Introduction
Gastric cancer is the third most common type of
cancer worldwide, with high incidence and mortality; ~989,600 new
cases of gastric cancer are diagnosed, of which 738,000 cases
succumb, per year, according to GLOBOCAN 2008 (1). Gastric cancer has developed into a
serious health problem worldwide, particularly in Eastern Asia,
Eastern Europe and South America. The transcriptional inactivation
of tumor suppressor genes is one of the main reasons for
carcinogenesis. Epigenetics studies have confirmed that DNA
methylation in the promoter region of a tumor suppressor gene leads
to transcriptional inactivation and is correlated with the
carcinogenesis of gastric cancer (2–5). This
phenomenon has also been simultaneously discovered in gastric
cancer tissue. Somatostatin (SST) is a gut peptide that is able to
inhibit the growth of tumor cells in gastric cancer and other types
of cancer, and is regarded as a new cancer repressive polypeptide
(6–9) However, the further mechanistic
interaction between gastric tumorigenesis and SST promoter
methylation remains unclear.
The present study investigated the expression of
SST, SST mRNA and SSTR mRNA in gastric cancer and utilized
methylation-specific PCR (MSP) technology for the analysis of SST
promoter DNA methylation.
Materials and methods
Tissues samples
A total of 51 pairs of fresh gastric tissue samples
were obtained from the Department of General Surgery, West China
Hospital (Sichuan University, Chengdu, China). Each pair of
samples, which consisted of a gastric cancer tissue and a normal
gastric tissue sample, was divided into a gastric cancer group and
a normal gastric tissue group, respectively. All the tumor and
normal gastric mucosal epithelial tissues were histologically
verified. The patients were not administered radiation, chemical or
biological treatment prior to surgery. Written informed consent was
obtained from each patient before enrollment and this study was
approved by the Ethics Committee of West China Hospital.
Radioimmunoassay analysis of SST
Following the homogenization of the normal and
gastric cancer tissues, the total protein level was treated with
the Total Protein Reagent kit (Biosino Bio-technology and Science
Inc., Hong Kong, China) at 37°C for 10 min and measured at a
wavelength of 546 nm using an allophanamide assay. The homogenate
was then treated using an SST radioimmunoassay kit (HY-104; Beijing
Sino-UK Institute of Biological Technology, Beijing, China). The
protein level of SST was measured using a γ-911 radioimmunoassay
counter (Zhongjia Optical and Electrical Instrument Company, Hefei,
China). The level of SST was corrected using the total protein
level.
Detection of SST and SSTR mRNA levels
using RT-PCR
Total RNA was isolated by Trizol (Invitrogen,
Carlsbad, CA, USA). First-strand cDNA was produced using the
Reverted Aid First Strand cDNA Synthesis kit (Fermentas,
Pittsburgh, PA, USA). The primer sequences and reaction conditions
for SST and SSTRs are listed in Table
I.
 | Table IPrimer sequences, reaction conditions
and cycles. |
Table I
Primer sequences, reaction conditions
and cycles.
| | Reaction
conditions |
|---|
| |
|
|---|
| Primer sequences
5′-3′ | Length (bp) | Annealing temperature
(°C) | Reaction cycles |
|---|
| RT-PCR |
| SST |
| Sense: GGC TGC GCT
GTC CAT CGT C | 285 | 58.0 | 36 |
| Antisense: CAG CCA
GCT TTG CGT TCT CG | | | |
| SSTR2 |
| Sense: GGT GAA GTC
CTC TGG AAT CC | 461 | 63.0 | 36 |
| Antisense: CCA TTG
CCA GTA GAC AGA GC | | | |
| SSTR3 |
| Sense: TCA TCT GCC
TCT GCT ACC TG | 221 | 65.0 | 36 |
| Antisense: GAG CCC
AAA GAA GGC AGG CT | | | |
| SSTR5 |
| Sense: GTG CAG GAG
GGC GGT ACC | 474 | 62.0 | 36 |
| Antisense: TGG ACG
CGG CTC CGT GGC | | | |
| β-actin |
| Sense: GAC TAC CTC
ATG AAG ATC CT | 312 | 53.0 | 35 |
| Antisense: GCG GAT
GTC CAC GTC ACA CT | | | |
| qMSP |
| SST |
| Sense: GGG GCG TTT
TTT AGT TTG ACG T | 102 | 58.2 | 40 |
| Antisense: AAC AAC
GAT AAC TCC GAA CCT CG | | | |
Detection of SST DNA methylation using
quantitative MSP (qMSP)
Genomic DNA was isolated using the Tissue Gen DNA
kit (ComWin Biotech, Beijing, China). Subsequently, the positive
methylated controlled DNA that was extracted from the placenta
tissue was incubated with CpG methyltransferase for 1–2 h at 37°C
and terminated following an incubation period at 65°C for 20 min.
Following this, all the genomic DNA, including the positive
methylated control (placenta tissue DNA) were managed by DNA
methylation modification using the Methylamp DNA Modification kit
(ComWin Biotech) and incubated for 60 min at 80°C in the dark. The
tissue DNA treated with CpG methyltransferase was defined as the
positive methylation control and the double distilled water as
negative. The determination of SST DNA methylation for all the
modified genomic DNA was performed using qMSP. The primer
sequences, reaction conditions and cycles for SST are listed in
Table I. The objective products of
qMSP were verified by a sequence assay.
Statistical analysis
SPSS 16.0 statistical software (SPSS Inc., Chicago,
IL, USA) was used for the statistical analysis. The measurement
data was analyzed using a paired-samples t-test and presented as
the mean ± standard deviation. P<0.05 was considered to
indicated a statistically significant difference. The count data
were applied using the χ2 and Fisher’s exact tests
(P<0.05).
Results
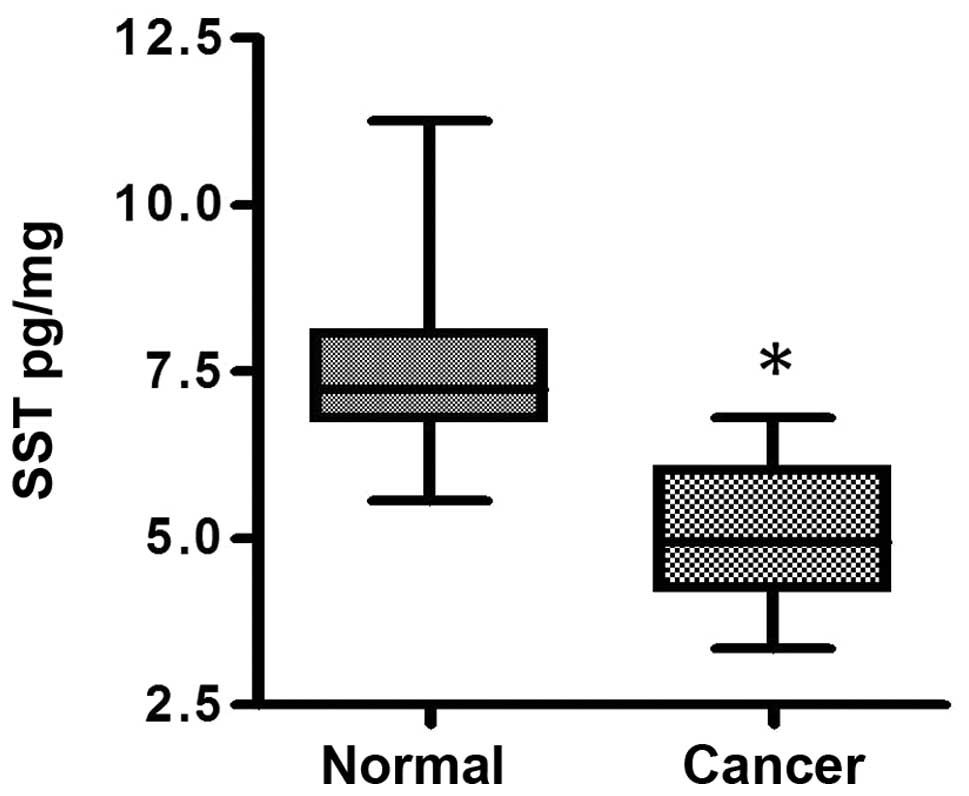
SST protein levels in gastric cancer
The SST protein level in the normal group was
significantly higher compared with that in the cancer group
(7.399±0.956 vs. 5.091±0.994 pg/mg; P<0.01; Fig. 1).
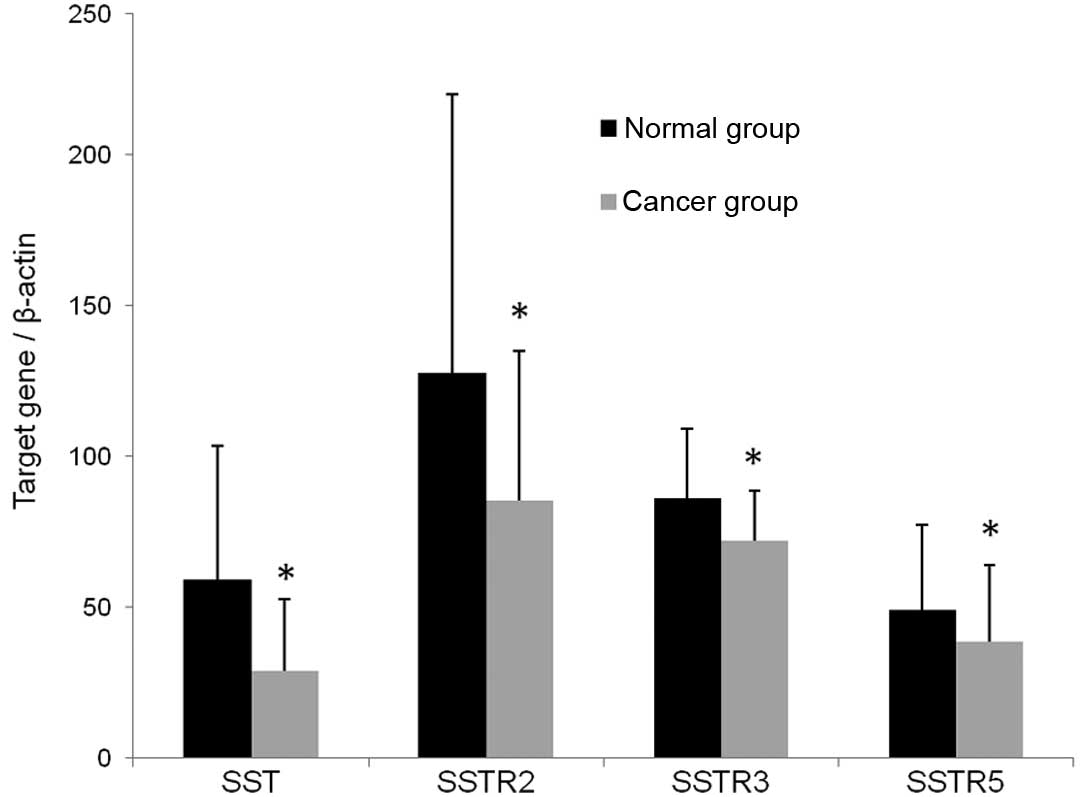
Detection of SST, SSTR2, SSTR3 and SSTR5
mRNA in the normal and cancer groups using RT-PCR
The expression of SST, SSTR2, SSTR3 and SSTR5 mRNA
in the normal and cancer groups was detected using RT-PCR. The
lengths of the amplified products of SST, SSTR subtypes and β-actin
are marked (Fig. 2). The mRNA
levels were analyzed using the integral optical density (IOD) by
the Quantity One software (Bio-Rad, Hercules, CA, USA). The IOD of
β-actin was used as the standard to correct the IOD levels of SST,
SSTR2, SSTR3 and SSTR5. The value indicated that the SST and SSTR
mRNA expression levels in the cancer group were lower than those of
the normal group. The IOD ratio of SST in the two groups was
0.456±0.331 vs. 0.218±0.183 (P<0.001). The IOD ratios of SSTR2,
SSTR3 and SSTR5 in two groups were (0.900±0.396, 0.647±0.174 and
0.364±0.202 vs. 0.646±0.375, 0.538±0.125 and 0.299±0.188,
respectively (P<0.01; Fig.
3).
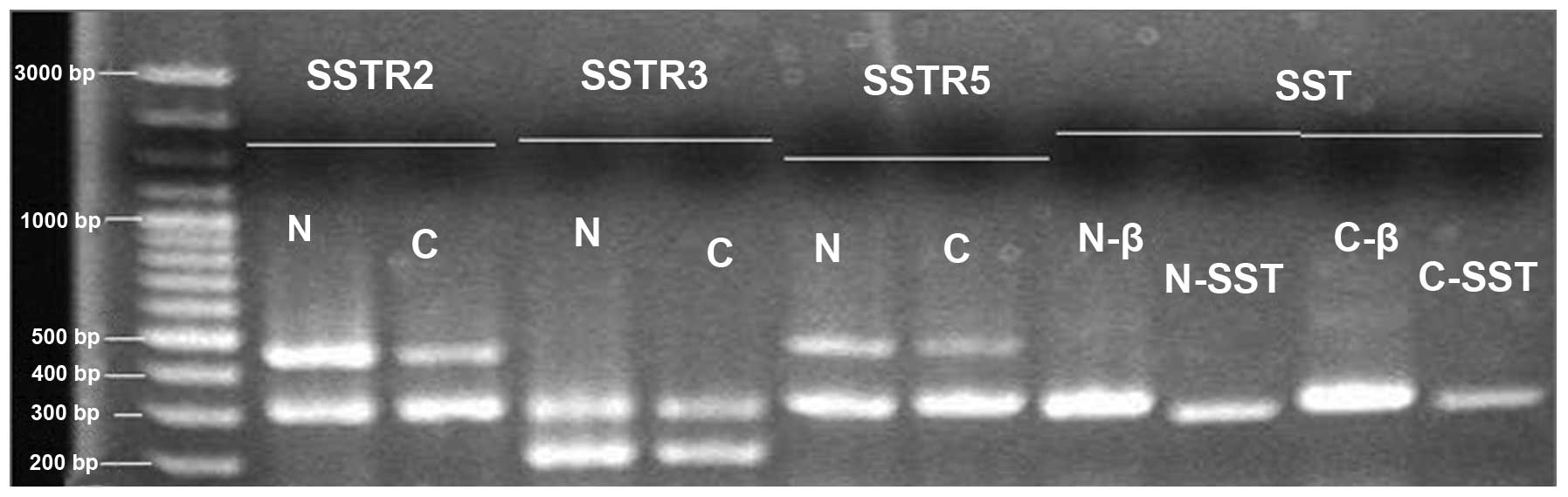 | Figure 2Expression of mRNA for SST, SSTR2,
SSTR3, SSTR5 in normal and cancer groups. Total RNA was isolated
from normal gastric tissue and gastric cancer. RT-PCR was used to
amplify SST and SSTR cDNAs with gene-specific primers. PCR products
were separated on 1.5% agarose gel and visualized following
ethidium bromide staining. N, normal group; C, cancer group; β,
β-actin; SST, somatostatin, SSTR, SST receptor; RT-PCR, reverse
transcription-PCR. |
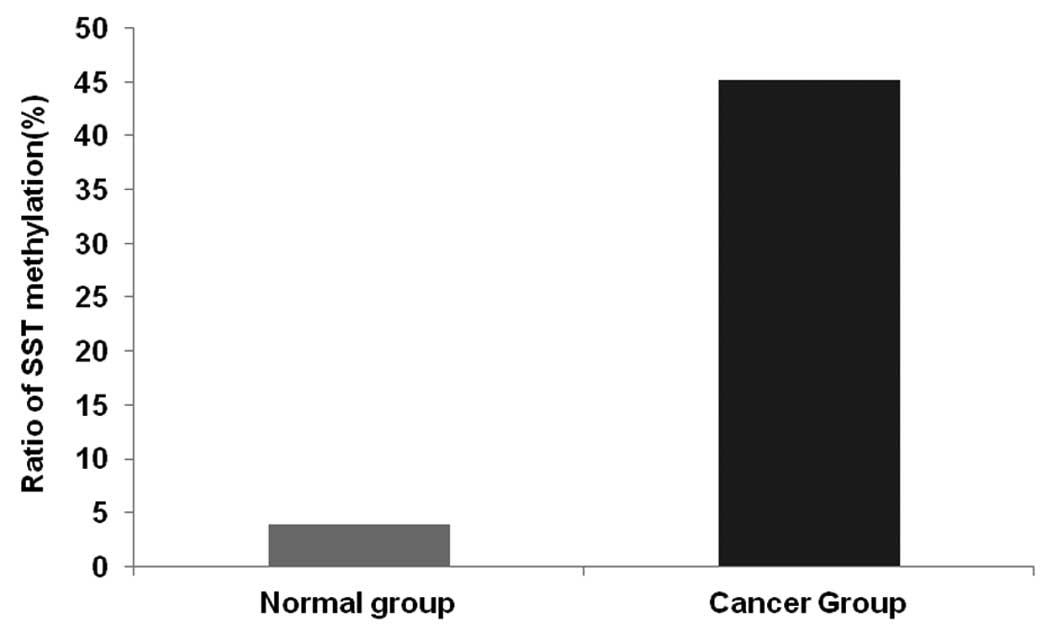
DNA methylation of the SST gene
correlates with SST expression
The DNA methylation level of SST in the two groups
was detected using qMSP. A single melt peak demonstrated that the
quality of the primer was high and no useless introductions were
identified in the assay. When the reaction reached the
concentration threshold (CT), the CT value was 10–13 reaction
cycles in the amplification curves, which demonstrated that the
amplification was effective (Table
II).
 | Table IIDistribution of methylation cases for
SST in pair of groups. |
Table II
Distribution of methylation cases for
SST in pair of groups.
| Normal group | |
|---|
|
| |
|---|
| Cancer group | + | − | Total |
|---|
| + | 0 | 23 | 23 |
| − | 2 | 26 | 28 |
| Total | 2 | 49 | 51 |
The positive rate of DNA methylation for SST in the
carcinoma group was markedly higher than that of the normal group
(23/51 vs. 2/51; Fig. 4). The
distribution of the positive methylation cases for SST in patients
with gastric cancer did not correlate with the age or gender of the
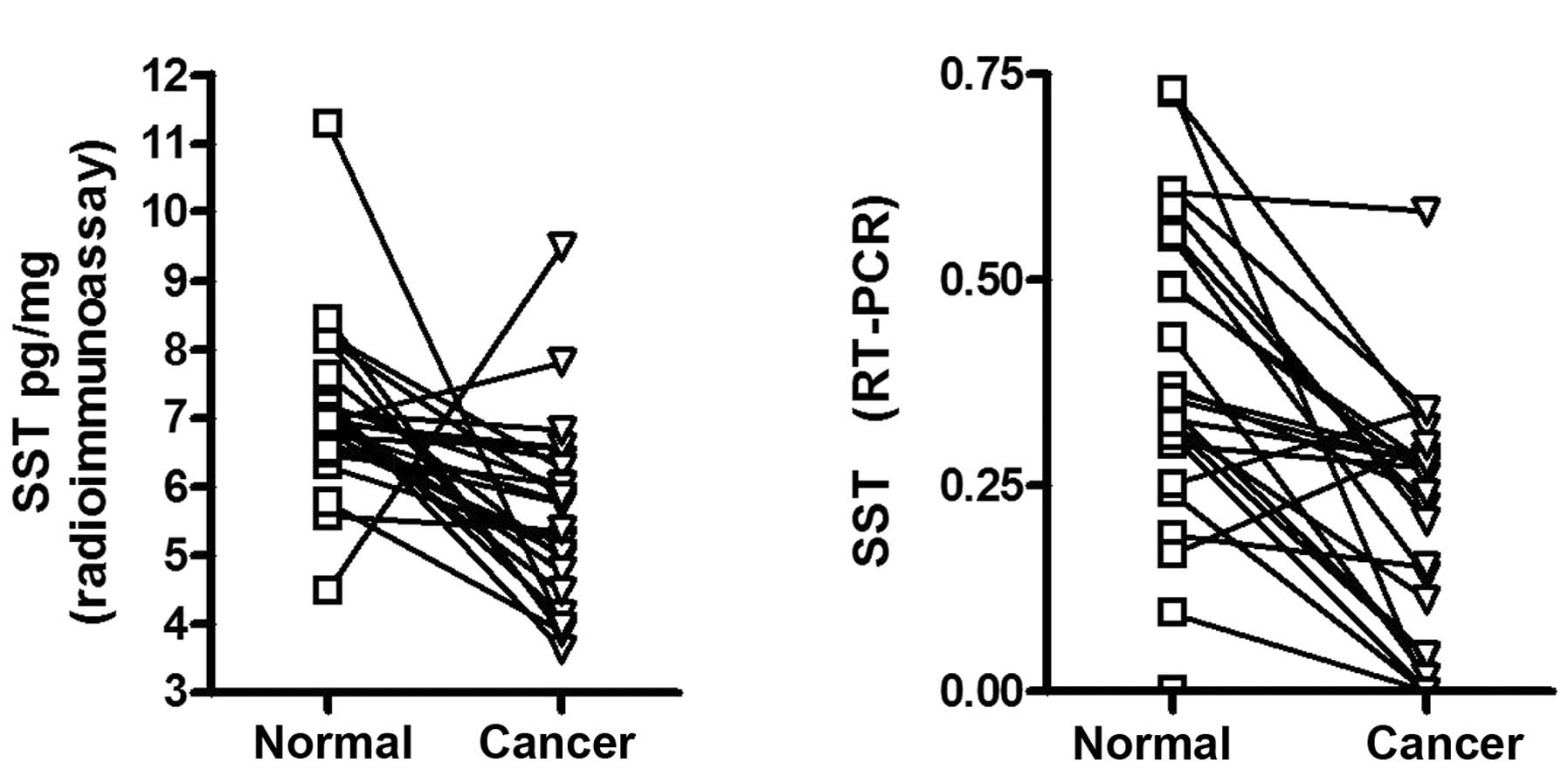
patients or the size, location and type of the carcinoma. In the 25
patients with DNA methylation of SST, the protein and mRNA levels
of SST were significantly lower in the non-methylation tissues
(Fig. 5).
Discussion
The occurrence and development of gastric cancer has
been regarded as a long-term, multi-phase and multi-gene
co-impacting process that is affected by various external factors
and genetic mutations, which manifest as gastrointestinal diseases
and external disorders (10). Among
them, the lack of genetic fragmentation, mutation and the
methylation of the DNA promoter region are considered as main
factors that lead to the emergence and development of human cancer
at a molecular level. A number of studies have suggested that
numerous tumor suppressor genes undergo methylation of the DNA
promoter region in gastric cancer, including p16, hMLH1, Runx3,
PTEN and XAF1 (11–15). The genes are involved in gene
repair, cell signal transduction, apoptosis, cell cycle regulation
and angiogenesis. Sections of the gene that are rich in CpG
dinucleotides are called the CpG islands, which are significant
targets for DNA methylation. CpG islands exist in >60% of gene
promoter regions, but are not evenly distributed within the genome
(16,17). CpG islands are often located in the
regulatory area of eukaryotic housekeeping genes, and the potential
inactivating effect of methylation has gained interest with regard
to the association between the DNA methylation of the CpG islands
in tumor suppressor gene promoter regions and cancer.
SST is widely distributed in the gastrointestinal
tract and plays a significant role in maintaining the internal
environment homeostasis. Several in vitro and in vivo
studies have suggested that SST functions as a tumor suppressor
gene in human cancers and may inhibit tumor growth through
mechanisms that involve the inhibition of growth factors and
hormones and a reduction in the vascularization (6,7,18). The
present study identified that the protein level of SST in the
normal group was 7.399±0.956 pg/mg, which was significantly higher
than that in the cancer group (5.091±0.994 pg/mg). Furthermore, the
current study demonstrated that SST mRNA expression was
significantly lower in the tumor group (0.218±0.183) compared with
that in the normal group (0.456±0.331), as measured using RT-PCR.
However, the role of DNA methylation in decreasing the expression
of SST in gastric cancer remains undetermined.
The present study used qMSP technology for the
analysis of promoter DNA methylation in SST. An increased DNA
methylation level was detected in the gastric cancer tissues
compared with that in the normal gastric mucosa samples. This
result suggested that epigenetic mechanisms may be the leading
cause of silencing SST expression in gastric cancer. In addition to
gastric cancer (9), DNA
hypermethylation has also been identified in colon and esophageal
cancer (19,20). The silencing of SST may be a
critical step in gastrointestinal tract carcinogenesis. Several
studies have confirmed that SST and its analogs may be able to
inhibit the growth of cancers (8,18). Our
previous studies also identified that octreotide, as one of the SST
analogs, was able to inhibit the growth of gastric cancer and
induce the apoptosis of gastric cancer cells in vitro and
in vivo(6,7). Further studies are required to
investigate the inhibition of endogenous SST expression in tumor
tissues, in addition to exogenous SST expression. As a reversible
DNA modification, methylation may be reversed using demethylation
drugs, including 5-Aza-dC. Treatment with the demethylation drug
reverses the status of methylation and recovers SST mRNA
expression. This may contribute to an enhanced curative effect in
gastric cancer. At present, this has been confirmed by certain
in vitro experiments (9,19,20).
However, further studies are required in order to apply it to
clinical treatment.
SST may predominantly function by directly combining
with specific SSTRs 1–5 and subsequently activating a variety of
signal transduction pathways. Among the receptors, SSTR2, SSTR3 and
SSTR5 are closely associated with gastrointestinal cancer.
Therefore, SSTR expression levels and the anti-proliferation
effects of SST are closely associated. In the present study, by
detecting the mRNA expression levels of SSTRs in the normal and
cancer groups using RT-PCR, the SSTR mRNA expression levels in the
cancer group were lower than those in the normal group, which
revealed that the reduction of mRNA expression occurred for not
only SST but also SSTRs. Whether the reduction of mRNA expression
for SSTRs was associated with the DNA methylation in the promoter
region or the other factors remains to be elucidated. Previous
studies have confirmed that the DNA methylation of SSTRs exists in
numerous types of cancer cells (21,22).
In summary, the present study demonstrated that SST
promoter methylation is frequently observed in gastric cancer
tissue and is associated with a decrease in SST protein expression.
This observation provides a foundation for targeting SST in the
treatment of gastric cancer. However, further studies are required
to confirm whether SSTR promoter methylation exists in gastric
cancer and how it may be demethylated effectively.
Acknowledgements
This study was funded by the National Scientific
Fund of China (grant no. 81070294).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Sapari NS, Loh M, Vaithilingam A and Soong
R: Clinical potential of DNA methylation in gastric cancer: a
meta-analysis. PLoS One. 7:e362752012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
González CA and Agudo A: Carcinogenesis,
prevention and early detection of gastric cancer: where we are and
where we should go. Int J Cancer. 130:745–753. 2012.PubMed/NCBI
|
|
4
|
Yamamoto E, Suzuki H, Takamaru H, Yamamoto
H, Toyota M and Shinomura Y: Role of DNA methylation in the
development of diffuse-type gastric cancer. Digestion. 83:241–249.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yamashita K, Sakuramoto S and Watanabe M:
Genomic and epigenetic profiles of gastric cancer: potential
diagnostic and therapeutic applications. Surg Today. 41:24–38.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang CH, Tang CW, Liu CL and Tang LP:
Inhibitory effect of octreotide on gastric cancer growth via MAPK
pathway. World J Gastroenterol. 9:1904–1908. 2003.PubMed/NCBI
|
|
7
|
Tang C, Liu C, Zhou X and Wang C: Enhanced
inhibitive effects of combination of rofecoxib and octreotide on
the growth of human gastric cancer. Int J Cancer. 112:470–474.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xie Y, Chen S, Wang CH and Tang CW: SOM230
combined with celecoxib prolongs the survival in nude mice with
HepG-2 xenografts. Cancer Biol Ther. 12:86–92. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jackson K, Soutto M, Peng D, Hu T, Marshal
D and El-Rifai W: Epigenetic silencing of somatostatin in gastric
cancer. Dig Dis Sci. 56:125–130. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fuchs CS and Mayer RJ: Gastric carcinoma.
N Engl J Med. 333:32–41. 1995. View Article : Google Scholar
|
|
11
|
Abbaszadegan MR, Moaven O, Sima HR,
Ghafarzadegan K, A’rabi A, Forghani MN, Raziee HR, Mashhadinejad A,
Jafarzadeh M, Esmaili-Shandiz E and Dadkhah E: p16 promoter
hypermethylation: a useful serum marker for early detection of
gastric cancer. World J Gastroenterol. 14:2055–2060. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fleisher AS, Esteller M, Tamura G, Rashid
A, Stine OC, Yin J, Zou TT, Abraham JM, Kong D, Nishizuka S, et al:
Hypermethylation of the hMLH1 gene promoter is associated with
microsatellite instability in early human gastric neoplasia.
Oncogene. 20:329–335. 2001. View Article : Google Scholar
|
|
13
|
Homma N, Tamura G, Honda T, Matsumoto Y,
Nishizuka S, Kawata S and Motoyama T: Spreading of methylation
within RUNX3 CpG island in gastric cancer. Cancer Sci. 97:51–56.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kang YH, Lee HS and Kim WH: Promoter
methylation and silencing of PTEN in gastric carcinoma. Lab Invest.
82:285–291. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zou B, Chim CS, Zeng H, Leung SY, Yang Y,
Tu SP, Lin MC, Wang J, He H, Jiang SH, et al: Correlation between
the single-site CpG methylation and expression silencing of the
XAF1 gene in human gastric and colon cancers. Gastroenterology.
131:1835–1843. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gardiner-Garden M and Frommer M: CpG
Islands in vertebrate genomes. J Mol Biol. 196:261–282. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bird AP: CpG-rich islands and the function
of DNA methylation. Nature. 321:209–213. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao B, Yang P, Yang J and Cai D: A
randomized trial of somatostatin to regulate the VEGFs/VEGFRs in
patients with gastric cancer. Hepatogastroenterology. 58:1425–1430.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jin Z, Mori Y, Hamilton JP, Olaru A, Sato
F, Yang J, Ito T, Kan T, Agarwal R and Meltzer SJ: Hypermethylation
of the somatostatin promoter is a common, early event in human
esophageal carcinogenesis. Cancer. 112:43–49. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mori Y, Cai K, Cheng Y, Wang S, Paun B,
Hamilton JP, Jin Z, Sato F, Berki AT, Kan T, et al: A genome-wide
search identifies epigenetic silencing of somatostatin,
tachykinin-1, and 5 other genes in colon cancer. Gastroenterology.
131:797–808. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Torrisani J, Hanoun N, Laurell H, Lopez F,
Maoret JJ, Souque A, Susini C, Cordelier P and Buscail L:
Identification of an upstream promoter of the human somatostatin
receptor, hSSTR2, which is controlled by epigenetic modifications.
Endocrinology. 149:3137–3147. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu Z, Marquez M, Nilsson S and Holmberg
AR: Incubation with somatostatin, 5-aza decitabine and trichostatin
up-regulates somatostatin receptor expression in prostate cancer
cells. Oncol Rep. 20:151–154. 2008.PubMed/NCBI
|