Introduction
Prostate cancer (CaP) is the most commonly diagnosed
malignancy in the United States, with the majority of cases
occurring in males over the age of 55 (1). In 2012, ~241,740 new cases of CaP were
predicted to be diagnosed, with ~28,170 men succumbing to CaP, in
the United States alone (1). Tumors
that are detected early via testing serum prostate-specific antigen
levels or digital rectal examination may be effectively treated by
prostatectomy or radiation therapy (2). Approximately 30% of treated patients
suffer relapse and progress to hormone refractory prostate cancer
(HRPC), which no longer responds to androgen ablation, whereas
early CaP growth is androgen-dependent. At that stage, there is no
curative therapy available for metastatic CaP (3,4).
Metastasis is a complex process by which cancer cells leave the
primary tumor and migrate to a secondary site where they
recolonize. It consists of multiple steps that are interconnected,
including invasion, migration, intravasation, extravasation and
recolonization (5,6). The shortcomings of treatment for such
highly invasive and metastatic disease have led to several
investigations of various molecular targets that directly affect
invasion and metastasis with the aim of developing safe and
effective treatments.
Numerous studies suggest that epithelial-mesenchymal
transition (EMT) may be an important step leading to cancer
metastasis (7–9). A notable mechanism by which E-cadherin
is downregulated in EMT is transcriptional repression by Snail
(10,11). Induction of Snail expression has
been noted in a number EMT processes that have been studied
(11–13). Additionally, increases in signaling
in survival pathways such as mitogen-activated protein kinase
(MAPK) is associated with increased Snail expression (14). Snail is composed of two interacting
domains (12,15,16);
the C-terminal domain is responsible for binding to DNA sequences
with a 5′-CAGGTG-3′ core, while the N-terminal is required for
transcriptional repression (16,17).
Overexpression of Snail is sufficient to induce EMT and is
associated with highly invasive tumors in mice and humans (18).
In order for tumors to colonize to a secondary site,
they must invade the extracellular matrix (ECM) (5,6).
Several proteolytic enzymes are involved in this process of
degradation. Among these enzymes is the plasminogen activation (PA)
system which leads to activation of matrix metalloproteases (MMPs)
(19,20). The members of the PA system include
urokinase-type plasminogen activator (uPA), plasminogen activator
inhibitors (PAIs) and the uPA receptor (uPAR) (19,20).
uPA, when bound to its cellular receptor uPAR, efficiently converts
plasminogen into the broad-spectrum serine protease plasmin; its
action on plasminogen is controlled by the serine protease
inhibitors PAI-1 and PAI-2 (13–15).
uPA catalyzes the activation of plasminogen into plasmin by
cleaving the arginine-valine bond. In turn, plasmin facilitates the
release of several proteolytic enzymes, including gelatinase and
fibronectin (19–21).
It has been well established that uPA and uPAR, both
members of the PA system, are involved in cancer invasion and
metastases (19–23). It has been shown that plasma levels
of uPA and uPAR are higher in males with CaP compared with healthy
controls and significantly declined after prostate removal
(24). Under normal conditions,
uPAR is considered to have fairly limited tissue expression
(25). Studies using mice and human
clinical samples have identified conditions in which uPAR
expression is induced (25,26). uPAR is induced during ECM
remodeling, stress, injury and inflammation, and is highly
expressed during tissue reorganization and inflammation, as well as
in virtually all human cancers (19,21,25).
Furthermore, it has been shown that uPAR is under an
extracellular-signal-regulated kinase (ERK)-dependent mechanism and
blocking uPAR’s activity leads to inhibition of motility in
hepatocellular carcinoma (27). In
human gastric cancer, studies have demonstrated that epidermal
growth factor (EGF) stimulates uPAR expression via the ERK pathway,
sequentially increasing cell invasion (28).
Several studies have shown that Snail mediates
invasion through MMP activation (29–31);
however, there are few studies that link Snail and uPA to cancer
progression. One study indicated that silencing uPA expression in
MDA-MB-231 breast cancer cells decreased expression of vimentin and
Snail, and induced changes in morphology characteristic of
epithelial cells (32). These
results demonstrate that uPAR-initiated cell signaling may be
targeted to reverse EMT in cancer (32). Another study suggested that when
Snail is blocked in the invasive breast cancer cell-line
MDA-MB-231, there is a decrease in the expression of PAI-1 and uPA
transcripts and reduced migration (33).
Previously, we have stably overexpressed Snail in
LNCaP and ARCaP CaP cell lines and shown that Snail led to EMT
associated with decreased/relocalized E-cadherin, increased
vimentin and increased migration (34–37).
In this study, we investigated the molecular mechanisms of
Snail-mediated cell invasion. We propose that Snail increases
invasion via uPA/uPAR signaling. The results showed that Snail
overexpression led to an increase in cell invasion, which was
antagonized by uPAR silencing. Snail also increased the levels of
uPA and uPAR protein, as well as uPA and ERK activities.
Furthermore, the inhibition of MAPK activity decreased uPA activity
and cell invasion. Our results show, for the first time, a link
between Snail, MAPK and uPA/uPAR in CaP. This demonstrates that
Snail regulates cell invasion via uPA-uPAR activites, possibly
through the MAPK pathway.
Materials and methods
Reagents and antibodies
RPMI-1640 medium and penicillin/streptomycin were
purchased from VWR International, Inc. (West Chester, PA, USA). The
protease inhibitor cocktail was obtained from Roche Molecular
Biochemicals (Indianapolis, IN, USA), while G418 and anti-human
actin antibodies were purchased from Sigma-Aldrich, Inc. (St.
Louis, MO, USA), and rabbit polyclonal anti-human Snail antibody
and rabbit anti-phospho-ERK1/2 (p-ERK) were obtained from Cell
Signaling Technology, Inc. (Danvers, MA, USA). Rabbit polyclonal
anti-uPA, anti-uPAR and anti-total-ERK1/2 were purchased from Santa
Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Horseradish
peroxidase-conjugated sheep anti-mouse, sheep anti-rabbit and the
ECL Prime or ECL Plus chemiluminescent reagents were obtained from
GE Healthcare Life Sciences (Little Chalfont, UK. Fetal bovine
serum (FBS) and dextran-coated charcoal-treated FBS (DCC-FBS) were
supplied by HyClone (South Logan, UT, USA). Control and Snail short
interfering RNA (siRNA) constructs were purchased from Dharmacon,
Inc. (Lafayette, CO, USA), and UO126 was purchased from
Sigma-Aldrich, Inc. The uPA Activity Assay kit was obtained from
Millipore (Billerica, MA, USA) and Matrigel was purchased from BD
Biosciences (Bedford, MA, USA).
Cell culture
Human CaP cell line ARCaP (Cedar Sinai Medical
Center, Los Angeles, CA, USA) stably transfected with
constitutively active Snail cDNA (ARCaP Snail representing an
aggressive cell line) or an empty vector Neo (ARCaP Neo
representing the less aggressive cell line), as well as LNCaP cells
overexpressing Snail, have been previously described as
representing an EMT model and were utilized in these experiments
(34–37). The 22Rv1 cells overexpressing Snail
utilized in the present experiments were previously generated
(35). The LNCaP human CaP cell
line was obtained from American Type Culture Collection (Manassas,
VA, USA) and maintained in RPMI-1640 (Corning Cellgro, Manassas,
VA, USA), supplemented with 10% FBS, 1% non-essential amino acids
and 1% antibiotics at 37°C in 5% CO2. The
Snail-transfected cells were maintained in RPMI-1640 supplemented
with 10% FBS, 1% non-essential amino acids and 1% antibiotics plus
400 μg/ml G418. All cells were maintained at 70–80% confluence.
Western blot analysis
Cells were cultured to 85–90% confluency;
subsequently, cells were washed with phosphate-buffered saline and
harvested in modified RIPA buffer (50 mM Tris, pH 8.0; 150 mM NaCl;
0.02% NaN3; 0.1% sodium dodecyl sulfate; 1% NP-40; 0.5%
sodium deoxycholate) containing 1.5X protease inhibitor cocktail, 1
mM phenylmethylsufonyl fluoride and 1 mM sodium orthovanadate.
Protein concentrations were calculated using the bicinchoninic acid
protein assay (Pierce, Rockford, IL, USA). Equal concentrations of
whole cell protein were separated on a 10% SDS-polyacrylamide gel
electrophoresis gel and transferred to a nitrocellulose membrane.
Non-specific antibody binding sites were blocked using 3 or 5%
non-fat dry milk and Tris-buffered saline and Tween-20 (TBST), and
washed with TBST. Membranes were incubated with primary antibodies
in 3% bovine serum albumin-TBST (p-ERK and Snail), or 5% non-fat
dry milk and TBST (uPA, uPAR, ERK1/2 and β-actin) overnight at 4°C.
Membranes were washed in TBST and incubated with HRP-conjugated
sheep anti-rabbit (Snail, uPA, uPAR and p-ERK) or anti-mouse
(actin) secondary antibody, then washed in TBST. Immunoblots were
detected using ECL Prime or ECL Plus chemiluminescent reagent (GE
Healthcare, Pittsburgh, PA, USA).
uPA activity assay
uPA activity was measured in conditioned medium from
the human CaP cell sublines LNCaP Neo/Snail, ARCaP Neo/Snail and
22Rv1 Neo/Snail using the uPA activity assay kit according to the
manufacturer’s instructions. A chromogenic substrate is cleaved by
active uPA to produce a colored product, which is detected on a
plate reader at 405 nm. The concentration of active uPA was
calculated relative to standards provided with the kit.
siRNA transfection
Transient transfection of uPAR siRNA was performed
on ARCaP Snail cells using DharmaFECT 1 reagent. Cells
(1×106/well) were seeded in a six-well plate and
transfected with 200 nm uPAR-siRNA or control-siRNA in serum free
media at 37°C with 5% CO2 for 5 h, followed by
replacement of transfection media with RPMI-1640 supplemented with
5% DCC-FBS. After 72 h, transfected cells were harvested for
western blot analysis of Snail, uPA, uPAR and β-actin; conditioned
media was collected for the uPA activity assay. Transfected cells
were also utilized for a subsequent invasion assay.
Invasion assay
The invasive properties of the cell lines were
measured using the BD BioCoat™ Matrigel™ Invasion guidelines.
Briefly, Boyden chamber inserts (Thermo Fisher Scientific, Waltham,
MA, USA) were coated with 50 μl 1:4 Matrigel and allowed to
solidify at 37°C for 1 h. Cells were seeded in quadruplicate at
5×104 (for ARCaP and 22Rv1) and 1×105 (for
LNCaP) in 0.1% FBS, while the lower chamber contained 10% FBS.
Cells were treated accordingly and allowed to invade through the
porous membrane coated with Matrigel at 37°C for 24–72 h. Inserts
were fixed, stained and photographed in two fields per insert. Cell
counts were performed for the determination of relative invasion or
the stain solubilized with Sorenson solution and optical density
measured at 590 nm.
ERK inhibitor asssay treatments
The human CaP cell subline ARCaP Snail
(1×106), was cultured overnight. The following day,
cells were treated with 20 μM ERK1/2 inhibitor (U0126) at the
following time-points (0 and 30 min, 2, 6, 24 and 72 h). The
conditioned media was collected and whole cell lysates were
collected as previously described.
Superarray analysis
Total RNA was isolated from ARCaP Neo or ARCaP Snail
cells using the Qiagen kit according to the manufacturer’s
instructions and 1 μg of which was reverse transcribed with
oligo(dT) using MMLV-reverse transcriptase (Invitrogen Life
Technologies, Carlsbad, CA, USA), to generate cDNA. The labeled
cDNA was incubated with GEArray Q Series cancer pathway membranes
(SuperArray, Valencia, CA, USA) at 60°C overnight. The membrane
used in the present study contained 96 genes that were closely
associated with cancer pathways, in addition to housekeeping
control genes (such as GAPDH). After being washed, the membrane was
incubated with streptavidin-alkaline phosphatase and was finally
exposed to CDP-Star chemiluminescent substrate (SuperArray). Signal
detection was performed using a high Performance chemiluminescence
film (Amersham Biosciences, Amersham, UK). Analysis of results was
performed using GEArray Expression Analysis Suite software
(http://geasuite.superarray.com).
Statistical analysis
All data are presented as the mean ± standard error
of at least three independent experiments. The data were analyzed
using two-way analysis of variance or Student’s t-test. All
statistical analyses were performed and all graphs generated using
GraphPad Prism 5.0 software (GraphPad Software Inc., San Diego, CA,
USA). P<0.05 was considered to indicate statistically
significant differences.
Results
Overexpression of Snail leads to an
increase in cell invasion
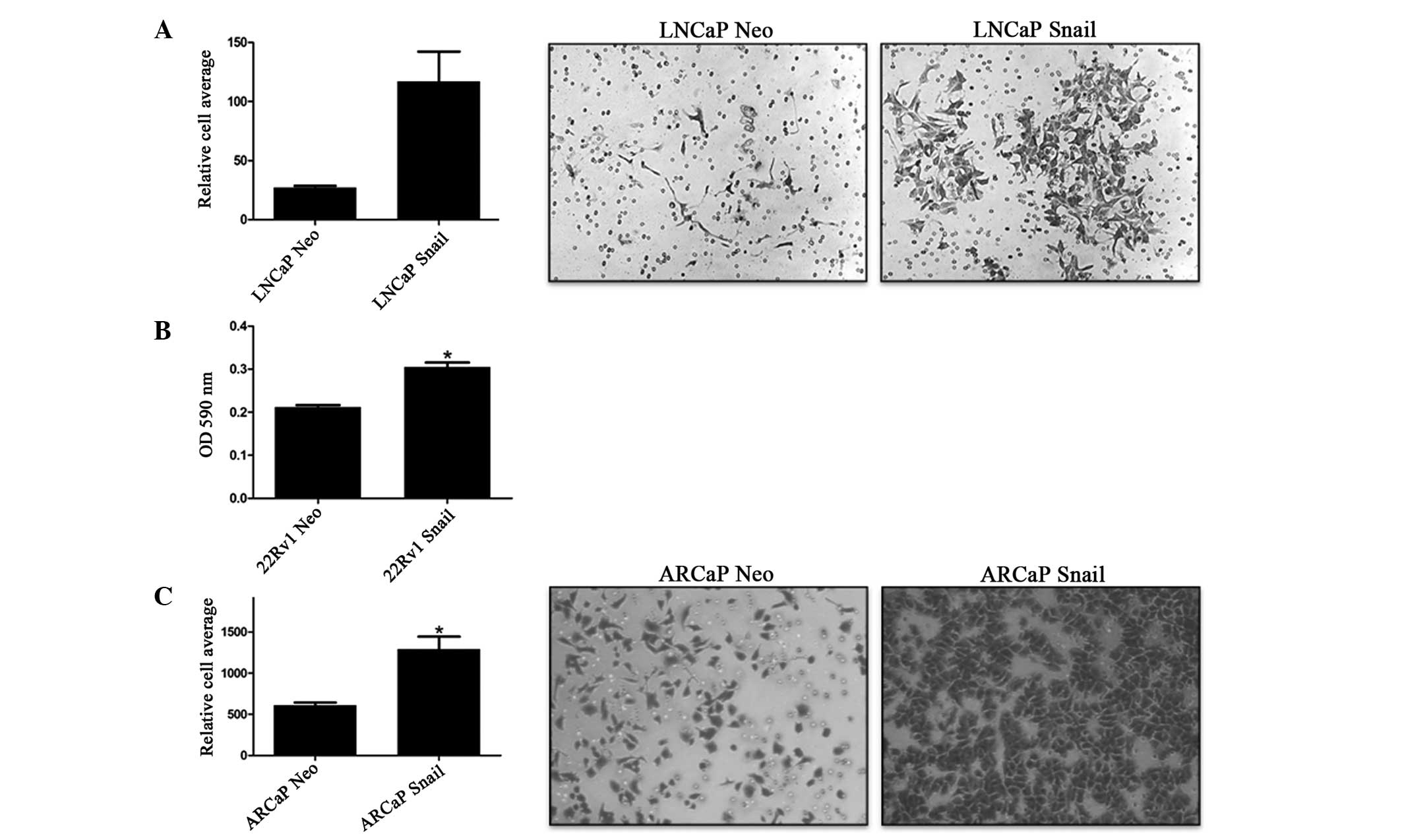
Previously, we have shown that Snail overexpression
increases cell invasion in 22Rv1 cells (35). To confirm these results and examine
the effect of Snail overexpression on LNCaP and ARCaP invasion
through the ECM, an invasion assay was performed where Matrigel
mimicked the ECM. As expected, Snail-transfected cells exhibited
significantly more cell invasion compared with the Neo
control-transfected cells in all three cell lines tested (Fig. 1). Therefore, Snail is associated
with increased cell invasion.
Overexpression of Snail leads to an
upregulation of uPA and uPAR
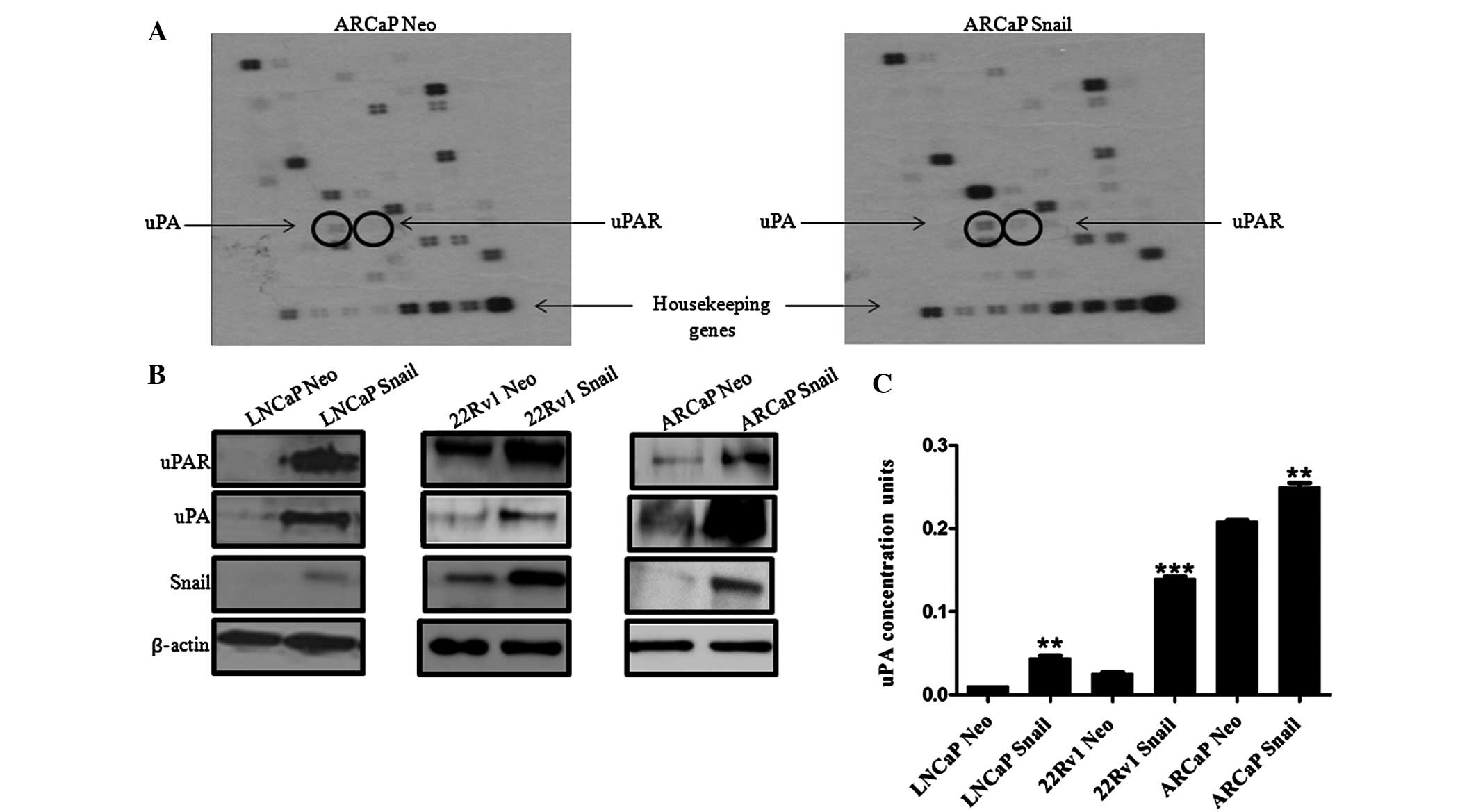
In order to examine the molecular mechanism by which
Snail may increase cell invasion, a superarray analysis was
performed on ARCaP Neo and ARCaP Snail CaP cells to identify genes
downstream of Snail that may be responsible for the increase in
cell invasion. Notably, a protein associated with cell invasion,
uPA, and its receptor, uPAR, were upregulated (Fig. 2A). Subsequently, the protein
expression levels of uPAR and its ligand uPA were evaluated in
Snail overexpressing LNCaP, 22Rv1 and ARCaP cells. In all three CaP
lines, Snail transfection increased uPA and uPAR protein expression
(Fig. 2B). Additionally,
measurement of secreted uPA activity in conditioned media showed
that LNCaP, 22Rv1 and ARCaP cell lines overexpressing Snail
exhibited higher uPA activity compared with that of the Neo control
(Fig. 2C). The results also
suggested that the androgen-independent ARCaP cells had a higher
active uPA concentration compared with that of the
androgen-dependent LNCaP and 22Rv1 cells. Therefore, Snail is
associated with increased uPa/uPAR protein levels and increased uPA
activity.
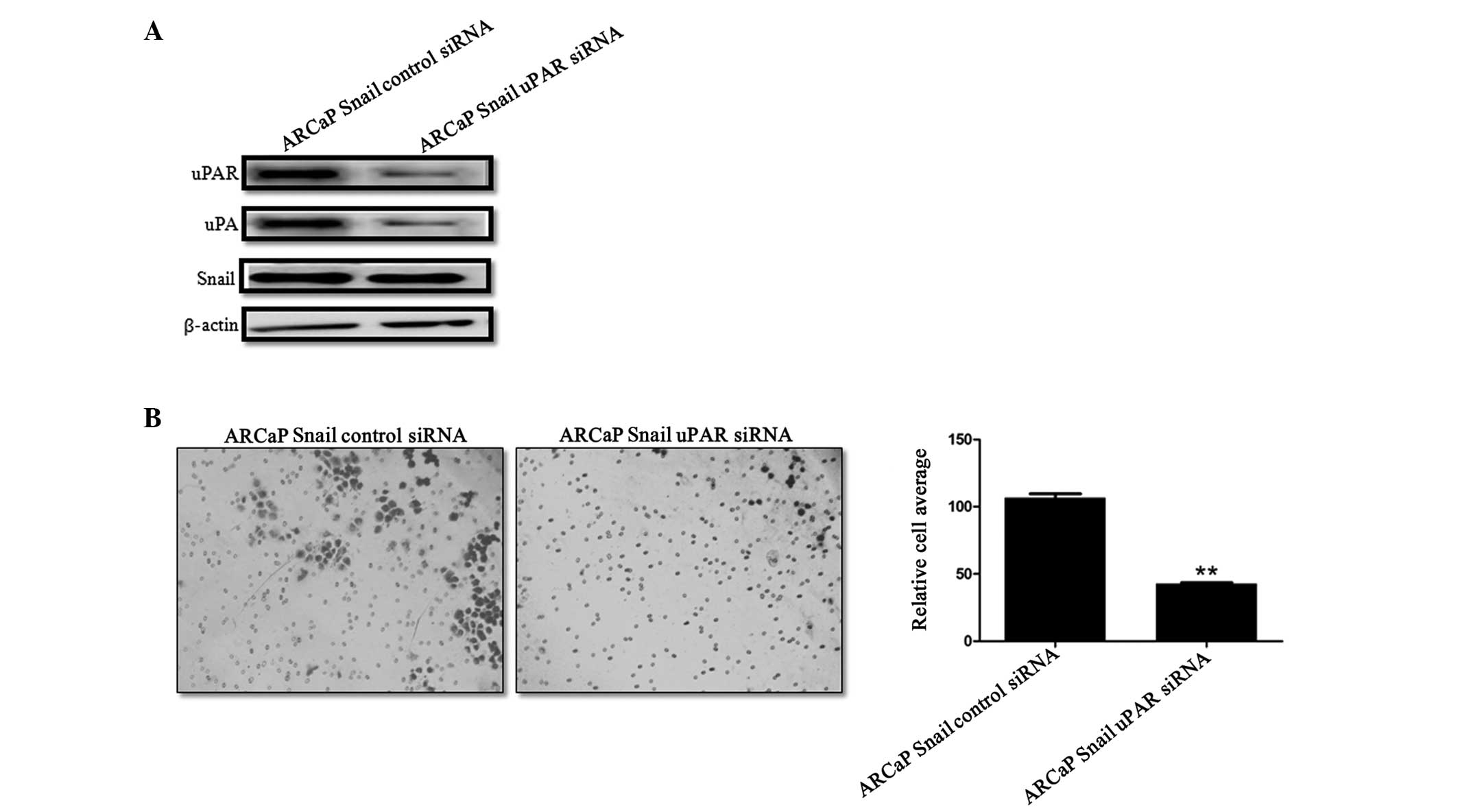
uPAR knockdown in Snail-overexpressing
ARCaP cells leads to decreased cell invasion
To evaluate the contribution of uPA/uPAR in the
increased invasion that was observed in the Snail overexpressed
cells, uPAR was transiently knocked down in ARCaP Snail cells.
Western blot analysis confirmed the knockdown of uPAR (Fig. 3A). Of note, uPAR knockdown was
accompanied by a decrease in uPA expression, while Snail expression
was not affected by this knockdown (Fig. 3A). Functionally, there was a
significant decrease in invasion following uPAR knockdown (Fig. 3B). Thus, uPAR contributes to
Snail-mediated cell invasion.
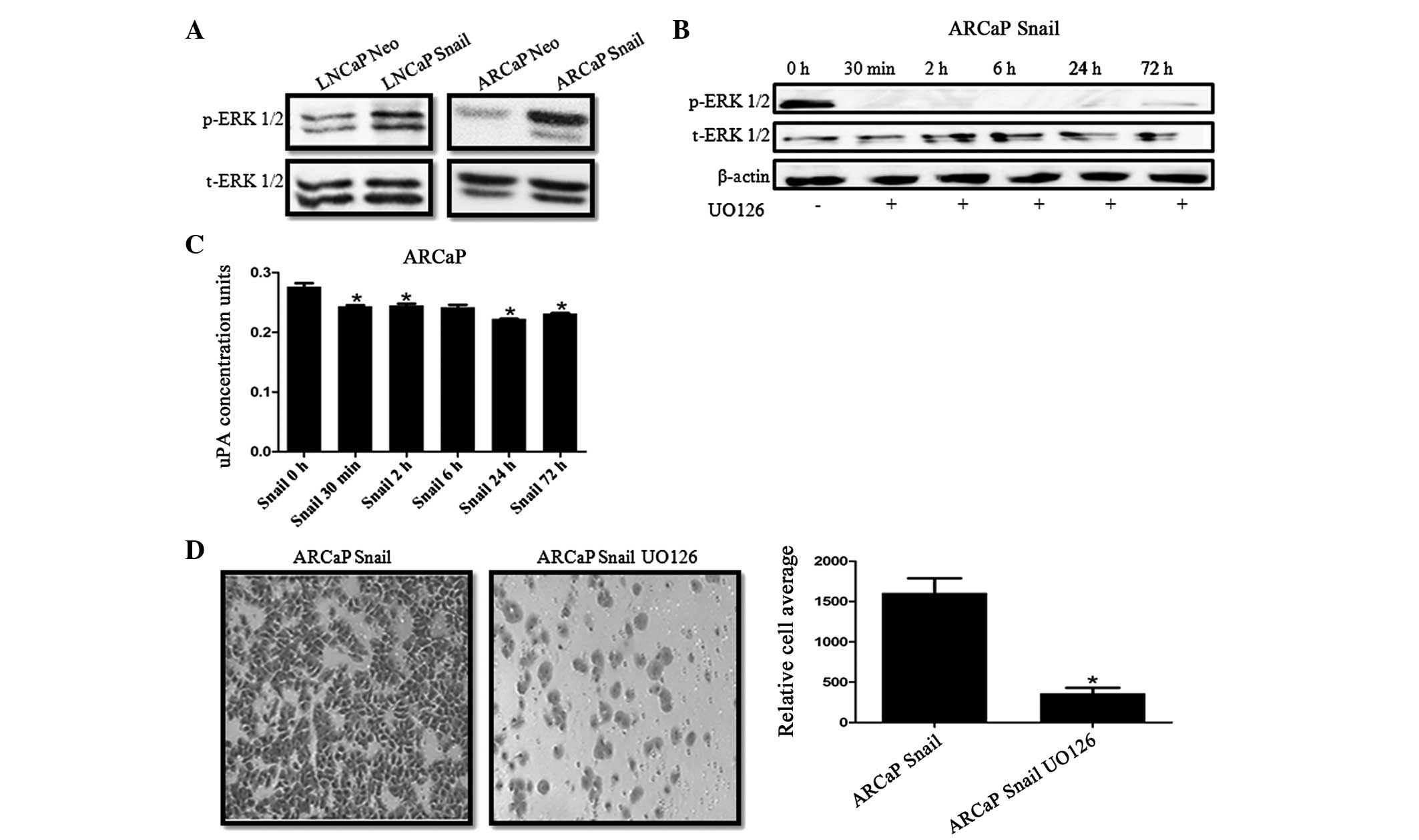
Inhibition of MAPK activity downregulates
uPA activity and decreases cell invasion
We have previously demonstrated that there is an
increase of phosphorylated MAPK (p-ERK) in CaP cells overexpressing
Snail (34,36). Therefore, we investigated whether
Snail regulation of uPA activity was mediated by MAPK signaling. It
was identified that Snail overexpression increases ERK activity in
LNCaP and ARCaP cell lines (Fig.
4A). Subsequently, Snail-transfected ARCaP cells were treated
with 20 μM UO126 MEK inhibitor for 30 min and 2, 6, 24 and 72 h.
Decreased ERK activity was observed by 30 min and persisted until
72 h as shown by the western blot analysis (Fig. 4B). It was also revealed that
inhibiting MAPK activity significantly decreased uPA activity
within 30 min (Fig. 4C). Finally,
ARCaP Snail cells treated with U1O26 for 24 h showed decreased
invasive potential compared with that of the ARCaP Neo control
(Fig. 4D).
Discussion
Studies have suggested that epithelial mesenchymal
transition (EMT) is an important step leading to cancer metastasis
(7–9). One mechanism by which E-cadherin is
downregulated in EMT is transcriptional repression by Snail
(10,11). In the present study, we have shown
that overexpression of Snail increases cell invasion in
androgen-dependent LNCaP and 22RV1 prostate cancer cell lines and
androgen-independent ARCaP prostate cancer cell lines. In
Snail-transfected ARCaP cells, certain genes that were upregulated
and downregulated were evaluated via superarray analysis, based on
their function. The results of the superarray demonstrated that the
overexpression of Snail leads to upregulation of genes involved
with invasion and metastasis, such as uPA and uPAR. It was
noteworthy that uPA and uPAR were upregulated in Snail-transfected
CaP cells, as in previous studies performed in PC3 and DU145 cells,
RNA interference of uPA and uPAR resulted in uPA and uPAR mRNA and
protein expression being completely inhibited and there was a
decline in metastasis (38).
Although the signaling cascade resulting in the expression of uPA
and uPAR being downregulated was not determined, the superarray
analysis and uPAR siRNA studies done in Snail-transfected cells
suggest that it may be through Snail. To confirm our superarray
studies, we showed that uPA and uPAR protein expression was
increased in Snail-overexpressing cells. Additionally, Snail
overexpression led to increased uPA activity. Although there was a
general increase in uPA activity in the Snail-transfected cells,
there was a greater level of uPA activity in the
androgen-independent ARCaP cells compared with that in the
androgen-dependent LNCaP and 22Rv1 cells.
To determine the effect the uPA/uPAR system has on
the increase in invasion in Snail-transfected cells, uPAR was
transiently knocked down. The most well known activator of uPA is
uPAR; therefore; knocking down uPAR inhibits the function of both
uPA and uPAR (19). We observed
that uPAR knockdown in ARCaP Snail cells led to a significant
decrease in cell invasion. It is noteworthy that Snail expression
was not affected by the knockdown of uPAR, suggesting that uPAR is
acting downstream of Snail to increase cell invasion; thus, for the
first time, we show that Snail relies on uPAR to increase invasion.
It may be suggested that uPA/uPAR signaling alone does not have an
important role in Snail-mediated invasion in ARCaP cells, as uPAR
knockdown did not completely eliminate invasion. Previously, we
have shown that ERK activity is increased in Snail-transfected
ARCaP cells (34,36). In the present study, in order to
determine whether Snail mediates invasion through the MAPK pathway,
Snail-transfected cells were treated with MEK inhibitor UO126 for
various time periods. uPA activity and invasion was significantly
decreased in ARCaP Snail cells treated with UO126 in a
time-dependent manner. This suggests that Snail may use the MAPK
pathway to mediate cell invasion through uPA/uPAR signaling in
ARCaP cells. Supporting these results, the literature suggest that
uPAR is under an ERK-dependent mechanism and blocking uPAR’s
activity leads to inhibition of motility in hepatocellular
carcinoma (27). Additionally, a
study on human gastric cancer has shown that EGF stimulates uPAR
expression via the ERK pathway, sequentially increasing cell
invasion (28). Although the
activity of uPA was decreased upon MAPK inhibition, it was not
completely eliminated, possibly since its activity may be mediated
by additional pathways, such as AKT. In breast cancer, studies have
shown that upon uPA binding to uPAR, AKT is activated (39,40).
Overall, the present results show, for the first
time, a link between Snail, MAPK and uPA/uPAR in CaP. Our studies
suggest that Snail overexpression increases cell invasion through
the upregulation of uPA/uPAR signaling, which is mediated in part
by the MAPK signaling pathway.
Acknowledgements
This study was supported by NIH grants 1P20MD002285
(VOM) and 8G12MD007590.
Abbreviations:
|
EMT
|
epithelial to mesenchymal
transition
|
|
uPA
|
urokinase plasminogen activator
|
|
uPAR
|
urokinase plasminogen activator
receptor
|
|
CaP
|
prostate cancer
|
|
MAPK
|
mitogen-activated protein kinase
|
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar
|
|
2
|
Siegel R, DeSantis C, Virgo K, et al:
Cancer treatment and survivorship statistics, 2012. CA Cancer J
Clin. 62:220–241. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mimeault M and Batra SK: Recent advances
on multiple tumorigenic cascades involved in prostatic cancer
progression and targeting therapies. Carcinogenesis. 27:1–22. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Culig Z, Steiner H, Bartsch G and Hobisch
A: Mechanisms of endocrine therapy-responsive and -unresponsive
prostate tumours. Endocr Relat Cancer. 12:229–244. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Clarke NW, Hart CA and Brown MD: Molecular
mechanisms of metastasis in prostate cancer. Asian J Androl.
11:57–67. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chambers AF, MacDonald IC, Schmidt EE, et
al: Steps in tumor metastasis: new concepts from intravital
videomicroscopy. Cancer Metastasis Rev. 14:279–301. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu J, Lamouille S and Derynck R:
TGF-beta-induced epithelial to mesenchymal transition. Cell Res.
19:156–172. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jing Y, Han Z, Zhang S, Liu Y and Wei L:
Epithelial-Mesenchymal Transition in tumor microenvironment. Cell
Biosci. 1:292011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Larue L and Bellacosa A:
Epithelial-mesenchymal transition in development and cancer: role
of phosphatidylinositol 3′ kinase/AKT pathways. Oncogene.
24:7443–7454. 2005.
|
|
10
|
Cho HJ, Baek KE, Saika S, Jeong MJ and Yoo
J: Snail is required for transforming growth factor-beta-induced
epithelial-mesenchymal transition by activating PI3 kinase/Akt
signal pathway. Biochem Biophys Res Commun. 353:337–343. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Batlle E, Sancho E, Francí C, et al: The
transcription factor snail is a repressor of E-cadherin gene
expression in epithelial tumour cells. Nat Cell Biol. 2:84–89.
2000. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nieto MA: The snail superfamily of
zinc-finger transcription factors. Nat Rev Mol Cell Biol.
3:155–166. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Haraguchi M: The role of the
transcriptional regulator snail in cell detachment, reattachment
and migration. Cell Adh Migr. 3:259–263. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Barrallo-Gimeno A and Nieto MA: The Snail
genes as inducers of cell movement and survival: implications in
development and cancer. Development. 132:3151–3161. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Domínguez D, Montserrat-Sentís B,
Virgós-Soler A, et al: Phosphorylation regulates the subcellular
location and activity of the snail transcriptional repressor. Mol
Cell Biol. 23:5078–5089. 2003.PubMed/NCBI
|
|
16
|
Peiró S, Escrivà M, Puig I, et al: Snail1
transcriptional repressor binds to its own promoter and controls
its expression. Nucleic Acids Res. 34:2077–2084. 2006.PubMed/NCBI
|
|
17
|
Peinado H, Ballestar E, Esteller M and
Cano A: Snail mediates E-cadherin repression by the recruitment of
the Sin3A/histone deacetylase 1 (HDAC1)/HDAC2 complex. Mol Cell
Biol. 24:306–319. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Heebøll S, Borre M, Ottosen PD, Dyrskjøt
L, Orntoft TF and Tørring N: Snail1 is over-expressed in prostate
cancer. APMIS. 117:196–204. 2009.
|
|
19
|
Dass K, Ahmad A, Azmi AS, Sarkar SH and
Sarkar FH: Evolving role of uPA/uPAR system in human cancers.
Cancer Treat Rev. 34:122–136. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Conese M and Blasi F: Urokinase/urokinase
receptor system: internalization/degradation of urokinase-serpin
complexes: mechanism and regulation. Biol Chem Hoppe Seyler.
376:143–155. 1995.PubMed/NCBI
|
|
21
|
Smith HW and Marshall CJ: Regulation of
cell signalling by uPAR. Nat Rev Mol Cell Biol. 11:23–36. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dong Z, Saliganan AD, Meng H, et al:
Prostate cancer cell-derived urokinase-type plasminogen activator
contributes to intraosseous tumor growth and bone turnover.
Neoplasia. 10:439–449. 2008.PubMed/NCBI
|
|
23
|
Lee KH, Kim SW and Kim JR: Reactive oxygen
species regulate urokinase plasminogen activator expression and
cell invasion via mitogen-activated protein kinase pathways after
treatment with hepatocyte growth factor in stomach cancer cells. J
Exp Clin Cancer Res. 28:732009. View Article : Google Scholar
|
|
24
|
Shariat SF, Roehrborn CG, McConnell JD, et
al: Association of the circulating levels of the urokinase system
of plasminogen activation with the presence of prostate cancer and
invasion, progression, and metastasis. J Clin Oncol. 25:349–355.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mazar AP, Ahn RW and O’Halloran TV:
Development of novel therapeutics targeting the urokinase
plasminogen activator receptor (uPAR) and their translation toward
the clinic. Curr Pharm Des. 17:1970–1978. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jo M, Takimoto S, Montel V and Gonias SL:
The urokinase receptor promotes cancer metastasis independently of
urokinase-type plasminogen activator in mice. Am J Pathol.
175:190–200. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bessard A, Frémin C, Ezan F, Coutant A and
Baffet G: MEK/ERK-dependent uPAR expression is required for
motility via phosphorylation of P70S6K in human hepatocarcinoma
cells. J Cell Physiol. 212:526–536. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Baek MK, Kim MH, Jang HJ, et al: EGF
stimulates uPAR expression and cell invasiveness through ERK, AP-1,
and NF-κB signaling in human gastric carcinoma cells. Oncol Rep.
20:1569–1575. 2008.PubMed/NCBI
|
|
29
|
Jordà M, Olmeda D, Vinyals A, et al:
Upregulation of MMP-9 in MDCK epithelial cell line in response to
expression of the Snail transcription factor. J Cell Sci.
118:3371–3385. 2005.PubMed/NCBI
|
|
30
|
Miyoshi A, Kitajima Y, Sumi K, et al:
Snail and SIP1 increase cancer invasion by upregulating MMP family
in hepatocellular carcinoma cells. Br J Cancer. 90:1265–1273. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yokoyama K, Kamata N, Fujimoto R, et al:
Increased invasion and matrix metalloproteinase-2 expression by
Snail-induced mesenchymal transition in squamous cell carcinomas.
Int J Oncol. 22:891–898. 2003.PubMed/NCBI
|
|
32
|
Jo M, Lester RD, Montel V, Eastman B,
Takimoto S and Gonias SL: Reversibility of epithelial-mesenchymal
transition (EMT) induced in breast cancer cells by activation of
urokinase receptor-dependent cell signaling. J Biol Chem.
284:22825–22833. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fabre-Guillevin E, Malo M, Cartier-Michaud
A, et al: PAI-1 and functional blockade of SNAI1 in breast cancer
cell migration. Breast Cancer Res. 10:R1002008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Neal CL, McKeithen D and Odero-Marah VA:
Snail negatively regulates cell adhesion to extracellular matrix
and integrin expression via the MAPK pathway in prostate cancer
cells. Cell Adh Migr. 5:249–257. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Neal CL, Henderson V, Smith BN, et al:
Snail transcription factor negatively regulates maspin tumor
suppressor in human prostate cancer cells. BMC Cancer. 12:3362012.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Barnett P, Arnold RS, Mezencev R, Chung
LW, Zayzafoon M and Odero-Marah V: Snail-mediated regulation of
reactive oxygen species in ARCaP human prostate cancer cells.
Biochem Biophys Res Commun. 404:34–39. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
McKeithen D, Graham T, Chung LW and
Odero-Marah V: Snail transcription factor regulates neuroendocrine
differentiation in LNCaP prostate cancer cells. Prostate.
70:982–992. 2010.
|
|
38
|
Pulukuri SM, Gondi CS, Lakka SS, et al:
RNA interference-directed knockdown of urokinase plasminogen
activator and urokinase plasminogen activator receptor inhibits
prostate cancer cell invasion, survival, and tumorigenicity in
vivo. J Biol Chem. 280:36529–36540. 2005. View Article : Google Scholar
|
|
39
|
Alfano D, Iaccarino I and Stoppelli MP:
Urokinase signaling through its receptor protects against anoikis
by increasing BCL-xL expression levels. J Biol Chem.
281:17758–17767. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lester RD, Jo M, Montel V, Takimoto S and
Gonias SL: uPAR induces epithelial-mesenchymal transition in
hypoxic breast cancer cells. J Cell Biol. 178:425–436. 2007.
View Article : Google Scholar : PubMed/NCBI
|