Introduction
Esophageal squamous cell carcinoma (ESCC) is a
malignancy that arises from esophageal epithelial cells and
represents ~2% of all tumor types by incidence (1). The treatment for esophageal cancer
includes surgery, radiotherapy and chemotherapy. Early to middle
stage esophageal cancer is often curable, with late stage disease
having a poor prognosis (2).
Despite advances in technology and an improvement in the survival
rate for esophageal cancer, the efficacy of treatment remains far
from satisfactory. The main reasons for treatment failure include a
change in respiratory and digestive function following surgery, and
the damage and side effects that are associated with chemotherapy
(1). Consequently, new methods for
the treatment of early and late stage disease are required.
Retinoblastoma protein-interacting zinc finger gene
1 (RIZ1) plays a significant role as a tumor suppressor gene in
esophageal cancer. RIZ1 has previously been reported to be
expressed at low levels in esophageal carcinoma tissues compared
with the adjacent non-cancerous tissues (3,4).
Furthermore, the expression of RIZ1 may also be regulated by
methylation of the gene promoter (4). The ESCC TE13 cell line was selected,
which expresses low levels of RIZ1, to confirm the existence of
methylation in the RIZ1 promoter in ESCC cells. To study the tumor
suppressor role of RIZ1, the TE13 cells were treated with a DNA
methyltransferase (DNMT) inhibitor, 5-aza-2′-deoxycytidine
(5-aza-CdR), in order to reverse the methylation of the RIZ1
promoter and re-express the protein. Furthermore, a eukaryotic
vector was constructed, which expressed human RIZ1. The effects of
re-expressing RIZ1 using the vector or by treatment with 5-aza-CdR
on apoptosis were investigated in the TE13 cells. The present study
aimed to identify a new therapeutic target and provide a foundation
for gene therapy in esophageal cancer.
Materials and methods
The study was conducted in accordance with the
Declaration of Helsinki. Approval for this study was obtained from
the Ethics Committee for the Use of Human Subjects of Tianjin
Medical University General Hospital (Tianjin, China). Patients
provided their written consent to participate in this study and
this consent was also approved by the Ethics Committee for the Use
of Human Subjects of Tianjin Medical University General
Hospital.
Cell lines and tissue samples
The human ESCC TE13 cell line was purchased from
American Type Culture Collection (Rockville, MD, USA) and cultured
in RPMI-1640 containing 4.76 g HEPES, 2.0 g NaCO3, 10.4
g RPMI-1640 and 1,000 ml ddH2O (Gibco, Carlsbad, CA,
USA), supplemented with 10% fetal bovine serum (Gibco), 1X
L-glutamine (2 mM), 100 U/ml penicillin and 100 μg/ml streptomycin.
The cells were incubated at 37°C in a 5% CO2 humidified
incubator.
The esophageal cancer tissues and the matched
adjacent non-cancerous tissue samples were obtained from the
Department of Cardiothoracic Surgery of Tianjin Medical University
General Hospital following the surgical excision of the tumors. All
the specimens were placed in liquid nitrogen immediately following
the resection and stored at −80°C until RNA or genomic DNA
extraction. None of the patients were administered chemotherapy or
radiation therapy prior to surgery and the diagnoses of all the
patients were pathologically confirmed to be esophageal squamous
carcinoma.
Isolation of RNA from cell lines and
tissue samples
RNA was isolated from cell lines or tissues using
TRIzol (Invitrogen, Carlsbad, CA, USA) according to the
manufacturer’s instructions. TRIzol (1 ml) was added to
5×106-1×107 cultured cells and the tissue
samples were ground into a fine powder using a pestle and mortar
prior to incubation in TRIzol (100 g/l). The RNA pellets were
resuspended in diethylpyrocarbonate-treated H2O. The
total RNA concentrations of the samples were quantified using a UV
spectrophotometer (DU-460, Beckman Coulter, Miami, FL, USA).
Reverse transcription amplification of
RIZ1 mRNA
Reverse transcription was performed to produce
complementary DNA (cDNA) using 2 μg RNA, molony murine leukemia
virus (M-MLV) reverse transcriptase, ribonuclease inhibitor and
dNTPs mixture (Takara Bio, Inc., Shiga, Japan) according to the
manufacturer’s instructions. Semi-quantitative polymerase chain
reaction (PCR) was performed using the cDNA templates.
According to the published NCBI RIZ1 mRNA sequence
(NM_012231), the size of the protein coding region is 5,157 base
pairs that are positioned between base pairs 857 and 6,013. Due to
the size of the amplicon, the open reading frame may be divided
into five sections, designated A603, A1200, B, C and D. The primers
were previously designed for the five amplicons of RIZ1 by Primer
5.0 software (Premier, Palo Alto, CA, USA) as follows: Forward:
5′-GTGGCTAGCATGAATCAGAACACTACTG-3′ and reverse:
5′-TTGGCCAGAGGTGAAATCTGG CTC-3′ for A603; forward:
5′-TGGCTGCGATATGTGA ATTG-3′ and reverse: 5′-CTCTACGCTGATGCCGTCTC-3′
for A1200; forward: 5′-GCTGATGGCAAAGCATCTG-3′ and reverse:
5′-AATTCCTTGCCTTCAGAGTCAC-3′ for B; forward:
5′-TCAAAGAAAGTCATTCAGTGC-3′ and reverse: 5′-CGGTGATGGTACTGAAATG-3′
for C; and forward: 5′-GCCTCAATCAGCATTACC-3′ and reverse:
5′-GTCTACTCTTTGAAGAATGGTC-3′ for D. PCR amplification for each
amplicon (A603, A1200, B, C and D) of RIZ1 was also performed using
cDNA from normal, control esophageal tissue. The PCR reactions were
performed in a volume of 50 μl, consisting of 5 μl 10X KOD buffer,
5 μl 2 M dNTPs, 3 μl 25 mM MgSO4, 2 μl each of the
forward and reverse primers, 1 μl cDNA, 1 μl KOD-Plus Ver. 2 enzyme
(Toyobo Co., Ltd., Osaka, Japan) and ddH2O. Each PCR
amplification required specific conditions according to the melting
temperature and size of the amplicon as follows: Initialization at
94°C for 2 min, 35 cycles of denaturation at 98°C for 10 sec,
annealing (A603, 60°C at 30 sec; A1200, 57°C at 30 sec; B, 55°C at
30 sec; C, 50°C at 30 sec and D, 50°C at 30 sec), amplification at
72°C 1 min and a final extension at 72°C for 10 min. The quality of
the amplified products was analyzed using 12 g/l agarose gels with
a UV spectrophotometer (Beckman Coulter) and the quantitative PCR
reaction products were sequenced.
Construction of pcDNA3.1(+)/RIZ1
The amplicons were extracted from the agarose gel
using the TIANgel Midi Purification kit (Tiangen Biotech Co., Ltd.,
Beijing, China) according to the manufacturer’s instructions. Each
of the five amplicons were separately inserted into a T Trans1-T1
Phage Resistant vector (Promega Biotech Co., Ltd., Beijing, China),
transformed into Trans1-T1 Phage Resistant competent cells, plated
on agar containing ampicillin, and X-gal and white colonies were
selected for further analysis. Following the expansion of the
selected bacterial colonies, plasmid DNA was extracted by alkaline
lysis (5). Restriction enzyme
digests were used to validate successful recombination with final
confirmation provided by sequencing. The sequencing results for
each plasmid were compared with the NCBI sequences using the BLAST
website. The five RIZ amplicons were digested from plasmids
containing the correct insert and ligated into the eukaryotic
pcDNA3.1(+) expression vector as described in Fig 3. Insertion was verified by
restriction enzyme digestion and sequencing.
Transfection of TE13 cells with
pcDNA3.1(+)/RIZ1
The TE13 cells were seeded in six-well culture
plates at a density of 2×105 in a volume of 2 ml media,
incubated at 37°C and allowed to reach a confluence of 90–95%.
After 24 h, the media were replaced with complete serum-free
RPMI-1640 or antibiotics-free medium in preparation for
transfection. Ultra-pure pcDNA3.1(+)/RIZ1 plasmid DNA was extracted
using the HighPure Mini Plasmid kit (Tiangen Biotech Co., Ltd.). A
liposome-mediated method (6) was
used to transfect the TE13 cells with the pcDNA3.1(+)/RIZ1 plasmid.
Empty pcDNA3.1(+) plasmid and untransfected cells were used as
negative controls. Following 6 h of incubation with media
containing the recombinant plasmid and transfection reagents, the
media were replaced with RPMI-1640, 10% FBS and antibiotics-free
medium. The transfected cells were incubated for 48 h and harvested
for further analysis.
Quantitative PCR (qPCR) for RIZ1
mRNA
The cDNA from 28 paired human ESCC tissues, matched
adjacent non-cancerous tissues and the TE13 cells were amplified
using SYBR Premix Ex Taq™ (Takara). The primer (10 μM) sets that
were used were as follows: Forward: 5′-TCTGCTGTTGACAAGACCC-3′ and
reverse: 5′-GCATCAATGCACATCCATC-3′ for RIZ1; and forward:
5′-GAAGGTGAAGGTCGGAGTC-3′ and reverse: 5′-GGGTGGAATCATATTGGAAC-3′
for glyceraldehyde 3-phosphate dehydrogenase. The reactions were
performed using a LightCycler (Roche Diagnostics, Mannheim,
Germany) qPCR system according to the manufacturer’s instructions.
Briefly, the reaction involved an initial denaturation step at 94°C
for 5 min followed by 45 cycles of denaturation at 95°C for 5 sec,
annealing at 59°C for 20 sec and extension at 72°C for 10 sec,
followed by the generation of thermal melting curves. Each sample
was run in triplicate for each gene.
Western blotting
The PcDNA3.1(+)/RIZ1-transfected TE13 cells were
homogenized in RIPA buffer (50 mM Tris-HCl, pH 7.4; 150 mM NaCl; 1%
Nonidet P-40; 0.5% sodium deoxycholate; 0.1% SDS; 1 mM EDTA; 1 mM
PMSF; 1 mg/ml aprotinin) and the protein concentrations were
determined using the bicinchoninic acid protein assay kit (Pierce
Biotechnology, Inc., Rockford, IL, USA). The cell lysates (30 μg)
were separated by 8% SDS-PAGE, transferred to nitrocellulose
membranes (Amersham Biosciences, Piscataway, NJ, USA) and
immunoblotted with the indicated antibodies. All the antibodies
were purchased from Abcam (Cambridge, UK), including the RIZ1 and
the β-actin primary antibody and the secondary antibody goat
anti-mouse. The bands were visualized using the PowerLook scanner
(UMAX, Taipei, Taiwan) and quantified with ImageQuant software. The
relative expression of RIZ1 was calculated as the gray value for
RIZ1 divided by the gray value for β-actin. The TE13-untransfected
and empty vector transfected cells were used as negative
controls.
Flow cytometric analysis
To determine the effect of overexpressing RIZ1 on
apoptosis, 2×105 TE13 cells were seeded in six-well
plates and allowed to attach for 12 h. Cell cycle synchronization
was achieved by serum starvation in serum-free RPMI-1640 media for
24 h. The cells were subsequently transfected with pcDNA3.1(+)/RIZ1
and harvested after 24 h. The cells were fixed in 70% ice-cold
ethanol overnight, treated with DNase-free Ribonuclease (Takara),
stained with propidium iodide (Sigma-Aldrich, St. Louis, MO, USA)
and subjected to analysis using a FACSAria™ (Becton-Dickinson,
Franklin Lakes, NJ, USA). The data were analyzed using ModFit LT
software (Verity Software House, Topsham, ME, USA). The
TE13-untransfected and empty vector transfected cells were used as
controls.
Treatment with 5-aza-CdR
The TE13 cells were seeded at a density of
2×105 in six-well plates and treated with 10 μM DNMT
inhibitor, 5-aza-CdR (Sigma-Aldrich), for 24–72 h. The drug was
refreshed daily. The 5-aza-CdR was removed and the cells were
subsequently incubated for 120 h.
Statistical analysis
Statistical analysis was performed using SPSS 18.0
(SPSS, Inc., Chicago, IL, USA). The data are presented as the mean
± standard deviation. The qPCR results are presented as
2−averageΔΔCT × 100%. T-tests and one-way ANOVA were
used to analyze parametric data. The statistical analysis of the
group comparisons involved one-way ANOVA and the χ2 test
was used to compare enumerated data; P<0.05 was considered to
indicate a statistically significant difference.
Results
Re-expression of RIZ1 by transfection of
pcDNA3.1(+)/RIZ1
In order to re-express RIZ1, a recombinant plasmid
was generated to enable the ectopic overexpression of RIZ1 in TE13
cells. Due to the size of the protein coding region of the RIZ1
gene, the target was divided into five amplicons, A603, A1200, B, C
and D. RNA was isolated, reverse transcribed into cDNA and
amplified by PCR. Each of the five amplicons were subsequently
ligated into the T Trans1-T1 Phage Resistant vector and used to
transform the competent bacterial cells. Blue-white screening and
ampicillin selection was used to select the potential positive
colonies. Following the expansion of the colonies in liquid
culture, the plasmid DNA was extracted by alkaline lysis.
Successful recombination was verified by restriction enzyme
digestion and sequencing. The five amplicons of the RIZ1 fragment
were then ligated into the eukaryotic pcDNA3.1(+) expression
vector. The RIZ1 fragment was successfully inserted into the
pcDNA3.1(+) plasmid to produce recombinant pcDNA3.1(+)/RIZ1, with
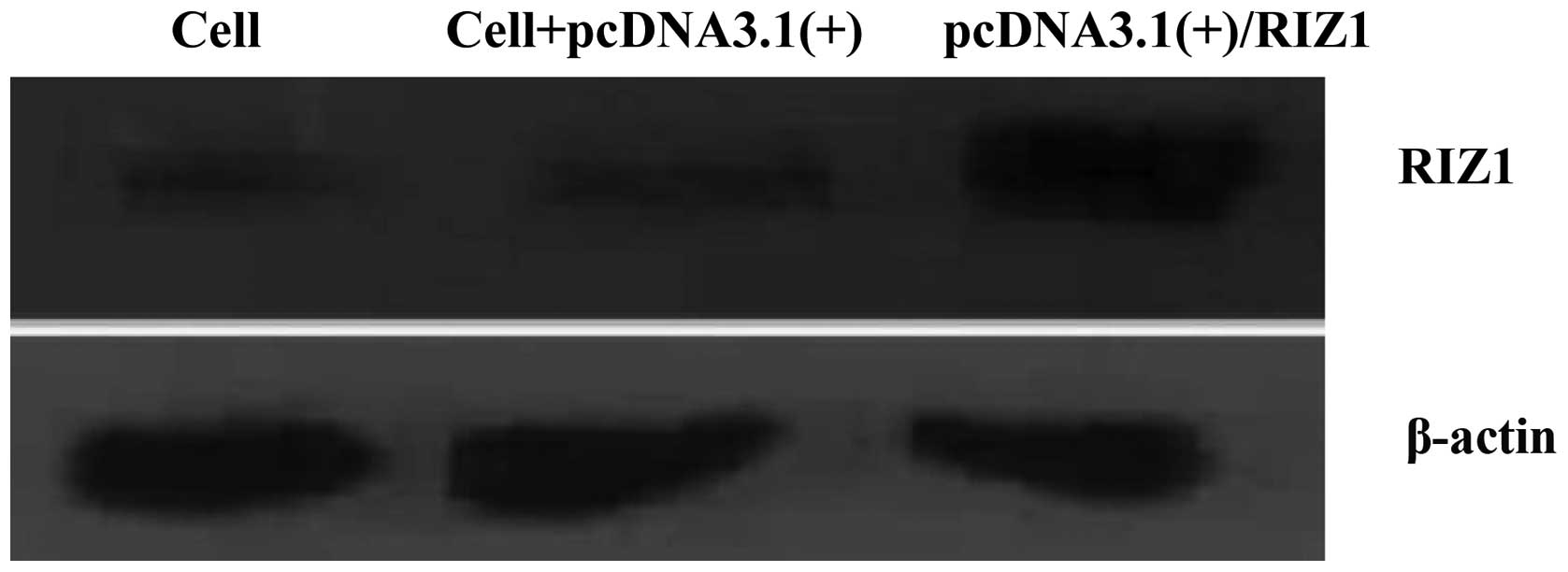
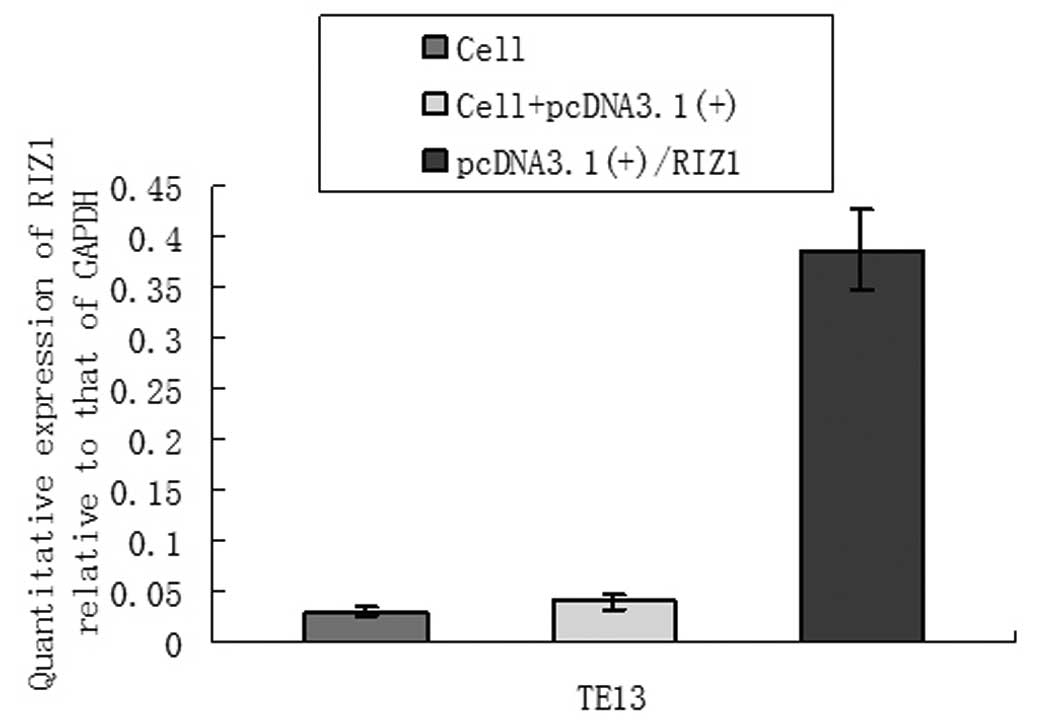
each amplicon in the correct order (Fig. 1). qPCR and western blot analysis
demonstrated an increase in RIZ1 mRNA and protein expression in the
TE13 cells that were transfected with the recombinant plasmid
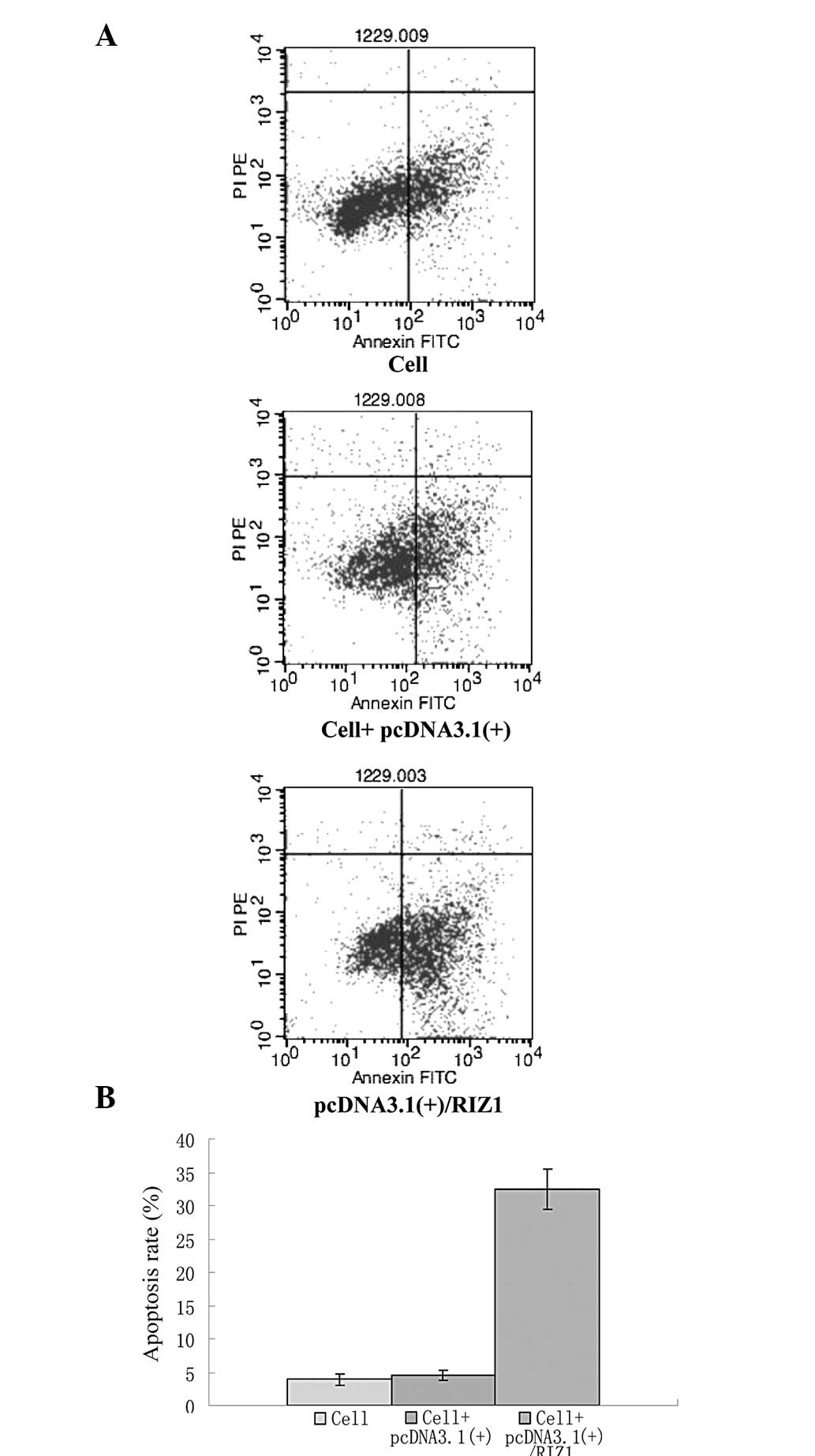
(Figs. 2 and 3). An analysis of the cell cycle using
flow cytometry demonstrated that the rate of apoptosis increased in
the TE13 cells subsequent to the transfection of the
pcDNA3.1(+)/RIZ1 plasmid (Fig.
4).
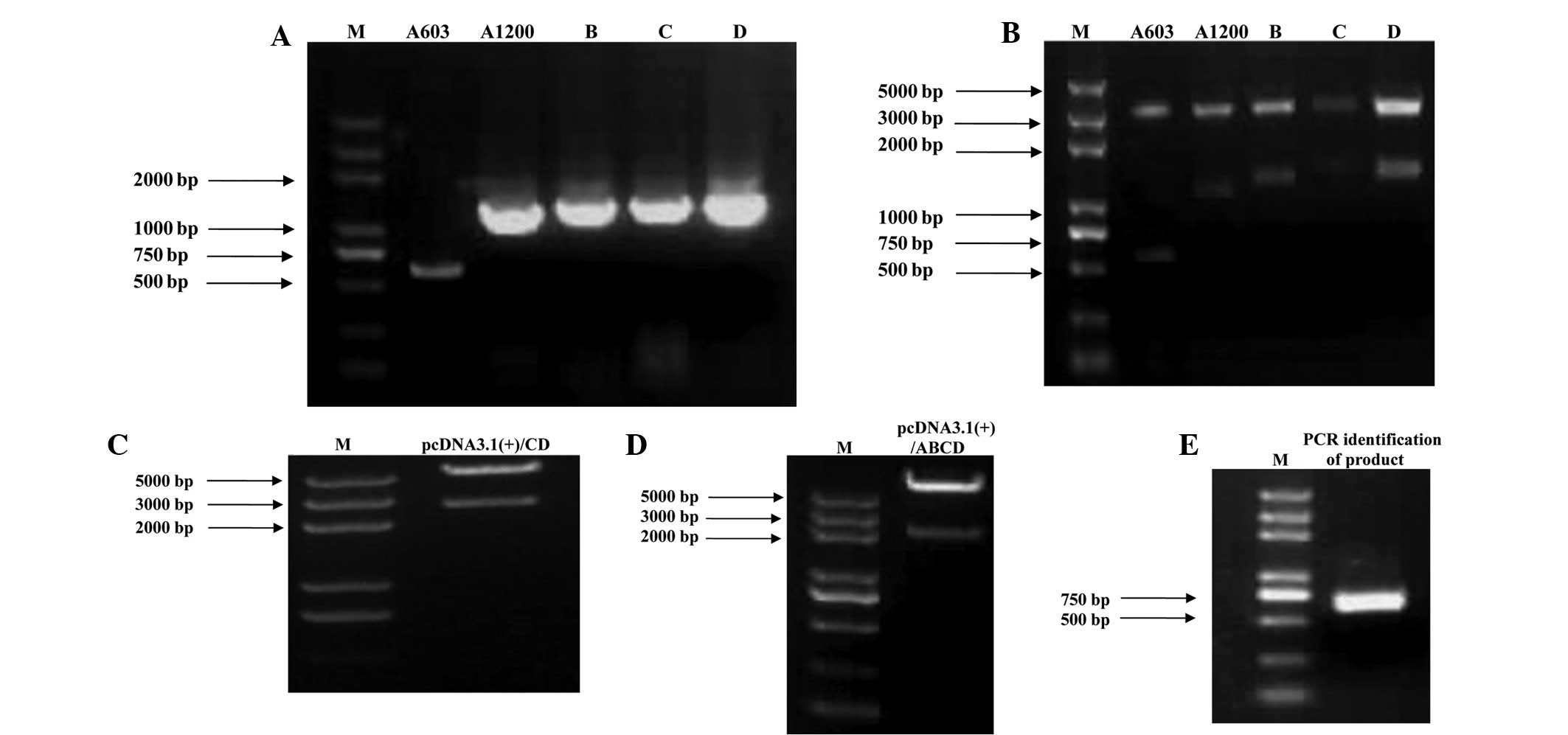 | Figure 1(A) qPCR results for the amplification
of each RIZ1 amplicon. The size of the protein coding region was
5,157 bp positioned between 857 and 6,013 bp. Due to the size of
the amplicon, the open reading frame was divided into five
sections, designated A603, A1200, B, C and D. (B) Amplification and
ligation of the RIZ1 amplicons into the pGEM-T vector. The five
amplicons were each inserted separately into the pGEM-T vector.
Restriction enzyme digestion verified that the digested products
were of the expected size. (C–E) Generation of the recombinant
pcDNA3.1(+)/RIZ1 construct. (C) The C and D segments were inserted
into the pcDNA3.1(+) vector and verified by restriction enzyme
digestion. The pcDNA3.1(+)/CD band of 2,583 bp was consistent with
the theoretical results. (D) The A1200 and B gene segments were
ligated into pcDNA3.1(+)/CD and verified by restriction enzyme
digestion. The pcDNA3.1(+)/ABCD band of 2,000 bp was consistent
with the theoretical results. (E) The A603 (PR domain) segment was
inserted into pcDNA3.1(+)/ABCD and verified by restriction enzyme
digestion. The 720 bp band was consistent with the theoretical
results. qPCR, quantitative polymerase chain reaction; RIZ1,
retinoblastoma protein-interacting zinc finger gene 1; M,
marker. |
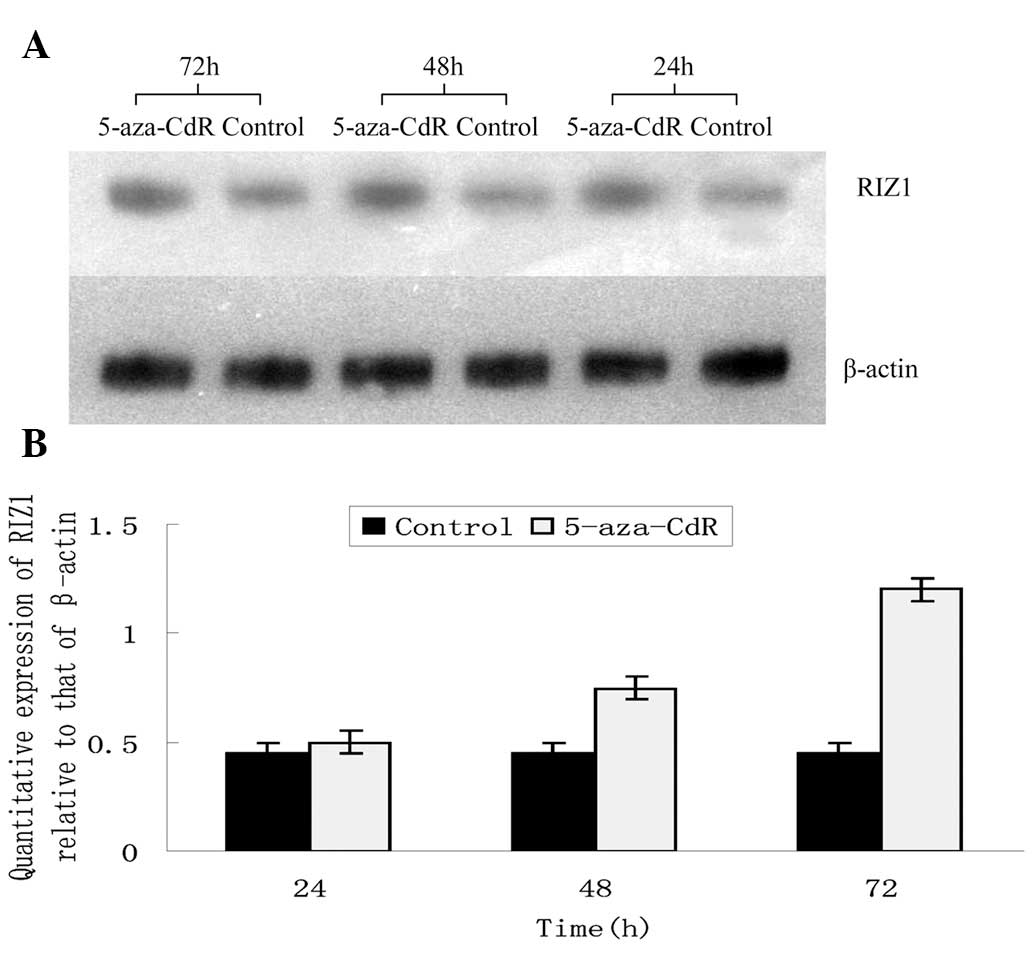
Re-expression of RIZ1 by 5-aza-CdR
treatment
The promoter of the RIZ1 gene in the TE13 cells,
which expressed low levels of RIZ1, was observed to be methylated.
The loss of this methylation was hypothesized to result in the
re-expression of RIZ1 (4).
Therefore, the TE13 cells were treated with 10 μM DNMT inhibitor,
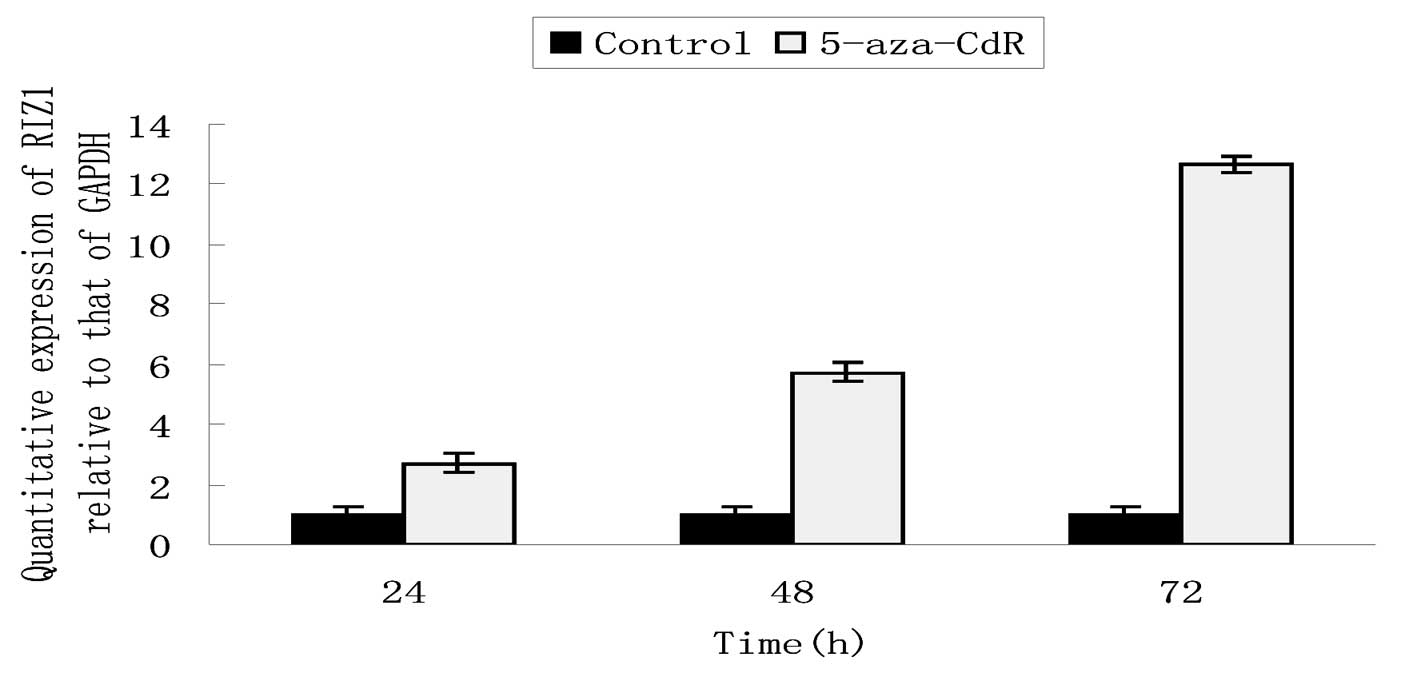
5-aza-CdR. qPCR and western blotting demonstrated that the mRNA and
protein levels of RIZ1 were significantly higher in the 5-aza-CdR
group compared with the control cells at every time point (Figs. 5 and 6). 5-aza-CdR increased the levels of RIZ1
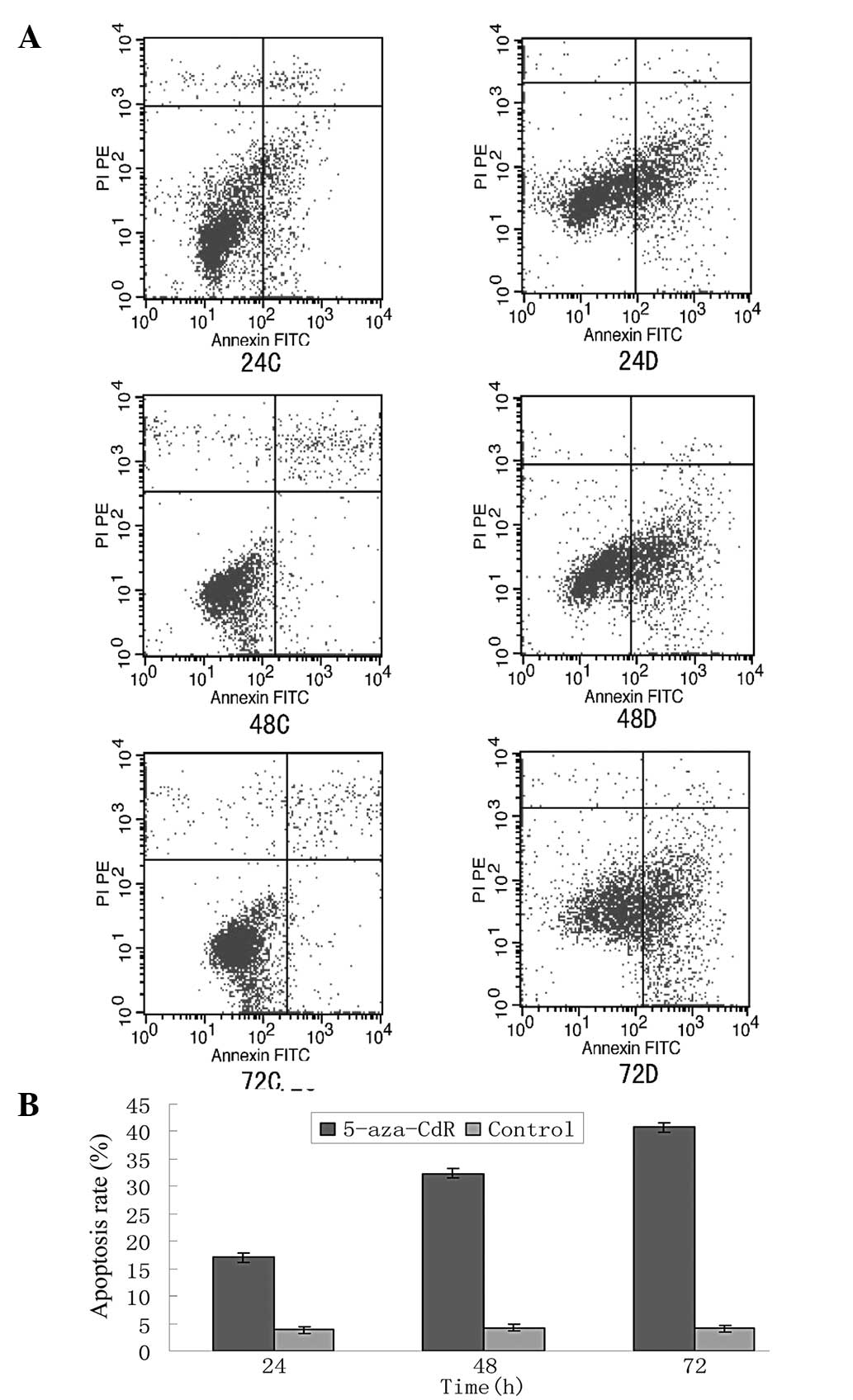
mRNA and protein expression in a time-dependent manner.
Furthermore, the rate of apoptosis in each 5-aza-CdR group
increased following the treatment with 5-aza-CdR. Additionally,
5-aza-CdR increased the rate of apoptosis in a time-dependent
manner between 24 and 72 h (Fig.
7).
Discussion
RIZ was first isolated by Buyse et
al(7) using retinoblastoma (Rb)
probes, whilst performing a functional screen for Rb, and it was
observed that RIZ was able to interact with Rb. Fluorescent in
situ hybridization demonstrated that the RIZ gene is
located on human chromosome 1p36. There are two variants of RIZ,
RIZ1 and RIZ2, due to the presence of two alternative initial
locations (8). The sequences of
RIZ1 and RIZ2 are identical, with the exception of the presence of
a PR domain in RIZ2. The PR domain in RIZ2 is termed the
PRDI-BF1-RIZ1 homology region and contains >100 amino acids. The
function of this domain is to act as a protein-binding-interface,
mediate protein-protein interactions, stabilize chromosomal
structures and, therefore, regulate gene expression (9). Gene families that contain a PR domain
contribute to tumorigenesis via a unique mechanism. The absence or
presence of a PR domain results in the differential expression of
proteins at an early stage of tumorigenesis, which provides a
mechanism for tumor initiation (10). Studies have shown that RIZ1 is able
to inhibit tumor development and is considered to be a significant
tumor suppressor gene. Furthermore, as RIZ1 acts in combination
with Rb, which induces the arrest of tumor cells in the
G2/M phase leading to cell death, the combined
re-expression of RIZ1 and Rb may halt tumor growth (11,12).
As a novel tumor suppressor, RIZ1 has been analyzed
in numerous studies, which have attempted to understand the
mechanism by which the gene is inactivated in cancer cells. Genetic
and epigenetic changes are believed to be responsible (13). From a genetic perspective, RIZ1 may
be deactivated by chromosomal instability and microsatellite
instability, as well as frameshift mutations, point mutations and
heterozygote deficiency (14). From
an epigenetic perspective, the deactivation of RIZ1 may occur due
to promoter methylation and histone acetylation. Changes to
chromosome 1p36, on which RIZ1 is located, are also associated with
numerous types of cancer, including breast cancer, ovarian cancer,
liver cancer, colorectal cancer, chronic myeloid leukemia,
melanoma, chromaffin tumor and neuroblastoma (15). In all these tumor types, the tumor
tissue samples and cancer cell lines display low expression levels
or deficiency of RIZ1. Furthermore, our previous study demonstrated
that esophageal cancer tissues expressed lower levels of RIZ1 mRNA
and protein compared with the normal tissues (3). Taken together, this evidence indicates
that the deactivation of the RIZ1 tumor suppressor gene may be
significant in the progression of esophageal cancer.
Treatment for ESCC may include surgery, radiotherapy
and chemotherapy. However, the efficacy of such treatment for later
stage disease is unsatisfactory. Furthermore, the side effects due
to radiotherapy and chemotherapy, including the depletion of bone
marrow, gastrointestinal toxicity, immunological suppression and
liver or kidney injury, are far from negligible (16). At present, there are a number of
noteworthy therapies that are being developed, including targeted
drugs, conjugated drugs and nanoparticles. Gene therapy is an
active area of research and holds much promise for the future of
anti-cancer therapy (17). The
development of a malignant tumor is caused by the inactivation of
tumor suppressor genes and the abnormal activation of oncogenes.
The main techniques that are being developed for the treatment of
cancer by gene therapy include immune, causal, suicide and
auxiliary gene therapy (18). The
re-expression of a specified gene in a target cell is a new
approach. However, if successfully applied, such approaches may
also be useful for the management of a number of other diseases, in
addition to cancer (19). Despite
much progress being made in the technology of the vehicles that are
used to deliver target genes to the appropriate cells, numerous
vehicles have significant shortcomings and extensive research is
required prior to the successful use of gene therapy in the clinic
(20).
The pcDNA3.1(+) vector is an efficient eukaryotic
expression vector. Transcription of the inserted sequence is
controlled by the human cytomegalovirus promoter, whilst there is a
transcription termination signal downstream (7,21,22).
Compared with the viral vectors that are used for gene therapy,
pcDNA3.1(+) does not stimulate an immune response, has no latent
toxic side effects and does not require the dissemination of live
viruses. Using a liposome-mediated transfection method, foreign
genes may easily be inserted into target cells. Therefore, the
pcDNA3.1(+) vector was selected to create pcDNA3.1(+)/RIZ1 for
transfection into the eukaryotic cells.
RIZ1 is a tumor suppressor gene that prevents the
progression of esophageal carcinoma. In the present study, the
eukaryotic pcDNA3.1(+)/RIZ1 expression plasmid was used to
transfect human squamous esophageal carcinoma TE13 cells, in order
to re-express RIZ1. Furthermore, the re-expression of RIZ1 was
attempted using the DNMT inhibitor, 5-aza-CdR, to reverse the
methylation of the RIZ1 promoter in the TE13 cells. The DNMT
inhibitor, 5-aza-CdR, has been used in the clinic for the treatment
of certain solid tumors and hematological diseases, including
myelodysplastic syndrome and acute myeloid leukemia (3,4). New
therapeutic approaches for the treatment of esophageal cancer may
be identified through further research into the epigenetic status
of genes and the appropriate application of 5-aza-CdR.
The present study demonstrated that the transfection
of pcDNA3.1(+)/RIZ1 or the application of 5-aza-CdR increased the
expression of RIZ1 and effectively increased the rate of apoptosis
in TE13 cells. This raises the possibility that the re-expression
of RIZ1 may induce apoptosis in malignant esophageal cancer cells.
Furthermore, the experiments have led to the development of a cell
line in which RIZ1 is overexpressed and the detailed biological
characterization of this cell line will follow. Future studies will
focus on in vivo models in order to establish new methods to
combat and potentially cure esophageal carcinoma using gene and
cellular transplantation therapy. Additionally, further research on
the mechanism of action of the RIZ1 tumor suppressor gene may lead
to the development of new biomarkers for early diagnosis and
prognostic evaluation in esophageal cancer.
Acknowledgements
This study was supported by a grant-in-aid from the
National Natural Science Foundation of China (no. 81201945), the
Science Foundation of Tianjin Medical University (no. 2011KY08) and
the Natural Science Foundation of Tianjin (no. 10JCYBJC11300). The
authors would also like to thank Dr Si-Cheng Zhao, from the Chinese
Academy of Sciences, with whom we discussed the study.
References
|
1
|
Amin HM, Hoshino K, Yang H, Lin Q, Lai R
and Garcia-Manero G: Decreased expression level of SH2
domain-containing protein tyrosine phosphatase-1 (Shp1) is
associated with progression of chronic myeloid leukaemia. J Pathol.
212:402–410. 2007. View Article : Google Scholar
|
|
2
|
Takeno S, Yamashita SI, Yamamoto S,
Takahashi Y, Moroga T, et al: Number of metastasis-positive lymph
node stations is a simple and reliable prognostic factor following
surgery in patients with esophageal cancer. Exp Ther Med.
4:1087–1091. 2012.PubMed/NCBI
|
|
3
|
Dong SW, Cui YT, Zhong RR, Liang DC, Liu
YM, Wang YG, He Z, Wang S, Liang SJ and Zhang P: Decreased
expression of retinoblastoma protein-interacting zinc-finger gene 1
in human esophageal squamous cell cancer by DNA methylation. Clin
Lab. 58:41–51. 2012.PubMed/NCBI
|
|
4
|
Dong SW, Zhang P, Liu YM, et al: Study on
RIZ1 gene promoter methylation status in human esophageal squamous
cell carcinoma. World J Gastroenterol. 18:576–582. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Klintschar M and Neuhuber F: Evaluation of
an alkaline lysis method for the extraction of DNA from whole blood
and forensic stains for STR analysis. J Forensic Sci. 45:669–673.
2000.PubMed/NCBI
|
|
6
|
Dean DA and Gasiorowski JZ:
Liposome-mediated transfection. Cold Spring Harb Protoc.
2011:prot55832011.PubMed/NCBI
|
|
7
|
Buyse IM, Shao G and Huang S: The
retinoblastoma protein binds to RIZ, a zincfinger protein that
shares an epitope with the adenovirus EIA protein. Proc Natl Acad
Sci USA. 92:4467–4471. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ng WT, Choi CW, Lee MC, Chan SH, Yau TK
and Lee AW: Familial nasopharyngeal carcinoma in Hong Kong:
epidemiology and implication in screening. Fam Cancer. 8:103–108.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Szende B and Tyihák E: Effect of
formaldehyde on cell proliferation and death. Cell Biol Int.
34:1273–1282. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Overmeer RM, Louwers JA, Meijer CJ, van
Kemenade FJ, Hesselink AT, et al: Combined CADM1 and MAL promoter
methylation analysis to detect (pre-)malignant cervical lesions in
high-risk HPV-positive women. Int J Cancer. 129:2218–2225. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Overmeer RM, Henken FE, Snijders PJ,
Claassen-Kramer D, Berkhof J, et al: Association between dense
CADM1 promoter methylation and reduced protein expression in
high-grade CIN and cervical SCC. J Pathol. 215:388–397. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Brait M, Ford JG, Papaiahgari S, Garza MA,
Lee JI, et al: Association between lifestyle factors and CpG island
methylation in a cancer-free population. Cancer Epidemiol
Biomarkers Prev. 18:2984–2991. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schoenhals M, Kassambara A, De Vos J, Hose
D, Moreaux J and Klein B: Embryonic stem cell markers expression in
cancers. Biochem Biophys Res Commun. 383:157–162. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang S, Balch C, Chan MW, Lai HC, Matei
D, et al: Identification and characterization of ovarian
cancer-initiating cells from primary human tumors. Cancer Res.
68:4311–4320. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen L, Ling BB, Alcorn J and Yang J:
Analysis of the expression of human N-myristoyltransferase 1
(hNMT-1) in cancers. Open Biomarkers J. 2:6–10. 2009. View Article : Google Scholar
|
|
16
|
Dalerba P, Cho RW and Clarke MF: Cancer
stem cells: models and concepts. Annu Rev Med. 58:267–284. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Noman AS, Koide N, Iftakhar-E-Khuda I,
Dagvadorj J, Tumurkhuu G, et al: Retinoblastoma protein-interacting
zinc finger 1 (RIZ1) participates in RANKL-induced osteoclast
formation via regulation of NFATc1 expression. Immunol Lett.
131:166–169. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Philippidis A: Gene therapy briefs. Hum
Gene Ther. 23:1221–1223. 2012. View Article : Google Scholar
|
|
19
|
Dong SW, Ma L, Xu N, Yan HQ, Liu HY, Li YW
and Zhang P: Research on the reactivation of Syk expression caused
by the inhibition of DNA promoter methylation in the lung cancer.
Neoplasma. 58:89–95. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Moran NL: First gene therapy approved. Nat
Biotechnol. 30:11532012. View Article : Google Scholar
|
|
21
|
Fujita J, Crane AM, Souza MK, Dejosez M,
Kyba M, et al: Caspase activity mediates the differentiation
ofembryonic stem cells. Cell Stem Cell. 2:595–601. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yu J, Vodyanik MA, Smuga-Otto K,
Antosiewicz-Bourget J, Frane JL, et al: Induced pluripotent stem
cell lines derived from human somatic cells. Science.
318:1917–1920. 2007. View Article : Google Scholar : PubMed/NCBI
|