Introduction
Myelodysplastic syndromes (MDSs) are clonal
hematopoietic stem cell disorders characterized by ineffective
dysplastic hematopoiesis involving one or more cell lineages, and
by peripheral-blood cytopenias with a high risk of progression to
acute myeloid leukemia (AML) (1).
MDS is most prevalent in white males and the incidence increases
markedly with age. The documented disease burden is expected to
increase in the near future, due to an aging population and
improving awareness of the disease. Approved therapies, including
lenalidomide, azacitidine and decitabine are now available for
patients who are ineligible for potentially curative hematopoietic
stem cell transplantation. These achieve hematological improvement
and enhance the quality of life of patients who previously would
have received supportive care alone. Thus, the mainstay of
treatment for patients with MDS is supportive red blood cell (RBC)
transfusions. Repeated transfusions eventually lead to iron
overload with an increased risk of associated comorbidity and
mortality that is independent of the underlying hematological
disease (2). Retrospective studies
have revealed that iron toxicity associated with transfusion burden
is associated with reduced survival in patients with MDS (3,4). This
is particularly problematic in patients with lower risk MDS due to
the longer life expectancy.
The most common non-leukemia-related causes of
mortality are cardiac failure (51%) and infections (31%) (3). Infections may be due to underlying
neutropenia or to transfusional iron overload (5). There is growing evidence that adequate
iron chelation therapy improves survival in International
Prognostic Scoring System (IPSS) lower-risk MDS patients with iron
overload and may delay AML transformation (1,6–9).
Guidelines recommend that iron overload be managed with chelation
therapy (10).
Until recently, desferoxamine and deferiprone were
the only drugs available for the treatment of transfusional iron
overload. However, neither provides satisfactory chelation therapy
for controlling iron toxicity. According to the pharmacokinetic
properties of desferoxamine, in order to be effective, it must be
administered as a slow infusion over the course of 8–12 h and this
must be repeated 5–7 days/week. This regimen is contraindicated in
patients with thrombocytopenia and the inconvenience often results
in low compliance (11).
Deferiprone is not approved for MDS and is not recommended, as it
causes neutropenia and agranulocytosis (12).
Deferasirox is a once-daily orally administered iron
chelator approved for the treatment of iron overload in patients
with transfusion-dependent anemias. It is efficacious and has an
acceptable safety profile in adult and pediatric patients with
transfusion-dependent thalassemia major and by various other
chronic anemias (13). Recent
guidelines from the Italian Society of Haematology recommend iron
chelation with deferasirox for the treatment of low or
intermediate-1 IPSS risk patients with MDS after they have received
≥20 units of packed RBCs (10). The
initial dose of 10 mg/kg may be increased to 20–30 mg/kg based on
iron transfusion load, serum ferritin (SF) levels and organ damage
due to iron overload. In addition to reducing markers of iron
overload, including SF, a number of recently published studies have
reported improvements in hematological parameters and transfusion
requirements in a portion of patients receiving iron chelation
therapy with deferasirox (14–16,
reviewed in 17). However,
experience outside of clinical trials is lacking. We have
investigated the safety and effectiveness of deferasirox therapy in
reducing iron overload and transfusion requirements in a
non-selected population of polytransfused low-risk MDS patients in
a routine clinical setting.
Patients and methods
Patients affected by low-risk MDS who had been
transfusion-dependent for ≥1 year, had SF levels ≥2,000 ng/ml
before starting iron chelation therapy and required ≥1 unit of
RBCs/month to maintain Hb levels ≥8 g/dl were enrolled. Thus, these
patients were eligible for analysis according to International
Working Group 2006 criteria (18).
The study was approved by the ethics committee of San Gennaro
Hospital (Naples, Italy) and written informed consent was obtained
from the patients or the patient’s family. Patients were treated at
the San Gennaro Hospital (Naples, Italy) or the San G. Moscati
Hospital (Avellino, Italy) between June 2006 and June 2012. All
patients had low-risk disease, with IPSS scores of low or
intermediate-1, and had been receiving only best supportive
care.
Patients were treated according to the standard
procedures at the enrolling centers. Initially, patients received
deferasirox (10 mg/kg) orally once daily, as recommended by the
Italian Society of Haematology, Italian Society of Sperimental
Haematology and Italian Group for Bone Marrow Transplantation,
Haematopoietic Stem Cells and Cell Therapy guidelines (10). Subsequent dosage adjustments in
increments of 10 mg/kg/day were based on efficacy in terms of
reduction in SF and safety parameters. The maximum dosage was 30
mg/kg/day (Table I).
 | Table IDeferasirox dosage for all 55
consecutive patients. |
Table I
Deferasirox dosage for all 55
consecutive patients.
| Dosage | Patients (n=55) |
|---|
| Starting dose,
mg/kg/day | 10 |
| Median daily
deferasirox dose over the course of the study, mg/kg/day
(range) | 23 (10–30) |
| Dose adjustments for
starting dosea, n (%) |
| Unchanged | 10 (18) |
| Increased | 45 (82) |
| Dose adjustments
during treatmentb, n (%) |
| Unchanged | 38 (69) |
| Increased | 11 (20) |
| Reduced | 6 (11) |
Patients had an initial visit at baseline and
follow-up visits at ~2-week intervals thereafter. At each visit,
the deferasirox dosage, number of blood transfusions received, and
changes in concomitant medications and iron intake were recorded.
Hematological response was determined according to International
Working Group 2006 criteria (18).
Laboratory examinations for renal function (serum creatinine, 24-h
creatinine excretion and creatinine clearance using the
Cockcroft-Gault and Modification of Diet in Renal Disease formulae)
and hepatic function (serum transaminases, bilirubin, alkaline
phosphatase and γ-glutamyl transpeptidase) were performed at
baseline and then weekly for the first month of treatment, monthly
for the next 5 months and bimonthly thereafter. Examination of the
ocular fundus and audiometric tests were performed at baseline and
then every 6 months.
Results
We enrolled 55 consecutive unselected patients
affected by low-risk MDS who had been transfusion-dependent for ≥1
year. All patients (33 males and 22 females; median age, 70 years)
had SF levels ≥2,000 ng/ml before starting iron chelation and
required ≥1 unit of RBCs/month to maintain Hb levels at ≥8 g/dl
(Table II). Four of the 55
patients had received prior chelation therapy with desferoxamine,
which had been discontinued due to renal toxicity. The median
transfusion requirement before starting treatment was 3
units/month.
 | Table IIDemographic and clinical
characteristics of 55 patients at diagnosis. |
Table II
Demographic and clinical
characteristics of 55 patients at diagnosis.
| Characteristics | Patients |
|---|
| Male, n (%) | 33 (60) |
| Female, n (%) | 22 (40) |
| Median age, years
(range, IQR) | 70 (58–79, 9) |
| WHO classification,
n |
| RA | 30 |
| RARS | 16 |
| RCMD | 8 |
| RCMD-RS | 1 |
| Transfusion
requirement, unit/month, mean ± SD | 2.9±0.95 |
| Prognosis (IPSS),
n | |
| Low | 32 |
| Intermediate-1 | 23 |
| Prognosis (WPSS),
n |
| Low | 41 |
| Intermediate-1 | 14 |
| Serum ferritin,
ng/ml, mean ± SD | 2362±172 |
| Hb, g/dl, mean ±
SD | 7.3±0.17 |
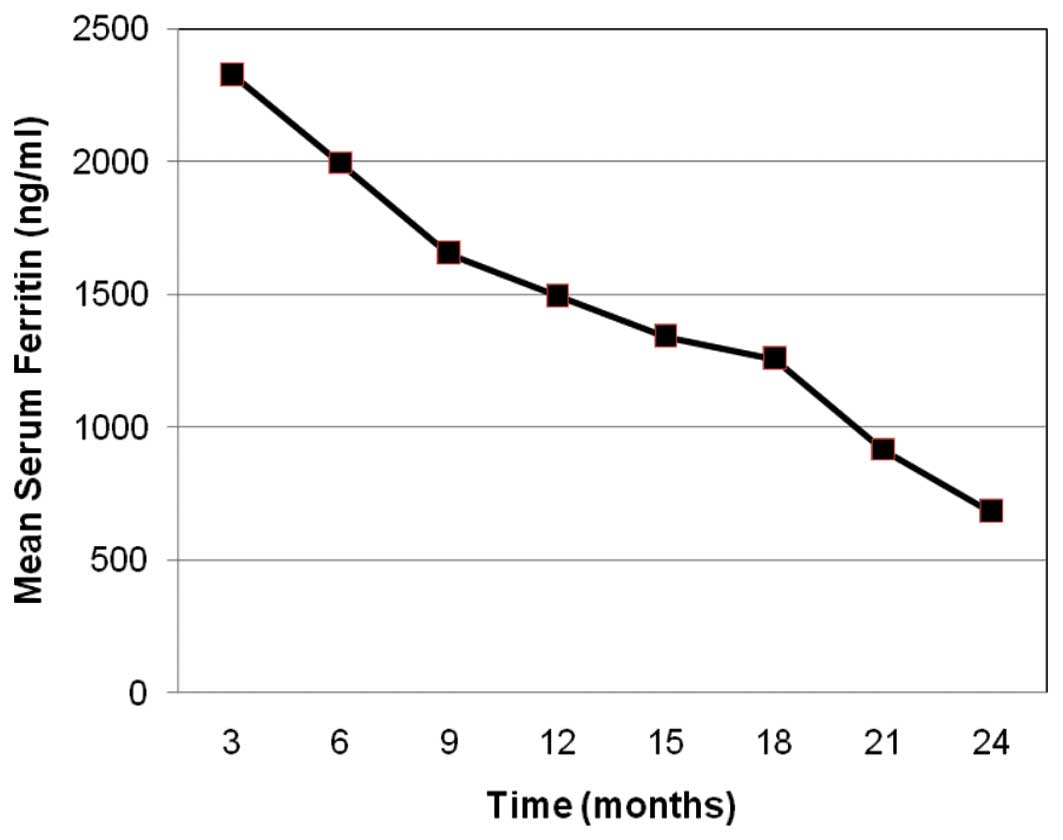
To date, all patients had >24 months of
follow-up. Mean iron intake was 0.31 mg/kg/day at the baseline and
0.26 mg/kg/day after 24 months of therapy. All patients exhibited
significant decreases in iron overload, as measured by SF, which
were maintained after 2 years of therapy (mean absolute decrease in
SF from baseline, 1,679±209 ng/ml; range 1,250–2,100 ng/ml),
corresponding to a mean reduction of 71%. The rate of decrease in
SF levels was relatively constant over the 24-month observation
period (Fig. 1).
A sustained reduction in transfusion requirement was
recorded in 16 of the 55 patients. Transfusion requirements were
reduced by at least one transfusion per month after 6 months of
therapy in all 16 patients who responded and were followed in all
cases by further improvements at 24 months, meeting 2006
International Working Group criteria for hematological response
(Table III). All 16 patients had
erythroid responses, including one in a patient with multilinear
dysplasia. There were no significant increases in neutrophil or
platelet counts.
 | Table IIICharacteristics of 16 patients meeting
2006 International Working Group criteria for haematological
improvement while receiving deferasirox. |
Table III
Characteristics of 16 patients meeting
2006 International Working Group criteria for haematological
improvement while receiving deferasirox.
| Gender | Age (years) | Histological
classification | Prognosis (IPSS) | Transfusion support
(units/month) | Serum ferritin
(ng/ml) | Haemoglobin
(g/dl) |
|---|
|
|
|
|---|
| Baseline | 6 months | 24 months | Baseline | 24 months | Baseline | 24 months | Change |
|---|
| M | 72 | RA | INT-1 | 3 | 2 | 1 | 2400 | 850 | 7.0 | 8.6 | 1.6 |
| M | 72 | RARS | INT-1 | 3 | 2 | 1 | 2420 | 850 | 7.3 | 8.8 | 1.5 |
| M | 72 | RA | INT-1 | 3 | 2 | 1 | 2440 | 850 | 7.3 | 8.8 | 1.5 |
| M | 60 | RA | LOW | 3 | 2 | 1 | 2350 | 750 | 7.3 | 8.9 | 1.6 |
| M | 73 | RCMD | INT-1 | 3 | 2 | 1 | 2450 | 850 | 7.3 | 8.8 | 1.5 |
| M | 60 | RA | LOW | 3 | 2 | 1 | 2350 | 750 | 7.4 | 9.0 | 1.6 |
| F | 72 | RA | LOW | 3 | 2 | 1 | 2100 | 450 | 7.3 | 8.8 | 1.5 |
| M | 60 | RA | LOW | 3 | 2 | 1 | 2450 | 750 | 7.2 | 8.8 | 1.6 |
| M | 67 | RARS | LOW | 4 | 3 | 2 | 2540 | 700 | 7.0 | 8.6 | 1.6 |
| M | 67 | RARS | LOW | 4 | 3 | 2 | 2540 | 700 | 7.0 | 8.6 | 1.6 |
| M | 67 | RARS | LOW | 4 | 3 | 2 | 2540 | 700 | 7.0 | 8.6 | 1.6 |
| M | 74 | RA | LOW | 3 | 1 | 1 | 2350 | 800 | 7.2 | 8.7 | 1.5 |
| F | 74 | RA | LOW | 3 | 1 | 1 | 2350 | 800 | 7.4 | 9.1 | 1.7 |
| M | 74 | RARS | INT-1 | 3 | 1 | 1 | 2350 | 800 | 7.0 | 8.6 | 1.6 |
| F | 67 | RA | LOW | 4 | 3 | 1 | 2340 | 700 | 7.0 | 8.5 | 1.5 |
| M | 74 | RA | LOW | 3 | 1 | 0 | 2350 | 800 | 7.0 | 8.8 | 1.8 |
The mean monthly transfusion requirement at baseline
was 3.25 units for the 16 patients who responded compared with 2.75
units among patients who did not have a hematological response;
mean age was 69 years in the two groups. Among the patients who
responded, less than one-fourth (3/16) were female, compared with
approximately half of non-responders (19/39).
At baseline, these 16 patients had a mean
transfusion requirement of 3.3 units/month, which decreased to 2.0
units/month after 6 months of treatment and to 1.1 units/month
after 24 months. These values were relatively unchanged in the
remaining patients, who required a mean 2.8, 2.7 and 2.8
units/month at baseline, 6 and 12 months, respectively. One patient
became transfusion-independent after 13 months of iron chelation
therapy. The patient was transfusion-free (SF, 800 ng/ml) with a
hemoglobin level of ~8.8 g/dl in December 2012, 30 months after
starting iron chelation therapy.
With regard to the onset of improvements in
erythropoiesis, in 7/16 patients this occurred by 6 months, while
all 16 patients had responses meeting International Working Group
2006 criteria after 12 months of treatment. Erythroid responses
were observed when SF levels had been reduced by ~40% compared with
baseline. There was a persistent reduction of transfusion
requirements in approximately one third of patients.
Adverse events (AE) were documented in 34 patients
(61.8%). All AEs were mild in severity (grade 1–2) and did not
require discontinuation of therapy. The most common drug-related
AEs were diarrhea, nausea, rash and headache (Table IV). There were no clinically
significant alterations of renal or hepatic function. Similarly,
optical and audiometric tests were normal.
 | Table IVAdverse events recorded in all 55
patients through 24 months of therapy with deferasirox. All adverse
events were grade 1–2. |
Table IV
Adverse events recorded in all 55
patients through 24 months of therapy with deferasirox. All adverse
events were grade 1–2.
| Adverse event | Events, n | Patients, n
(%) |
|---|
| Diarrhea | 20 | 15 (27.0) |
| Nausea | 11 | 10 (18.0) |
| Rash | 8 | 8 (14.5) |
| Headache | 8 | 8 (14.5) |
Discussion
Iron overload in patients with transfusion-dependent
MDS is an important clinical problem, as secondary hemosiderosis
may lead to organ failure in patients with a long life expectancy.
Moreover, several studies have shown that the survival of patients
with MDS is affected by transfusion dependence, which is a poor
independent prognostic factor (19). Iron overload also has a negative
impact on the success of stem cell transplantation (20).
In order to prevent iron toxicity, current
guidelines indicate that iron chelation therapy should be
considered in patients with SF levels of 1,000 ng/ml and who
require 2 units RBC transfusions per month for ≥1 year.
Our findings are in agreement with results of the
Evaluation of Patients’ Iron Chelation with Exjade®
(EPIC) study, the largest prospective evaluation of an iron
chelation therapy conducted to date, which confirmed that
deferasirox is an efficacious and generally well-tolerated
treatment for iron overload in patients with transfusion-dependent
disorders, including β-thalassemia and MDS (19). Our results, based on over 24 months
of follow-up in non-selected MDS patients treated in a routine
clinical setting, confirm the effectiveness and safety of
deferasirox therapy in reducing the iron overload in polytransfused
MDS patients.
We also identified a subset of 16 patients who had a
reduction in transfusion requirement meeting International Working
Group criteria for hematological response. This is in agreement
with previous observations of hematological improvement with
chelation therapy (reviewed in 17).
Moreover, a post hoc analysis of a subgroup of 341
patients with myelodysplastic syndromes enrolled in the EPIC study
also identified hematological responses to deferasirox in a cohort
of iron-overloaded patients (15).
In this study, erythroid hematopoietic responses, according to
International Working Group 2006 criteria, were observed in 53 of
247 patients eligible for analysis (21.5%) and there was a trend
toward greater reductions in SF in patients with hematological
responses. The erythroid response rate using the same criteria was
similar in our study (29%, 16/55).
The results of the multicenter prospective Gimema
trial of 152 consecutive patients (median age, 72 years) with low
or intermediate-1 risk MDS were reported (16). After one year of deferasirox
treatment, 22 patients had achieved transfusion independence,
defined as no transfusion requirements for three consecutive
months. The probability of acquiring transfusion independence after
one year was 19.7% (95% CI, 19.4–20). While a similar percentage of
our patients achieved a hematological response, only one patient
achieved complete transfusion independence.
List et al(14) enrolled 176 patients with low or
intermediate-1-risk MDS and SF levels >1,000 mg/l to receive
treatment with deferasirox. The median reduction in SF was 36.7% in
the 49 patients (28%) who completed 24 months of treatment. This is
considerably less than our finding of a median reduction in SF of
70.2% at 24 months. Additionally, the rate of hematological
erythroid responses defined by International Working Group 2006
criteria was lower in this study (30%, 51/176), compared with that
in our patients (29%, 16/55).
However, the mechanism underlying the hematological
response is not known. Reduction of oxidative stress caused by
excess free iron in bone marrow has been implicated in bone marrow
toxicity (21). Reduction of labile
iron with chelation therapy has been proposed as the mechanism
underlying hematological responses (17). Ghoti et al(22) measured labile iron (the redox-active
form) and reactive oxygen species in blood cells from patients who
had received deferasirox therapy for 3 months. The authors
identified significant reductions in reactive oxygen species and
lipid peroxidation, as well as an increase in the antioxidant,
reduced glutathione.
A direct effect of deferasirox on hematopoiesis
through the nuclear factor-κB (NF-κB) pathway has been proposed,
based on in vitro experiments showing strong,
iron-independent inhibition of NF-κB by deferasirox, but not other
chelators (23). However, there is
also limited evidence of hematological improvement in patients with
MDS treated with desferoxamine (24), which argues against a direct role
for deferasirox. Gattermann et al(15) found that, while treatment with
deferasirox reduced labile iron to <0.4 μmol/l, there was no
difference between hematological responders and non-responders. If
removal of iron is labile, since it is the underlying mechanism of
the hematological response, one may expect to see a difference
between these groups.
The timing of hematological responses may give an
indication of the mechanism. Seven of the patients in the present
study had responded after 6 months of treatment and all 16 patients
responded after 12 months. Patients appeared to respond when they
had reached an ~40% reduction in SF. In the 3-year study by List
et al(14), the median time
to any type of hematological response was also ~6 months.
Further studies are warranted to define the role of
deferasirox in reducing the blood transfusion request in a subset
of patients affected by MDS that is refractory to other
conventional therapies.
Acknowledgements
The authors would like to thank all the patients and
investigators who took part in the study.
References
|
1
|
Neukirchen J, Fox F, Kündgen A, Nachtkamp
K, Strupp C, Haas R, Germing U and Gattermann N: Improved survival
in MDS patients receiving iron chelation therapy - a matched pair
analysis of 188 patients from the Düsseldorf MDS registry. Leuk
Res. 36:1067–1070. 2012.PubMed/NCBI
|
|
2
|
Dreyfus F: The deleterious effects of iron
overload in patients with myelodysplastic syndromes. Blood Rev.
22(Suppl 2): S29–S34. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Malcovati L, Porta MG, Pascutto C,
Invernizzi R, Boni M, Travaglino E, Passamonti F, Arcaini L,
Maffioli M, Bernasconi P, et al: Prognostic factors and life
expectancy in myelodysplastic syndromes classified according to WHO
criteria: a basis for clinical decision making. J Clin Oncol.
23:7594–7603. 2005. View Article : Google Scholar
|
|
4
|
Delea TE, Hagiwara M and Phatak PD:
Retrospective study of the association between transfusion
frequency and potential complications of iron overload in patients
with myelodysplastic syndrome and other acquired hematopoietic
disorders. Curr Med Res Opin. 25:139–147. 2009. View Article : Google Scholar
|
|
5
|
Toma A, Fenaux P, Dreyfus F and Cordonnier
C: Infections in myelodysplastic syndromes. Haematologica.
97:1459–1470. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rose C, Brechignac S, Vassilief D, et al:
Does iron chelation therapy improve survival in regularly
transfused lower risk MDS patients? A multicenter study by the GFM
(Groupe Francophone des Myélodysplasies). Leuk Res. 34:864–870.
2010.PubMed/NCBI
|
|
7
|
Komrokji RS, Al Ali NH, Padron E, Lancet
JE and List AF: Impact of iron chelation therapy on overall
survival and AML transformation in lower risk MDS patients treated
at the Moffitt Cancer Center. Blood (ASH Annual Meeting Abstracts).
118:27762011.
|
|
8
|
Lyons RM, Marek BJ, Paley C, et al:
Relationship between chelation and clinical outcomes in 600
lower-risk MDS ratients: registry analysis at 36 months. Blood (ASH
Annual Meeting Abstracts). 120:38002012.
|
|
9
|
de Swart L, Smith A, Fenaux P, et al:
Early mortality in 1000 newly diagnosed MDS patients with low- and
intermediate-1 risk MDS in the European Leukemianet MDS (EUMDS)
registry. Blood (ASH Annual Meeting Abstracts). 120:38302012.
|
|
10
|
Santini V, Alessandrino PE, Angelucci E,
Barosi G, Billio A, Di Maio M, et al: Clinical management of
myelodysplastic syndromes: update of SIE, SIES, GITMO practice
guidelines. Leuk Res. 34:1576–1588. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Arboretti R, Tognoni G and Alberti D:
Italian Colaborative Group on Thalassarmia Pharmacosurveillance and
quality of care of thalassaemic patients. A large scale
epidemiological survey. Eur J Clin Pharmacol. 56:915–922. 2001.
View Article : Google Scholar
|
|
12
|
Cappellini MD and Piga A: Current status
in iron chelation in hemoglobinopathies. Curr Mol Med. 8:663–674.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Galanello R, Campus S and Origa R:
Deferasirox: pharmacokinetics and clinical experience. Expert Opin
Drug Metab Toxicol. 8:123–134. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
List AF, Baer MR, Steensma DP, Raza A,
Esposito J, Martinez-Lopez N, et al: Deferasirox reduces serum
ferritin and labile plasma iron in RBC transfusion dependent
patients with myelodysplastic syndrome. J Clin Oncol. 30:2134–2139.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gattermann N, Finelli C, Della Porta M, et
al: Hematologic responses to deferasirox therapy in
transfusion-dependent patients with myelodysplastic syndromes.
Haematologica. 97:1364–1371. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Angelucci E, Santini V, Di Tucci AA, et
al: Deferasirox chelation therapy in transfusion dependent MDS
patients. Final report from the GIMEMA MDS0306 prospective trial.
Blood (ASH Annual Meeting Abstracts). 120:4252012.
|
|
17
|
Guariglia R, Martorelli MC, Villani O,
Pietrantuono G, Mansueto G, D’Auria F, et al: Positive effects on
hematopoiesis in patients with myelodysplastic syndrome receiving
deferasirox as oral iron chelation therapy: a brief review. Leuk
Res. 35:566–570. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cheson BD, Greenberg PL, Bennett JM, et
al: Clinical application and proposal for modification of the
International Working Group (IWG) response criteria in
myelodysplasia. Blood. 108:419–425. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gattermann N, Finelli C, Della Porta M, et
al: Deferasirox in iron-overloaded patients with
transfusion-dependent myelodysplastic syndromes: Results from the
large 1-year EPIC study. Leuk Res. 34:1143–1150. 2010.PubMed/NCBI
|
|
20
|
Armand P, Kim HT, Cutler CS, Ho VT, Koreth
J, Alyea EP, Soiffer RJ and Antin JH: Prognostic impact of elevated
pretransplantation serum ferritin in patients undergoing
myeloablative stem cell transplantation. Blood. 109:4586–4588.
2007. View Article : Google Scholar
|
|
21
|
Yahata T, Takanashi T, Muguruma Y, Ibrahim
AA, Matsuzawa H, Uno T, Sheng Y, Onizuka M, Ito M, Kato S and Ando
K: Accumulation of oxidative DNA damage restricts the self-renewal
capacity of human hematopoietic stem cells. Blood. 118:2941–2950.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ghoti H, Fibach E, Merkel D, Perez-Avraham
G, Grisariu S and Rachmilewitz EA: Changes in parameters of
oxidative stress and free iron biomarkers during treatment with
deferasirox in iron-overloaded patients with myelodysplastic
syndromes. Haematologica. 95:1433–1434. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Messa E, Carturan S, Maffé C, Pautasso M,
Bracco E, Roetto A, et al: Deferasirox is a powerful NF-kappaB
inhibitor in myelodysplastic cells and in leukemia cell lines
acting independently from cell iron deprivation by chelation and
reactive oxygen species scavenging. Haematologica. 95:1308–1316.
2010. View Article : Google Scholar
|
|
24
|
Jensen PD, Jensen IM and Ellegaard J:
Desferoxamine treatment reduces blood transfusion requirements in
patients with myelodysplastic syndrome. Br J Haematol. 80:121–124.
1992. View Article : Google Scholar : PubMed/NCBI
|