Introduction
Colorectal cancer is a common malignant tumor of the
gastrointestinal tract, constituting ~10% of new colorectal cancer
cases each year. Despite major breakthroughs in the treatment of
colorectal cancer, 40–50% of patients are likely to develop local
or distant tumor recurrences, and survival rates are poor (1). Therefore, the development of a novel
therapeutic target is required, with the aim of increasing the
survival rates of colorectal cancer patients.
Ca2+-dependent phospholipid-binding
proteins, Annexins, are involved in cellular processes, including
apoptosis, proliferation and differentiation (2–4).
Overexpression of Annexin A1 has been found in numerous cancer
types (5,6). Annexin A2 induces angiogenesis by
regulating the extracellular matrix metalloproteinase inducer.
Furthermore, overexpression of Annexin A2 has been associated with
the development, invasion and distant metastases of various tumors
(7–11). Compared with other Annexins, few
previous studies have investigated the function of Annexin A3.
Köllermann et al previously reported that Annexin A3
staining was markedly decreased or absent in prostate cancer and
was found to correlate inversely with pT stage and Gleason grade
(12).
Yan et al(13) previously showed that the increased
expression of Annexin A3 is a mechanism of platinum resistance in
ovarian cancer. Similarly, the Annexin A3 gene is silenced by miRNA
induced apoptosis and inhibits the growth of human gallbladder
cancer cells (14). However, to
date, a correlation between Annexin A3 expression and colorectal
cancer has not been reported.
Hypoxia-inducible factor-1α (HIF-1α) has been found
to correlate with the increased expression of vascular endothelial
growth factor (VEGF) in various cancer types (15,16).
The overexpression of HIF-1α has been associated with the poor
prognosis of various types of cancer (17,18).
Considering the important role of HIF-1α in the angiogenesis of
tumors, HIF-1α is a target for cancer therapy. Similarly, Annexin
A3 has been associated with the stimulation of VEGF expression and
may be a regulator of angiogenesis. Park et al(19) found that Annexin A3-overexpressing
human embryonic kidney (HEK) 293 cells induce the migration and
tube formation of human umbilical vein endothelial cells.
Furthermore, in HEK 293 cells, the expression of Annexin A3
activates HIF-1 transactivation activity, which indicates that
Annexin A3 may regulate the HIF-1 signaling pathway. Therefore,
Annexin A3 and HIF-1α have been hypothesized to be vital in the
angiogenesis of cancer. However, no previous studies have
investigated the correlation between Annexin A3 and HIF-1α
expression in types of human cancer. In this study, the expression
of Annexin A3 and HIF-1α was detected in colorectal cancer. The
objective was to ascertain whether Annexin A3 is involved in the
progression of colorectal cancer, and to investigate its
correlation with HIF-1α. Additionally, the impact of Annexin A3 and
HIF-1α on patient outcome was evaluated.
Materials and methods
Patients and tissues
A total of 60 unselected formalin-fixed and
paraffin-embedded samples from colorectal cancer patients were
obtained from the Department of Pathology, Xiangya Hospital of
Central-South University (Changsha, China), between 1999 and 2001.
All samples were diagnosed by two pathologists. Clinical and
pathological results were investigated prospectively and the
specific tumor registry was determined after surgery and follow-up.
The follow-up deadline was December, 2010. Survival rate was
calculated between the date of surgery and the last follow-up or
date of mortality. Each case was classified according to the tumor
size and localization, Dukes’ stage and differentiation degree. The
study was approved by the ethics committee of Central-South
University. Informed written consent was obtained from each
patient.
Immunohistochemistry
Annexin A3 and HIF-1α staining was performed using
formalin-fixed and paraffin-embedded serial sections. Sections (4
μm) were cut from the selected paraffin blocks and deparaffinized
by routine techniques. The slides were microwaved in citrate buffer
for 6 min for antigen retrieval. Annexin A3 was detected using a
rabbit polyclonal antibody (1:150) and HIF-1α was detected using a
mouse polyclonal antibody (1:100; both Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA). The primary antibody reaction was
performed at 4°C overnight. Labeling was detected by adding
biotinylated secondary antibodies, avidin-biotin complex and
diaminobenzidine (all Maxim-Bio, Fuzhou, China). Sections were then
counterstained with hematoxylin. Staining results for each antibody
were examined by two authors independently.
Evaluation of Annexin A3 and HIF-1α
expression
Annexin A3 and HIF-1α immunoreactivity was detected
in the cytoplasm of tumor cells. The expression of Annexin A3 and
HIF-1α was scored according to the positive percentage and staining
intensity of the stained tumor cells. The percentage positivity was
scored as follows: 0, 0–25%; 1, 26–50%; 2, 51–75%; and 3, >75%.
The staining intensity was scored as follows: 0, no staining; 1,
weak; 2, moderate; and 3, strong. If the product of multiplication
between staining intensity and the percentage of positive cells was
≥2, it was considered as positive (+) immunoreactivity.
Statistical analysis
Annexin A3 expression was assessed for a correlation
with clinicopathological parameters and HIF-1α expression using the
Fisher’s exact and Spearman’s correlation coefficient tests. For
the survival analysis, the survival curves were calculated using
the Kaplan-Meier method. All statistical analyses were performed
using SPSS 11.0 for Windows (SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Correlation between Annexin A3 expression
and clinicopathological variables
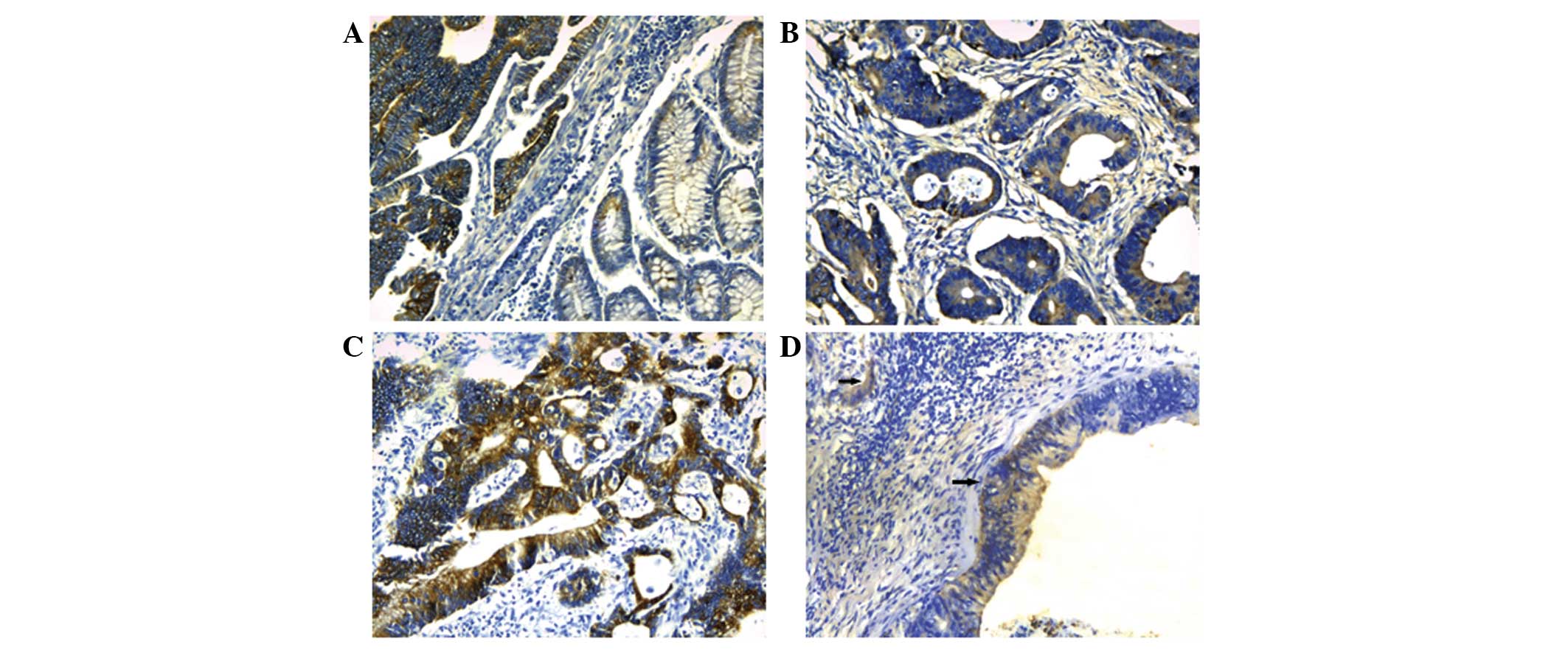
Of the 60 colorectal cancer tissues, Annexin A3
staining was undetected in 21 cases. Compared with weak or no
expression in normal colorectal tissues, 65% (39/60) of colorectal
cancer specimens showed Annexin A3 immunoreactivity (Fig. 1). Annexin A3 was predominantly
expressed in the cytoplasm of cancer cells. To improve the
investigation of the role of Annexin A3 in colorectal cancer, the
correlation between Annexin A3 expression and clinicopathological
factors was analyzed. Tumor size and Dukes’ stage exhibited
statistically significant correlations with Annexin A3 expression
(Table I). However, no significant
correlation was identified between the levels of Annexin A3
expression and other clinical and pathological features, including
gender, age, tumor localization, tumor differentiation degree and
lymph node metastasis.
 | Table ICorrelation between Annexin A3 or
HIF-1α expression and clinicopathological features of the
colorectal cancer patients. |
Table I
Correlation between Annexin A3 or
HIF-1α expression and clinicopathological features of the
colorectal cancer patients.
| Annexin A3
expression | | HIF-1α
expression | |
|---|
|
| |
| |
|---|
| Variable | Negative | Positive | P-value | Negative | Positive | P-value |
|---|
| Gender |
| Male | 15 | 25 | | 19 | 21 | 0.274 |
| Female | 6 | 14 | 0.775 | 13 | 7 | |
| Age, years |
| ≤60 | 11 | 18 | | 17 | 12 | 0.451 |
| >60 | 10 | 21 | 0.788 | 15 | 16 | |
| Tumor size, cm |
| ≤2 | 13 | 11 | | 18 | 6 | 0.008 |
| >2 | 8 | 28 | 0.014 | 14 | 22 | |
| Tumor
localization |
| Right colon | 7 | 15 | | 8 | 14 | 0.062 |
| Left colon | 14 | 24 | 0.783 | 24 | 14 | |
| Tumor
differentiation |
| Grade 1 | 4 | 10 | | 11 | 3 | 0.037 |
| Grades 2 and 3 | 17 | 29 | 0.751 | 21 | 25 | |
| Dukes’ stage |
| A+B | 15 | 11 | | 13 | 13 | 0.795 |
| C+D | 6 | 28 | 0.002 | 19 | 15 | |
| Lymph node
metastasis |
| No | 13 | 20 | | 21 | 12 | 0.118 |
| Yes | 8 | 19 | 0.587 | 11 | 16 | |
Correlation between HIF-1α expression and
clinicopathological variables
HIF-1α expression was observed in 28 (47%) of the 60
cases. In positive cases, HIF-1α immunoreactivity was present in
the cytoplasm of tumor cells. The correlation between HIF-1α and
clinicopathological factors is exhibited in Table I. HIF-1α expression was found to
significantly correlate with tumor size and differentiation degree.
No significant correlation was identified between HIF-1α expression
and the other clinicopathological factors, including gender, age,
tumor localization, Dukes’ stage and lymph node metastasis.
Correlation between Annexin A3 and HIF-1α
expression
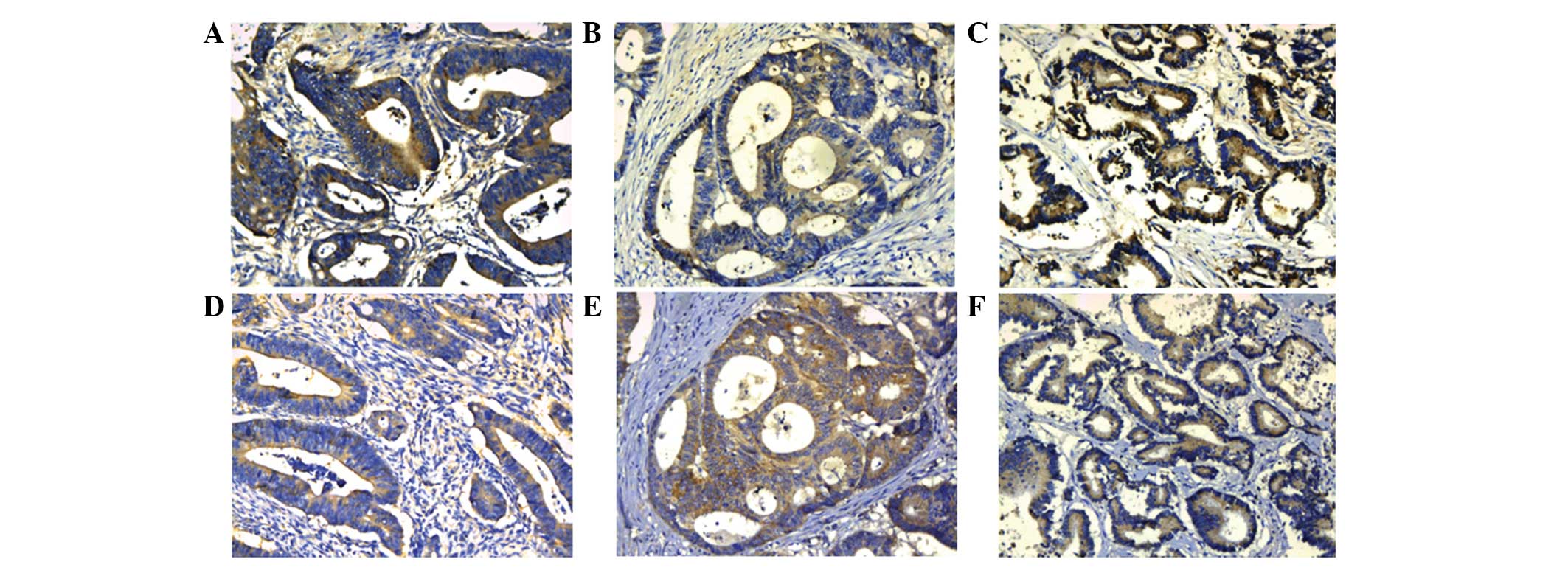
Due to the important roles of Annexin A3 and HIF-1α
in cancer progression, the correlation between Annexin A3 and
HIF-1α expression was analyzed. In the same samples, Annexin A3 and
HIF-1α exhibited similar expression patterns (Fig. 2). In total, 60 colorectal cancer
samples were detected for Annexin A3 and HIF-1α staining. Of the 60
cases, 23 (38%) were positive for Annexin A3 and HIF-1α, whereas,
Annexin A3 and HIF-1α were not expressed in 16/60 (27%) of cases.
Furthermore, 16 of the 60 (27%) cases were Annexin A30-positive but
HIF-1α-negative, whereas only HIF-1α was stained in 5/60 (8%)
cases. A significant correlation was observed between Annexin A3
and HIF-1α expression (P=0.009; rs=0.336).
Correlation between Annexin A3 expression
and overall survival in patients with colorectal cancer
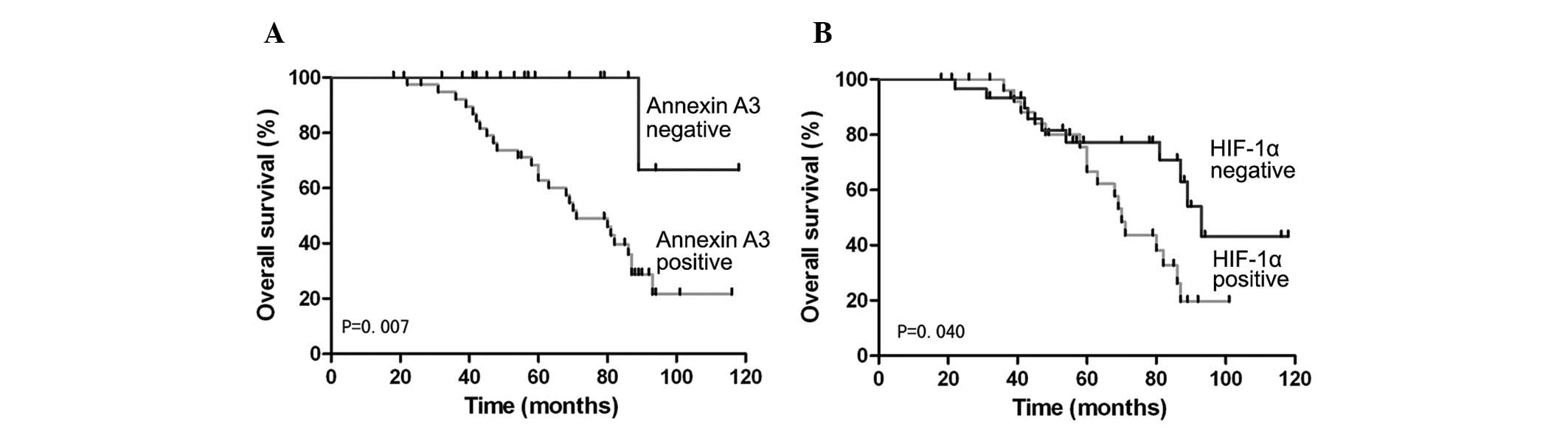
To investigate the prognostic role of Annexin A3 in
colorectal cancer, overall survival rates were estimated by
Kaplan-Meier survival. Within this group, patients with positive
expression of Annexin A3 exhibited significantly shorter overall
survival than patients negative for Annexin A3 (P=0.007) (Fig. 3). Similarly, overall survival in
colorectal cancer patients was significantly different between
patients positive for HIF-1α and patients negative for HIF-1α
(P=0.040) (Fig. 3). Univariate and
multivariate analyses were performed to evaluate the impact of
Annexin A3 expression and pathological factors on the prognosis of
colorectal cancer (Table II).
Univariate Cox regression analysis indicated that tumor
differentiation, Dukes’ stage, lymph node status and Annexin A3
expression were significantly associated with overall survival. In
the multivariate analyses, Dukes’ stage, lymph node status and
Annexin A3 expression were associated with poor overall survival.
Annexin A3 expression levels are an indicator of a poor prognosis
for overall survival (hazard ratio, 3.331; 95% CI, 1.213–9.098)
(Table II). Of all the
clinicopathological factors, Dukes’ stage was the most independent
factor predicting prognosis (P=0.021).
 | Table IIUnivariate and multivariate analysis
of overall survival in patients with colorectal cancer. |
Table II
Univariate and multivariate analysis
of overall survival in patients with colorectal cancer.
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|---|
| Variable | Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value |
|---|
| Gender | 1.678 | 0.742–4.126 | 0.268 | | | |
| Age, years | 1.012 | 0.971–1.055 | 0.560 | | | |
| Tumor
localization | 0.886 | 0.264–2.971 | 0.844 | | | |
| Tumor
differentiation | 5.641 | 2.278–12.981 | 0.000 | 2.627 | 0.897–6.154 | 0.059 |
| Tumor size, cm | 1.001 | 0.325–3.084 | 0.999 | | | |
| Dukes’ stage | 0.117 | 0.021–0.448 | 0.002 | 0.188 | 0.037–0.861 | 0.021 |
| Lymph node
status | 6.896 | 2.405–20.863 | 0.001 | 3.586 | 1.056–10.231 | 0.029 |
| HIF-1α
expression | 1.552 | 0.600–4.015 | 0.365 | | | |
| Annexin A3
expression | 6.875 | 2.471–20.057 | 0.001 | 3.331 | 1.213–9.098 | 0.031 |
Discussion
Annexin A3 has been previously identified as an
oncoprotein, based on its overexpression in lung adenocarcinoma and
its ability to promote lymph node metastasis (20). However, it has been reported that
Annexin A3 may play a different role in tumor development or
progression among various human organs. Köllermann et al
previously found that Annexin A3 protein expression was essentially
reduced in prostate cancer and exhibited a negative correlation
with the prognosis of patients (12). These contradictory studies led the
present study to investigate the roles of Annexin A3 expression in
colorectal cancer.
In the present study, a subset of the colorectal
cancer cases with follow-up data were analyzed to show a
correlation between the absence of Annexin A3 and clinical outcome.
The results indicated that Annexin A3 protein expression was
significantly increased in colorectal cancer tissues compared with
the paired noncancerous tissues, which is consistent with the
results in lung adenocarcinoma (20). These results indicate that Annexin
A3 may act as an oncogene in colorectal cancer. Furthermore,
overexpression of Annexin A3 was found to significantly correlate
with tumor size and Dukes’ stage, which indicated that Annexin A3
expression may be important in the development of colorectal
cancer. Furthermore, the high expression of Annexin A3 in
colorectal cancer tissues was found to significantly correlate with
shorter overall survival in patients, compared with that of low
Annexin A3 expression. Cox regression analysis revealed that Dukes’
stage, lymph node metastases and Annexin A3 expression were
independent prognostic factors in colorectal cancer patients. These
results illustrate that Annexin A3 is a potentially important
factor in the progression of colorectal cancer, as well as a novel
molecular target of gene therapy for colorectal cancer.
Angiogenesis is strictly regulated via a balance of
numerous angiogenic and anti-angiogenic factors. When the balance
is disrupted, it leads to tumor development. It is known that
HIF-1α is a major factor in the regulation of VEGF expression
(16). Previously, Park et
al(19) found that Annexin A3
may affect vascular formation by directly or indirectly regulating
VEGF expression. Furthermore, luciferase assay results show that
Annexin A3 activates HIF-1 activity in HEK 293 cells and HIF-1 may
be a target of Annexin A3. Consistent with these observations, the
current study also found a close correlation between Annexin A3 and
HIF-1α expression in colorectal cancer tissues. The results
indicate that Annexin A3 may be involved in tumor angiogenesis by
regulating HIF-1α. Future studies are required to elucidate the
correlation between Annexin A3 and HIF-1α. In addition, HIF-1α
expression has been found to correlate significantly with tumor
size and differentiation. However, in multivariate Cox model
analysis, HIF-1α was not an independent prognostic factor for the
overall survival of colorectal cancer patients.
In conclusion, the present study is the first to
evaluate Annexin A3 expression and its correlation with
clinicopathological factors in colorectal cancer tissues. The
results indicate that the increased expression of Annexin A3 in
colorectal cancer significantly correlates with tumor progression
and poor prognosis. In addition, Annexin A3 and HIF-1α proteins
appear to be important in the advancement of colorectal cancer. It
was also found that Annexin A3 expression is associated with HIF-1α
expression. These observations highlight an improved understanding
of the carcinogenesis of colorectal cancer and Annexin A3 may be a
potential biomarker for predicting the prognosis of colorectal
cancer patients.
References
|
1
|
Saif MW: Targeted agents for adjuvant
therapy of colon cancer. Clin Colorectal Cancer. 6:46–51. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Raynal P and Pollard HB: Annexins: the
problem of assessing the biological role for a gene family of
multifunctional calcium- and phospholipid-binding proteins. Biochim
Biophys Acta. 1197:63–93. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gerke V and Moss SE: Annexins: from
structure to function. Physiol Rev. 82:331–371. 2002.PubMed/NCBI
|
|
4
|
Gerke V, Creutz CE and Moss SE: Annexins:
linking Ca2+ signalling to membrane dynamics. Nat Rev
Mol Cell Biol. 6:449–461. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bai XF, Ni XG, Zhao P, et al:
Overexpression of annexin 1 in pancreatic cancer and its clinical
significance. World J Gastroenterol. 10:1466–1470. 2004.PubMed/NCBI
|
|
6
|
Zimmermann U, Woenckhaus C, Teller S, et
al: Expression of annexin AI in conventional renal cell carcinoma
(CRCC) correlates with tumour stage, Fuhrman grade, amount of
eosinophilic cells and clinical outcome. Histol Histopathol.
22:527–534. 2007.
|
|
7
|
Chen R, Brentnall TA, Pan S, et al:
Quantitative proteomics analysis reveals that proteins
differentially expressed in chronic pancreatitis are also
frequently involved in pancreatic cancer. Mol Cell Proteomics.
6:1331–1342. 2007. View Article : Google Scholar
|
|
8
|
Esposito I, Penzel R, Chaib-Harrireche M,
et al: Tenascin C and annexin II expression in the process of
pancreatic carcinogenesis. J Pathol. 208:673–685. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bae SM, Lee CH, Cho YL, et al:
Two-dimensional gel analysis of protein expression profile in
squamous cervical cancer patients. Gynecol Oncol. 99:26–35. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Domoto T, Miyama Y, Suzuki H, et al:
Evaluation of S100A10, annexin II and B-FABP expression as markers
for renal cell carcinoma. Cancer Sci. 98:77–82. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wamunyokoli FW, Bonome T, Lee JY, et al:
Expression profiling of mucinous tumors of the ovary identifies
genes of clinicopathologic importance. Clin Cancer Res. 12:690–700.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Köllermann J, Schlomm T, Bang H, et al:
Expression and prognostic relevance of Annexin A3 in prostate
cancer. Eur Urol. 54:1314–1323. 2008.
|
|
13
|
Yan X, Yin J, Yao H, Mao N, Yang Y and Pan
L: Increased expression of Annexin A3 is a mechanism of platinum
resistance in ovarian cancer. Cancer Res. 70:1616–1624. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tan Y, Meng HP, Teng FM, et al: Effect of
miRNA interference to AnnexinA3 gene on growth of human gallbladder
cancer cells. Chin Hepatobiliary Surg. 16:853–856. 2010.(In
Chinese).
|
|
15
|
Katsuta M, Miyashita M, Makino H, et al:
Correlation of hypoxia inducible factor-1alpha with lymphatic
metastasis via vascular endothelial growth factor-C in human
esophageal cancer. Exp Mol Pathol. 78:123–130. 2005. View Article : Google Scholar
|
|
16
|
Kimura S, Kitadai Y, Tanaka S, et al:
Expression of hypoxia-inducible factor (HIF)-1alpha is associated
with vascular endothelial growth factor expression and tumour
angiogenesis in human oesophageal squamous cell carcinoma. Eur J
Cancer. 40:1904–1912. 2004. View Article : Google Scholar
|
|
17
|
Kyzas PA, Stefanou D, Batistatou A and
Agnantis NJ: Prognostic significance of VEGF immunohistochemical
expression and tumor angiogenesis in head and neck squamous cell
carcinoma. J Cancer Res Clin Oncol. 131:624–630. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shibaji T, Nagao M, Ikeda N, et al:
Prognostic significance of HIF-1 alpha overexpression in human
pancreatic cancer. Anticancer Res. 23:4721–4727. 2003.PubMed/NCBI
|
|
19
|
Park JE, Lee DH, Lee JA, et al: Annexin A3
is a potential angiogenic mediator. Biochim Biophys Res Com.
337:1283–1287. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu YF, Xiao ZQ, Li MX, et al:
Quantitative proteome analysis reveals Annexin A3 as a novel
biomarker in lung adenocarcinoma. J Pathol. 217:54–64. 2009.
View Article : Google Scholar : PubMed/NCBI
|