Introduction
Lung cancer is one of the leading causes of cancer
mortality worldwide (1). Non-small
cell lung cancer (NSCLC) accounts for ~85% of all lung cancer
cases, with a five-year survival rate of 15.1% in China (2). The main cause of mortality for NSCLC
is cancer metastasis, which is difficult to treat and is currently
a popular area for lung cancer research. Despite maximal therapy,
surgically treated patients with stage I–III NSCLC are at risk for
developing metastatic disease. Clinicians and researchers should
urgently explore treatment targets in order to inhibit cancer
metastasis, thereby establishing more reliable and effective
therapies for NSCLC.
Glypican-5 (GPC5) is one of the six members of the
glypican family that are bound to the external surface of the
plasma membrane by glycosyl-phosphatidylinositol (GPI) linkage
(3). The GPC5 gene has eight exons
encoding 572 amino acids and spans a large genomic region of 1.47
Mb at chromosome 13q31.3. GPC5 expression is developmentally
regulated, with a general role in the control of growth and
differentiation during mammalian development (4). A genome-wide association study (GWAS)
has reported that genetic variations of GPC5 may contribute to an
increased risk of lung cancer in patients who have never smoked
(5). GPC5 has also been found to be
abnormally expressed in various human tumors. Studies have shown
that GPC5 expression was lower in tumors with a relatively better
prognosis, while it was higher in tumors with greater metastatic
potential, such as in small cell lung cancer and rhabdomyosarcoma
(6–9). However, the effect of GPC5 expression
on NSCLC prognosis has yet to be defined. We hypothesized that GPC5
affects the prognosis of NSCLC patients by involving the metastatic
process.
The aim of the present study was to investigate the
expression pattern of GPC5 in NSCLC cell lines and tumor tissue
samples. The effects of GPC5 expression on cancer cell migration
were assessed. The correlation of GPC5 expression levels with NSCLC
survival was also examined.
Materials and methods
Cell culture, vector construction and
plasmid transfection
The NSCLC cell lines A549, H3255 and SPC-A1 were
cultured in RPMI-1640 (Wisent Inc., St- Bruno, QC, Canada)
supplemented with 10% fetal bovine serum (HyClone, Logan, UT, USA),
100 U/ml penicillin and 100 μg/ml streptomycin at 37°C in an
atmosphere of 5% CO2. Coding sequences of human GPC5 was
cloned into pEGFP-N1 and designated as pEGFP/GPC5. GPC5 targeted
three shRNAs, these were predicted to be
5′-GATCCGTTCGGAAACTTTTCCAGTTCAAGAGACTGGAAAAGTTTCCGAACTTTTTTTTCAA-3′,
5′-GATCCTTTGTAAACAGATTTTTTGTCAAGAGCAAAAAATCTGTTTACAAATTTTTTTTCAA-3′
and
5′-GATCCAAAGTTATACTCAGCGTGTTCAAGAGACACGCTGAGTATAACTTTTTTTTTTTCAA-3′.
All three shRNAs targeting GPC5 were constructed into
pRNAT-U6.1/Neo, as pRNAT-shRNA-GPC5-1,2,3. The cell lines A549,
H3255 and SPC-A1 were transfected with pEGFP/GPC5, pEGFP-N1,
pRNAT-shRNA-GPC5-1,2,3 or pRNAT-U6.1 using TurboFect (Fermentas,
Vilnius, Lithuania) according to the manufacturer’s instructions.
GPC5 expression was examined using reverse transcription-polymerase
chain reaction (RT-PCR) and western blot analysis.
RNA isolation, reverse transcription and
PCR
Total RNA was extracted using TRIzol reagent
(Invitrogen, Carlsbad, CA, USA) and reverse transcribed into cDNA
using a PrimeScript RT reagent kit (Takara, Dalian, China)
according to the manufacturer’s instructions. PCR was performed
using the Mastercycler Gradient (Eppendorf, Hamburg, Germany).
Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was amplified as
the endogenous control. The primer sequences used were: GPC5:
forward, 5′-CCCTCGAGGGAGGATGGACGCACAGACC-3′ and reverse,
5′-CGGGATCCCGCCAGGCATATGCAGA GAGAGAG-3′; GAPDH: forward,
5′-CAATGACCCC TTCATTGACC-3′ and reverse, 5′-TGGAAGATGGTGAT
GGGATT-3′.
Western blot analysis
Protein lysates were separated by 12% sodium dodecyl
sulphate-polyacrylamide gel electrophoresis (SDS-PAGE), and
electrophoretically transferred to a polyvinylidene difluoride
(PVDF) membrane (Millipore, Billerica, MA, USA). Subsequently, the
membrane was incubated with rabbit monoclonal antibody against
human GPC5 (1:100; Abcam, Cambridge, UK) followed by horseradish
peroxidase-labeled goat anti-rabbit IgG (Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA) and detected by chemiluminescence. GAPDH
was used as a protein loading control. The intensity of protein
fragments was quantified with ImageJ software (http://rsbweb.nih.gov/ij/).
Wound scratch and transwell assays
Cells were seeded in 12-well plates and cultured
overnight to form a confluent monolayer. After being scratched with
a sterile pipette tip, the cells were rinsed gently with PBS to
remove the detached cells and subsequently incubated with medium
containing 1% FBS at 37°C in an atmosphere of 5% CO2.
Images of the wounded areas were captured at 0 and 24 h after
incubation. The distances between the two edges of the scratched
cells were measured and the healing rate was calculated using the
formula: healing rate = (the distance prior to healing - the
distance following healing)/the distance prior to healing × 100. A
Transwell (8 μm pore size; Costar, Corning, USA) assay was used to
further analyze cell migration according to the manufacturer’s
instructions. Fifty thousand cells were placed in the upper
chambers in serum-free media, and the lower chambers were filled
with RPMI-1640 + 10% FBS. Following incubation for 24 h at 37°C,
non-migrating cells on the top surface of the membrane were removed
with a cotton swab. The membranes were fixed with methanol for 10
min and stained with 0.5% crystal violet for 5 min. The number of
cells that migrated to the bottom of the filter were counted
manually under an inverted microscope.
Study population and data collection
A panel of formalin-fixed paraffin-embedded (FFPE)
NSCLC tissues from patients undergoing curative resection between
January, 2003 and December, 2008 were obtained from the The
Affiliated Drum Tower Hospital of Nanjing University Medical School
(Nanjing, China). Full medical record abstraction was performed in
order to obtain the following patient variables: age, gender,
smoking status, respiratory symptoms upon lung cancer diagnosis,
cell type, tumor differentiation, vascular invasion, regional lymph
node metastasis, pTNM stage, adjuvant treatment and other medical
conditions. Smokers were defined as those having smoked at least
100 cigarettes in their lifetime. pTNM staging designations were
made according to the postsurgical pathological staging system
according to the 7th edition of the TNM classification of malignant
tumors (10). Complete removal of
the primary lesion with negative resection margins was requested.
All patients had an Eastern Cooperative Oncology Group (ECOG)
performance status of 0 or 1. A total of 127 patients with complete
data were identified in the current analysis. All patients enrolled
in the study were newly diagnosed with NSCLC and none had received
neoadjuvant chemotherapy, radiation therapy or immunotherapy prior
to surgical therapy. Informed consent was obtained from each
patient in this study. Their contact materials and the study
protocol were reviewed and approved by the Ethics Committee of Drum
Tower Hospital Institutional Review Board (Nanjing, China).
Immunohistochemistry (IHC)
FFPE tissue samples were deparaffinized and
rehydrated. The endogenous peroxydase activity was blocked with 3%
hydrogen peroxide in methanol for 15 min. For antigen retrieval,
the slides were boiled under pressure for 3 min in 10 mM citrate
acid buffer (pH 6.0). Non-specific binding was blocked with 10%
normal goat serum (Abcam) for 30 min at room temperature. The
slides were subsequently incubated with a rabbit polyclonal
antibody to GPC5 (1:100; Abcam) for a further 30 min at room
temperature and incubated overnight at 4°C. The slides were then
washed in Tris-buffered saline and incubated sequentially with
anti-rabbit IgG (dilution 1:400; Dako, Carpinteria, CA, USA). Color
was developed by 15 min incubation with 3,3′-diaminobenzidine.
Samples were counterstained with hematoxylin, followed by
dehydration and mounting. Negative controls were included by
replacing the primary antibody with PBS. Normal human brain tissue
was detected as a positive control. The quality of IHC was
confirmed using H&E staining. The evaluation of immunostaining
of these samples was performed by two trained pathologists (J Yang
and FQ Meng) who were unaware of the clinical background of the
samples. The intensity and percentage of positive cells were
considered. Five visual fields were randomly observed, and 100
cells in each field were counted (magnification, ×400). Positive
cells from the 100 tumor cells in each field were counted. Tumor
cells with a brown cell membrane were considered to be positive and
were scored as: 3+, strong; 2+, moderate; 1+, weak; and 0, no
staining. The average percentages of positively stained tumor cells
were classified as: 0 for 0%; 1 for 1–33%; 2 for 34–66%; and 3 for
67–100%. The intensity and percentage scores were multiplied to
yield a composite score of 1–9 for each sample. Composite scores of
1–3 were defined as indicating a low GPC5 expression, while scores
of 4–9 were considered to indicate a high GPC5 expression.
Statistical analysis
Data from in vitro studies were expressed as
the means ± SD and statistical significance was assessed by the
Student’s t-test. Survival was assessed up to December 31, 2011.
Overall survival (OS) was calculated as the period from the date of
surgery for NSCLC until death. Patients who were alive at the last
contact were censored. Associations between clinicopathological
variables and GPC5 protein expression were examined using Pearson’s
χ2 test for categorical variables (or Fisher’s exact
test if any sample number was <5), and the Student’s t-test for
continuous variables. Univariate Cox proportional hazard models
were used to evaluate the prognostic impact on survival of all
factors of interest. Factors were included in multivariate models
if P<0.05 in the univariate analysis. Kaplan-Meier analysis was
performed for survival curves and statistical significance was
assessed using the log-rank test. All analyses were performed with
SPSS software, version 16.0 (SPSS Inc., Chicago, IL, USA). All
tests were two-sided and performed at a significance level of
0.05.
Results
Expression of GPC5 in NSCLC cell
lines
GPC5 expression in three NSCLC cell lines (two
invasive: A549 and H3255, one less invasive: SPC-A1) were analyzed.
GPC5 was highly expressed in A549 and H3255 cells, compared with
SPC-A1 cells (Fig. 1A and B).
Following transfection with pEGFP/GPC5, the expression of GPC5 was
elevated in SPC-A1 (Fig. 1C and D).
After screening three shRNA vectors, pRNAT-shRNA-GPC5-1 was
identified as the most appropriate interfering plasmid for
subsequent use. As indicated in Fig. 1C
and D, pRNAT-shRNA-GPC5-1 may significantly downregulate the
expression of GPC5.
GPC5 enhances the migration ability of
NSCLC cells
Following transfection with pRNAT-shRNA-GPC5-1, A549
and H3255 cells migrated at a markedly slower rate than parental
cells transfected with control vectors (Fig. 2A). The mean migration rate of A549
cells transfected with pRNAT-shRNA-GPC5-1 was (61±0.6%) while the
mean rate of the control was (83±0.6%) (P<0.001). The mean
migration rate of H3255 cells transfected with pRNAT-shRNA-GPC5-1
was (13±4.0%) while the mean rate of the control was (36±2.1%)
(P=0.001). SPC-A1 with GPC5 overexpression demonstrated a higher
migration rate than that of the control [(37±3.5%) vs. (18±0.6%),
P=0.001] (Fig. 2A). Accordingly,
the downregulation of GPC5 impeded the transmigration of A549 and
H3255 cells from the top of the transwell membrane (Fig. 2B). The upregulation of GPC5
expression promoted transmembrane invasion compared with the
control in SPC-A1 (Fig. 2B).
Correlation between GPC5 protein
expression and clinicopathological parameters in NSCLC
Typical immunohistochemical staining patterns
observed for GPC5 protein are shown in Fig. 3. Positive staining for GPC5 was
mainly localized in the cell membrane. Tumor cells with high levels
of GPC5 expression were more invasive compared to those with low
levels of GPC5 expression (Fig.
3B). The median survival time of the 127 resected NSCLC
patients was 33.0 months (95% CI: 20.65–45.35). The overall 5-year
survival rate was 37.4%. High levels of GPC5 expression were
detected in 58/127 (45.7%) of the NSCLC tissues.
Clinicopathological characteristics of NSCLC are listed in Table I according to GPC5 expression
status. There were no significant differences in age, gender,
smoking status, cell type and adjuvant treatment between patients
with high and low levels of GPC5 expression (P>0.05). Patients
with high levels of GPC5 expression tended to have respiratory
symptoms upon lung cancer diagnosis compared to those with low
levels of GPC5 expression (P=0.03). Tumor cells with high GPC5
expression levels exhibited poor differentiation (P=0.04). The
correlation between vascular invasion and GPC5 expression was also
examined, with more cases demonstrating vascular invasion in the
high GPC5 expression group (34.5 vs. 17.4%, P=0.03). A high GPC5
expression was found to correlate significantly with regional lymph
node metastasis (P=0.007).
 | Table ICharacteristics of 127 non-small cell
lung cancer patients. |
Table I
Characteristics of 127 non-small cell
lung cancer patients.
| GPC5 expression | |
|---|
|
| |
|---|
| Patient
characteristics | High [No. (%)] | Low [No. (%)] | P-value |
|---|
| Total patients | 58 | 69 | |
| Age (years) | | | 0.93 |
| Mean (SD) | 63.45 (11.61) | 63.28 (11.32) | |
| Gender | | | 0.97 |
| Female | 17 (29.3) | 20 (29.0) | |
| Male | 41 (70.7) | 49 (71.0) | |
| Respiratory
symptoms | | | 0.03 |
| No | 12 (20.7) | 27 (39.1) | |
| Yes | 46 (79.3) | 42 (60.9) | |
| Smoking status | | | 0.31 |
| Never smokers | 25 (43.1) | 36 (52.2) | |
| Smokers | 33 (56.9) | 33 (47.8) | |
| Tumor
differentiation | | | 0.04 |
| Well + moderate | 31 (53.4) | 49 (71.0) | |
| Poorly | 27 (46.6) | 20 (29.0) | |
| Cell type | | | 0.92 |
| Adenocarcinoma | 30 (51.7) | 36 (27) | |
| Squamous | 24 (41.4) | 27 (39.1) | |
| Othersa | 4 (6.9) | 6 (8.7) | |
| Vascular
invasion | | | 0.03 |
| No | 38 (65.5) | 57 (82.6) | |
| Yes | 20 (34.5) | 12 (17.4) | |
| Regional lymph node
metastasis | | | 0.007 |
| No | 23 (39.7) | 44 (63.8) | |
| Yes | 35 (60.3) | 25 (36.2) | |
| Stage | | | 0.003 |
| I | 18 (31.0) | 37 (53.6) | |
| II | 12 (20.7) | 18 (26.1) | |
| III | 28 (48.3) | 14 (20.3) | |
| Adjuvant chemotherapy
and/or radiation | | | 0.32 |
| No | 37 (63.8) | 38 (55.1) | |
| Yes | 21 (36.2) | 31 (44.9) | |
Survival analysis
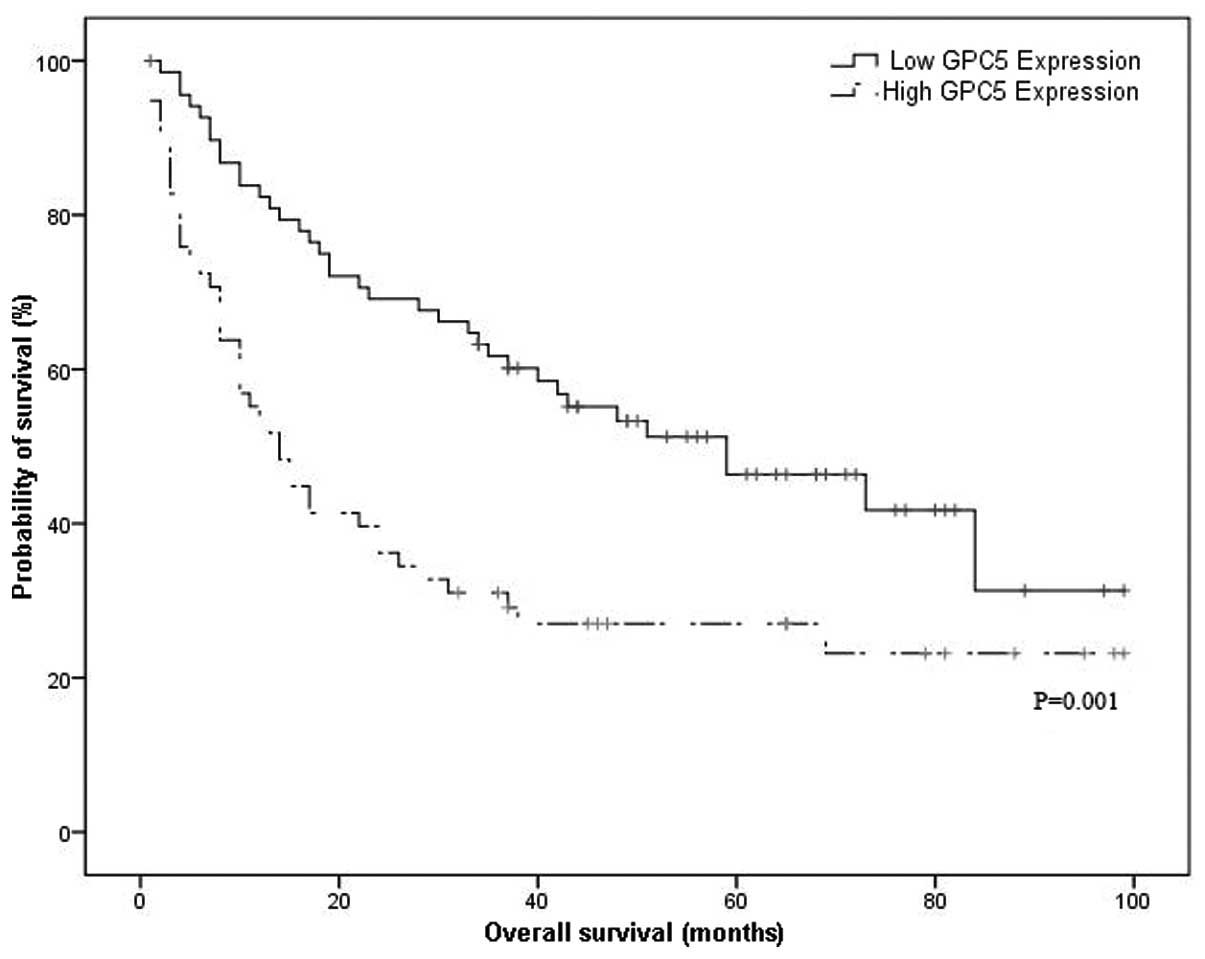
The median OS time was 14.0 months (95% CI:
9.0–19.0) in patients with high levels of GPC5 expression and 59.0
months (95% CI: 30.2–87.8) in those with low levels of GPC5
expression, which demonstrated a significant difference (P=0.001,
Fig. 4). For the univariate
analysis, as shown in Table II,
gender, respiratory symptoms, tumor differentiation, cell type,
regional lymph node metastasis and stage all correlated
significantly with OS (P<0.05). There was a significant
correlation between GPC5 expression and OS (hazard ratio: 2.11; 95%
CI: 1.35–3.30; P=0.001). Following adjustment for variables
significant to univariate analysis, a significant correlation
between GPC5 expression and OS remained, with a markedly larger
effect (adjusted hazard ratio: 2.18; 95% CI: 1.35–3.52; P=0.001,
data not shown).
 | Table IIUnivariate analysis. |
Table II
Univariate analysis.
| Patient
characteristics | Unadjusted HR (95%
CI) | P-value |
|---|
| Age (years) |
| <65 | Reference | |
| >65 | 1.12 (0.72–1.74) | 0.62 |
| Gender |
| Female | Reference | |
| Male | 1.74
(1.02–2.98) | 0.04 |
| Respiratory
symptoms |
| No | Reference | |
| Yes | 1.80
(1.06–3.05) | 0.03 |
| Smoking status |
| Never smokers | Reference | |
| Smokers | 1.39
(0.89–2.17) | 0.15 |
| Tumor
differentiation |
| Well +
moderate | Reference | |
| Poorly | 1.91
(1.23–2.99) | 0.004 |
| Cell type |
|
Adenocarcinoma | Reference | |
| Othersa | 1.85
(1.18–2.89) | 0.007 |
| Vascular
invasion |
| No | Reference | |
| Yes | 1.51
(0.92–2.45) | 0.10 |
| Regional lymph node
metastasis |
| No | Reference | |
| Yes | 2.17
(1.38–3.41) | 0.001 |
| Stage |
| I | Reference | |
| II | 2.53
(1.42–4.52) | 0.002 |
| III | 2.71
(1.57–4.66) | <0.001 |
| Adjuvant
chemotherapy and/or radiation |
| No | Reference | |
| Yes | 1.00
(0.64–1.56) | 1.00 |
| GPC5 expression
level |
| Low | Reference | |
| High | 2.11
(1.35–3.30) | 0.001 |
Discussion
In the present study, we identified GPC5 expression
in NSCLC tumor cells and these expression levels may have affected
tumor cell migration. To the best of our knowledge, this is the
first study to investigate the role of GPC5 on NSCLC survival. High
expression levels of GPC5 were identified as negative prognostic
markers in patients with resected NSCLC.
The 8-exon GPC5 gene is located at chromosome
13q31.3, encoding a 572-amino acid product. The GPC5 protein
belongs to the glypican gene family (GPC1-GPC6), the members of
which have been reported to be overexpressed in several human
malignancies (11). The function of
GPC5 has yet to be established and studies of its role in tumors
have been limited, although the 13q31–32 amplification has been
observed in lung carcinomas (12),
breast tumors (7), neurological
tumors (8), liposarcomas (13) and rhabdomyosarcomas (9). In this study, GPC5-overexpressing
NSCLC cells exhibited a high rate of migration, whereas GPC5
downregulated cells exhibited a low rate of migration. The
enhancement of the migratory ability of cancer cells is an
important factor in the promotion of tumor metastasis. Using IHC
methods, high GPC5 expression levels were observed in patients with
vascular invasion and regional lymph node metastasis. These
findings suggest that GPC5 overexpression is likely a mechanism
activated by NSCLC in order to promote cancer cell metastasis via
vessels and lymph nodes, which requires confirmation with further
molecular experiments. We explored the value of GPC5 as a molecular
prognostic indicator and found that high levels of GPC5 expression
predicted poor postsurgical survival times for curatively resected
NSCLC patients.
These findings reveal the crucial role of GPC5 in
NSCLC metastasis and survival. However, the mechanism responsible
for GPC5-mediated effects on tumor metastasis remain to be
clarified. Studies have highlighted potentially significant roles
for the Wnt signaling pathway in the development of lung cancer in
addition to being involved in mammalian limb development (14,15).
The Wnt signaling pathway is also involved in numerous biological
processes, such as cell proliferation, differentiation, survival,
apoptosis and migration. In NSCLC, abnormal Wnt signaling has been
observed (16,17). Certain Wnt proteins are expressed
abnormally in NSCLC samples, including Wnt1, −2 and −7a. Wnt1 and
Wnt2 were overexpressed in NSCLC samples and cancer cells, with
Wnt1 expression exhibiting resistance to apoptosis-inducing therapy
(18). Conversely, inhibiting Wnt1
and Wnt2 may lead to the apoptosis of cancer cells and decrease
tumor growth in vivo and in vitro(17). As the Wnt pathway is involved in
epithelial-mesenchymal transition (EMT), the activation of the Wnt
signaling pathway may enhance the invasiveness of tumor cells
(19). A study by Williamson et
al(9) revealed that GPC5
overexpression increased proliferation in rhabdomyosarcoma by
potentiating the effects of Wnt1. We hypothesize that GPC5 may
promote tumor cell EMT by facilitating the activation of the Wnt
pathway, which subsequently may enhance tumor invasiveness and
metastasis, which requires further studies.
A recent GWAS suggested that genetic variants at
13q31.3 modulate GPC5 expression that had been downregulated in the
adenocarcinomas in patients who had never smoked. The GPC5
expression levels were reduced in the mammary tumors of breast
cancer patients (20). Associations
of GPC5 in distinct phenotypes suggests that GPC5 may have multiple
roles in different diseases. There may be numerous genes and
modulators involved in GPC5 gene expression, which should be
incorporated into the future studies.
In conclusion, our findings suggest that the
evaluation of GPC5 expression may be useful clinically in
recognizing patients who are more likely to have a poor NSCLC
outcome. Specifically, higher levels of GPC5 expression suggest a
tendency for a shorter survival time. Furthermore, GPC5 may be
involved in NSCLC metastasis through enhancing cancer cell
migration.
Acknowledgements
The authors would like to thank Fanqing Meng, MD,
(Department of Pathology, The Affiliated Drum Tower Hospital of
Nanjing University Medical School, Nanjing, China) for her
technical assistance in the evaluation of the immunostained
samples. This study was supported by a grant from the Natural
Science Foundation of Jiangsu Province (No. BK20130089) and the
Basic Research Funding for the Central Universities of China from
Nanjing University (No.1117021411).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar
|
|
2
|
Herbst RS, Heymach JV and Lippman SM: Lung
cancer. N Engl J Med. 359:1367–1380. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Esko JD and Selleck SB: Order out of
chaos: assembly of ligand binding sites in heparan sulfate. Annu
Rev Biochem. 71:435–471. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Veugelers M, Vermeesch J, Reekmans G,
Steinfeld R, Marynen P and David G: Characterization of glypican-5
and chromosomal localization of human GPC5, a new member of the
glypican gene family. Genomics. 40:24–30. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li Y, Sheu CC, Ye Y, et al: Genetic
variants and risk of lung cancer in never smokers: a genome-wide
association study. Lancet Oncol. 11:321–330. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ota A, Tagawa H, Karnan S, et al:
Identification and characterization of a novel gene, C13orf25, as a
target for 13q31-q32 amplification in malignant lymphoma. Cancer
Res. 64:3087–3095. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ojopi EP, Rogatto SR, Caldeira JR,
Barbiéri-Neto J and Squire JA: Comparative genomic hybridization
detects novel amplifications in fibroadenomas of the breast. Genes
Chromosomes Cancer. 30:25–31. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Reardon DA, Jenkins JJ, Sublett JE, Burger
PC and Kun LK: Multiple genomic alterations including N-myc
amplification in a primary large cell medulloblastoma. Pediatr
Neurosurg. 32:187–191. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Williamson D, Selfe J, Gordon T, Lu YJ,
Pritchard-Jones K, Murai K, Jones P, Workman P and Shipley J: Role
for amplification and expression of glypican-5 in rhabdomyosarcoma.
Cancer Res. 67:57–65. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Goldstraw P, Crowley J, Chansky K, et al:
The IASLC Lung Cancer Staging Project: proposals for the revision
of the TNM stage groupings in the forthcoming (seventh) edition of
the TNM classification of malignant tumours. J Thorac Oncol.
2:706–714. 2007. View Article : Google Scholar
|
|
11
|
Li Y and Yang P: GPC5 gene and its related
pathways in lung cancer. J Thorac Oncol. 6:2–5. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ullmann R, Petzmann S, Sharma A, Cagle PT
and Popper HH: Chromosomal aberrations in a series of large-cell
neuroendocrine carcinomas: unexpected divergence from small-cell
carcinoma of the lung. Hum Pathol. 32:1059–1063. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schmidt H, Bartel F, Kappler M, et al:
Gains of 13q are correlated with a poor prognosis in liposarcoma.
Mod Pathol. 18:638–644. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Quélin C, Bendavid C, Dubourg C, et al:
Twelve new patients with 13q deletion syndrome: genotype-phenotype
analyses in progress. Eur J Med Genet. 52:41–46. 2009.PubMed/NCBI
|
|
15
|
Saunders S, Paine-Saunders S and Lander
AD: Expression of the cell surface proteoglycan glypican-5 is
developmentally regulated in kidney, limb, and brain. Dev Biol.
190:78–93. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
He B, You L, Uematsu K, et al: A
monoclonal antibody against Wnt-1 induces apoptosis in human cancer
cells. Neoplasia. 6:7–14. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
You L, He B, Xu Z, et al: Inhibition of
Wnt-2-mediated signaling induces programmed cell death in
non-small-cell lung cancer cells. Oncogene. 23:6170–6174. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen S, Guttridge DC, You Z, et al: Wnt-1
signaling inhibits apoptosis by activating beta-catenin/T cell
factor-mediated transcription. J Cell Biol. 152:87–96. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Micalizzi DS, Farabaugh SM and Ford HL:
Epithelial- mesenchymal transition in cancer: parallels between
normal development and tumor progression. J Mammary Gland Biol
Neoplasia. 15:117–134. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang C, Zhang S, Zhang D, Zhang Z, Xu Y
and Liu S: A lung cancer gene GPC5 could also be crucial in breast
cancer. Mol Genet Metab. 103:104–105. 2011. View Article : Google Scholar : PubMed/NCBI
|