanced non-squamous non-bleeding NSCLC (3). In 2007, in a European multi-center
based phase III clinical trial, Reck et al(5,6)
reported the survival differences between two doses of bevacizumab
and gemcitabine/carboplatin (G/C) treatments (7.5 mg/kg and 15
mg/kg) and a pure G/C chemotherapy. This pilot study was performed
using 1,043 cases of previously untreated advanced non-squamous
NSCLC without brain metastases. The results demonstrated that
chemotherapy combined with various doses of bevacizumab may
significantly prolong disease PFS. The low-dose group had a 25%
lower risk of mortality (HR=0.75; P=0.003) and a PFS time of 6.7
months compared with 6.1 months in the placebo group, while the
high-dose group with a PFS time of 6.5 months had an 18% lower risk
of mortality compared with the placebo group (HR=0.82; P=0.83).
Currently, pre-clinical studies have confirmed that subsequent to
stopping an anti-VEGF treatment, the tumor blood vessels tend to
re-grow and the structures of re-grown tumor vascularization are
similar to patients without the anti tumor vascularization
treatment, while the VEGF-A expression and VEGF dependence are also
noticed (7–10). These findings provide the
theoretical rationale of a bevacizumab maintenance therapy and
explain why bevacizumab may extend the disease PFS time. In 2011,
Nadler et al reported in the results from the U.S. community
practice network that advanced NSCLC may achieve a favorable
clinical outcome following the completion of chemotherapy with
bevacizumab maintenance therapy until further disease progression
(11). Almost all clinical studies
have used bevacizumab combined with first-line chemotherapies
following maintenance therapy, hence the National Comprehensive
Cancer Network (NCCN) and European Society for Medical Oncology
(ESMO) guidelines and others recommend the use of bevacizumab
maintenance therapy once the disease is stabilized by a first-line
chemo-treatment. However, a clear long-term beneficial effect of
bevacizumab maintenance therapy has not yet been confirmed. The
present case was part of the SAiL (2) clinical trial, and the primary endpoint
results suggested that bevacizumab combined with chemotherapy
regimens may delay the time to progression (TTP) by 7.2–7.8 months
and achieve 14.6–15.3 months OS. The objective response rates (ORR)
and disease control rates (DCR) were as high as 50.8 and 88.7%,
respectively. These data revealed a breakthrough compared with
previous chemotherapy effectiveness levels (ORR, 30–35%; TTP or
PFS, 4–6 months; and OS, 9–11 months) (12–17).
Following a C/P-combined bevacizumab treatment, the
tumor status of the patient in the present study was stable. The
bevacizumab maintenance therapy lasted for the long duration of 50
months, and three months subsequent to withdrawing the treatment
due to proteinuria, the disease re-started progression. The total
PFS was 54 months, which was much longer than the reported levels
and confirms the safeness of long-term bevacizumab application.
Although the tumor did not significantly narrow, it was maintained
in a long progression-free period and the results agree with the
‘survival with tumor’ concept. This concept refers to the survival
of advanced patients through effective anti-tumor treatment to
prolong the stable disease. The tumor re-start progress following
the withdrawal of bevacizumab also illustrates that the prolonged
SD status is associated with bevacizumab maintenance therapy. The
present case revealed that the proteinuria level was positively
correlated with the cumulative bevacizumab dose and therefore may
contribute to side-effect studies of bevacizumab in the future.
In summary, bevacizumab maintenance therapy was
identified to be correlated with a 54-month prolonged PFS in the
patient of the present study. The long-term use of bevacizumab led
to a certain level of adverse reaction, which was controlled and
completely reversed subsequent to withdrawing the treatment.
Currently there is no clear indication for using bevacizumab in
NSCLC treatments and therefore the present case of prolonged PFS
may only be an individual case. A large-scale clinical trial is
necessary to validate the possibility of using bevacizumab as a
standard maintenance therapy for lung cancer.
Acknowledgements
The authors would like to thank Dr Luo Fang for the
analysis and interpretation of the data.
References
|
1
|
Bareschino MA, Schettino C, Rossi A, et
al: Treatment of advanced non small cell lung cancer. J Thorac Dis.
3:122–133. 2011.PubMed/NCBI
|
|
2
|
Crinò L, Dansin E, Garrido P, et al:
Safety and efficacy of first-line bevacizumab-based therapy in
advanced non-squamous non-small-cell lung cancer (SAiL, MO19390): a
phase 4 study. Lancet Oncol. 11:733–740. 2010.PubMed/NCBI
|
|
3
|
Sandler A, Gray R, Perry MC, et al:
Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell
lung cancer. N Engl J Med. 355:2542–2550. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sandler A, Yi J, Dahlberg S, et al:
Treatment outcomes by tumor histology in Eastern Cooperative Group
Study E4599 of bevacizumab with paclitaxel/carboplatin for advanced
non-small cell lung cancer. J Thorac Oncol. 5:1416–1423. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Reck M, von Pawel J, Zatloukal P, et al:
Phase III trial of cisplatin plus gemcitabine with either placebo
or bevacizumab as first-line therapy for nonsquamous non-small-cell
lung cancer: AVAil. J Clin Oncol. 27:1227–1234. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Reck M, von Pawel J, Zatloukal P, et al;
BO17704 Study Group. Overall survival with cisplatin-gemcitabine
and bevacizumab or placebo as first-line therapy for nonsquamous
non-small-cell lung cancer: results from a randomised phase III
trial (AVAiL). Ann Oncol. 21:1804–1809. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yuan F, Chen Y, Dellian M, et al:
Time-dependent vascular regression and permeability changes in
established human tumor xenografts induced by an anti-vascular
endothelial growth factor/vascular permeability factor antibody.
Proc Natl Acad Sci USA. 93:14765–14770. 1996. View Article : Google Scholar
|
|
8
|
Mancuso MR, Davis R, Norberg SM, et al:
Rapid vascular regrowth in tumors after reversal of VEGF
inhibition. J Clin Invest. 116:2610–2621. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bagri A, Berry L, Gunter B, et al: Effects
of anti-VEGF treatment duration on tumor growth, tumor regrowth,
and treatment efficacy. Clin Cancer Res. 16:3887–3900. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ebos JM, Lee CR, Cruz-Munoz W, et al:
Accelerated metastasis after short-term treatment with a potent
inhibitor of tumor angiogenesis. Cancer Cell. 15:232–239. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nadler E, Yu E, Ravelo A, Sing A, Forsyth
M and Gruschkus S: Bevacizumab treatment to progression after
chemotherapy: outcomes from a U.S. community practice network.
Oncologist. 16:486–496. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kelly K, Crowley J, Bunn PA Jr, et al:
Randomized phase III trial of paclitaxel plus carboplatin versus
vinorelbine plus cisplatin in the treatment of patients with
advanced non - small-cell lung cancer: a Southwest Oncology Group
trial. J Clin Oncol. 19:3210–3218. 2001.PubMed/NCBI
|
|
13
|
Scagliotti GV, De Marinis F, Rinaldi M, et
al; Italian Lung Cancer Project. Phase III randomized trial
comparing three platinum-based doublets in advanced non-small-cell
lung cancer. J Clin Oncol. 20:4285–4291. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schiller JH, Harrington D, Belani CP, et
al; Eastern Cooperative Oncology Group. Comparison of four
chemotherapy regimens for advanced non-small-cell lung cancer. N
Engl J Med. 346:92–98. 2002. View Article : Google Scholar
|
|
15
|
Fossella F, Pereira JR, von Pawel J, et
al: Randomized, multinational, phase III study of docetaxel plus
platinum combinations versus vinorelbine plus cisplatin for
advanced non-small-cell lung cancer: the TAX 326 study group. J
Clin Oncol. 21:3016–3024. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gridelli C, Ardizzoni A, Douillard JY, et
al: Recent issues in first-line treatment of advanced
non-small-cell lung cancer: Results of an International Expert
Panel Meeting of the Italian Association of Thoracic Oncology. Lung
Cancer. 68:319–331. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Scagliotti GV, Parikh P, von Pawel J, et
al: Phase III study comparing cisplatin plus gemcitabine with
cisplatin plus pemetrexed in chemotherapy-naive patients with
advanced-stage non-small-cell lung cancer. J Clin Oncol.
26:3543–3551. 2008. View Article : Google Scholar : PubMed/NCBI
|