Introduction
Ovarian carcinomas account for only 15–20% of female
malignant carcinomas in the US; however, these tumors have the
highest mortality rate. Ovarian serous carcinoma (OSC) accounts for
40% of all ovarian carcinomas in the US and is the leading cause of
morbidity and mortality in malignant carcinoma of the female
reproductive system (1). Despite
applied chemotherapies and cytoreductive surgery combined
therapies, the five-year survival rate of OSC is only 30–40%
(2). Recent studies have found that
ovarian carcinoma is a disease that is characterized by
simultaneous excessive cell proliferation and decreased apoptosis,
with uncontrolled proliferation and blocked apoptosis playing a
crucial role in tumorigenesis (3,4). It is
important to study the mechanisms and genes associated with
proliferation and apoptosis in ovarian carcinoma, particularly for
OSC, which may highlight new gene-therapeutic methods that
simultaneously inhibit proliferation and induce apoptosis of cancer
cells.
The phosphatidylinositol-3-kinase/protein kinase B
(PI3K/Akt) signaling pathway was identified in 1987 by Staal who
found an oncogene known as serine/threonine-specific protein kinase
(Akt) in the murine retrovirus AKT8 after producing foci of
malignant transformation in the mink lung epithelial cell line, CCL
64 (5). Akt is a 60-kDa
serine/threonine-specific protein kinase prevalent in eukaryotic
regulatory networks and has an important junction point which links
multiple signal transduction pathways, regulates multiple
extracellular cytokine signal transductions and is important for
Ras-mediated oncogenic transformation (6). The PI3K/Akt signaling pathway is also
involved in cell metabolism, regulation of the cell cycle and
angiogenesis, and is associated with the development of diabetes
and multiple autoimmune diseases, including rheumatoid arthritis.
Deficiency or inactivation of the PI3K/Akt signaling
pathway-associated regulatory genes, mutation or amplification of
the PI3K gene and activation of receptors or junction molecules of
its downstream signaling pathway have been identified in multiple
tumor cell lines (7).
Cyclin-dependent kinases (CDKs) are crucial for the
cell division cycle (8,9). The cell cycle is coregulated by CDKs
and cyclin-dependent kinase inhibitors (CDKIs), as well as cyclin
D1 levels, which are essential for the G1 to S phase cell cycle
transition. However, little is known with regard to the association
of Akt with the cell cycle progression.
Based on a previous study (10), pathomorphology and
immunohistochemistry were used in the current study to investigate
the expression of p-Akt and cyclin D1 in normal ovarian tissue
(NOT), ovarian serous cystadenoma (OSA), ovarian serous borderline
tumors (OS-BT) and OSC samples to further investigate the role of
p-Akt in the development of ovarian epithelial cancer and its
possible effect on cyclin D1 signaling pathway activation.
Materials and methods
Tissue samples
Paraffin-embedded tissues were collected from the
First Hospital of Shijiazhuang (Shijiazhuang, China). All tissues
originated from patients aged between 35 and 64 years old without
history of radiotherapy, chemotherapy and hormone therapy prior to
surgery. Among the collected samples, 12 were OSA, 18 were OS-BT
and 46 were OSC. Of the 46 OSC samples, 16 were
well-differentiated, 20 were moderately differentiated and 10 were
poorly differentiated, while 22 OSC samples led to lymphatic
metastasis and 24 were metastasis-free. An additional 10
paraffin-embedded NOTs were used as control. The current study was
approved by the ethics committee of the First Hospital of
Shijiazhuang and the Sixth Hospital of Shijiazhuang. Informed
written consent was obtained from all participants.
Immunohistochemistry
Immunohistochemistry S-P assays were used to
investigate the expression of p-Akt in NOT, OSA, OS-BT and OSC
samples. In total, 10 high power fields were randomly selected from
each paraffin section and examined under a light microscope
(Olympus, Tokyo, Japan) using the double-blind method. All slides
were processed with polylysine prior to immunohistochemical
staining for p-Akt and cyclin D1 protein visualization. Rabbit
anti-human p-Akt monoclonal antibodies (Cell Signaling Technology,
Inc., Danvers, MA, USA) and rabbit anti-human cyclin D1 monoclonal
antibodies (ready-to-use; Fuzhou Maixin Biotechnology Development
Co., Ltd., Fuzhou, China) were used as primary antibodies, and goat
anti-rabbit IgG/biotin (Fuzhou Maixin Biotechnology Development
Co., Ltd.) was used as the secondary antibody. Phosphate-buffered
saline (PBS; 0.01 mol/l) only was used as a control for primary
antibodies.
Sample preparation
Paraffin-embedded samples were prepared into 4-μm
sections and deparaffinized by a standard method. The paraffin
sections were then submerged in hydrogen peroxide methanol
solutions and vortexed at room temperature for 15 min to block the
bioactivity of endogenous peroxidases. Next, sections were rinsed
twice for 5 min with distilled water and placed into plastic boxes
filled with antigen retrieval buffer (0.01 mol/l citric acid/sodium
citrate solution, pH 6.0; Fuzhou Maixin Biotechnology Development
Co., Ltd.) for initial microwave treatment at 7th gear for 5 min
followed by a second treatment at 4th gear for 3 min. The boxes
were then cooled at room temperature for 15–20 min. Next, sections
were rinsed three times for 5 min with 0.01 mol/l PBS and normal
goat serum was added at 37°C for 30 min to block endogenous biotin.
Serum was discarded and primary antibodies (rabbit anti-human p-Akt
monoclonal antibody, 1:200; and rabbit anti-human cyclin D1
monoclonal antibody, ready-to-use) were added separately for
overnight incubation in a humid atmosphere at 4°C. Sections were
then rinsed three times for 5 min with 0.01 mol/l PBS, and
secondary antibody (goat anti-rabbit IgG/biotin) was added for 25
min at 37°C, followed rinsing three times for 5 min with 0.01 mol/l
PBS. Next, streptavidin/horseradish peroxidase was added for 20 min
at 37°C and the sections were rinsed four times for 5 min with 0.01
mol/l PBS. Finally, freshly prepared DAB-H2O2
(Fuzhou Maixin Biotechnology Development Co., Ltd.) was added for
color development, which was monitored under a light microscope
(Olympus CX21; Olympus Corporation, Tokyo, Japan) and rinsed again
with distilled water to terminate the reaction. Hematoxylin (BASO
Precision Optics Ltd., Taiching, Taiwan) was added for slight
re-staining. Sections were differentiated with hydrochloric acid
alcohol, dehydrated with an ascending series of ethanol, cleared in
xylene and mounted in neutral balsam. Positive samples and negative
controls were set up during the process. The procedure was used for
all sample sections.
Immunochemistry analysis
Selecting a homogeneously stained positive region
and grading the proportion of stained cells compared with all cells
within the field of vision was scored as follows: 0, no positively
stained cells; 1, <25% positively stained cells; 2, 25–50%
positively stained cells; and 3, >50% positively stained cells.
Grading was determined by the color intensity of stained cells and
was as follows: 0, negative; 1, weak light yellow; 2, medium brown
yellow; and 3, strong dark brown. Results were analyzed by adding
the above values together and were determined as follows: 0–2,
negative (−); 3–4, weak-positive (+); and 5–6, strong-positive
(++). Weak-positive (+) and strong-positive (++) were considered as
positive.
Statistical analysis
Data were analyzed using SPSS version 11.0 (SPSS,
Inc., Chicago, IL, USA) and quantitative data are presented as the
medium ± standard deviation. Values between groups were compared by
one-way analysis of variance and counting data were analyzed by the
χ2 test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of p-Akt in ovarian
carcinoma
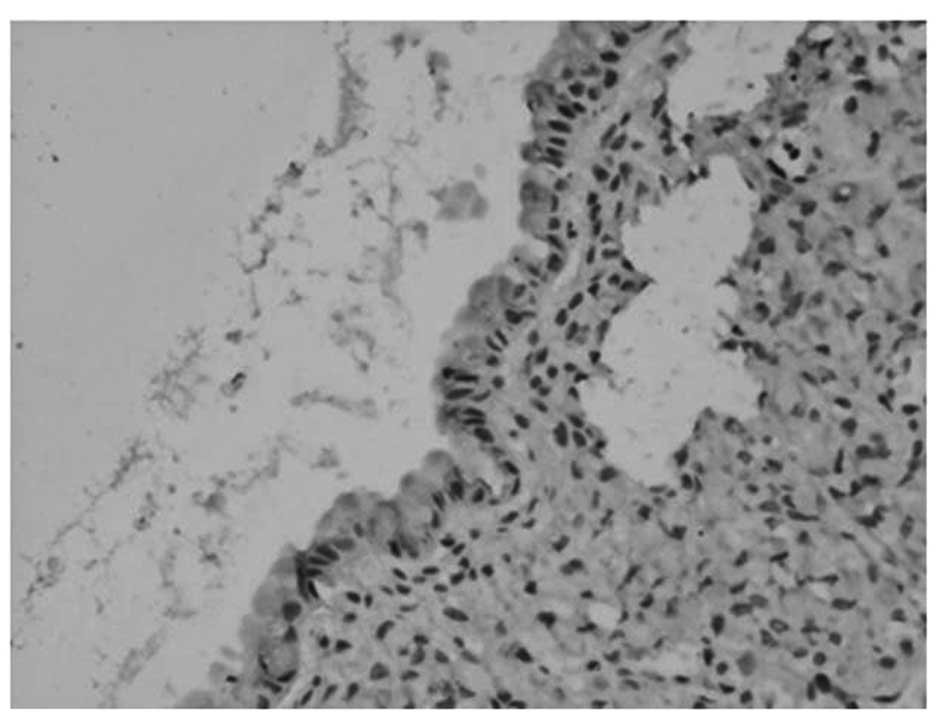
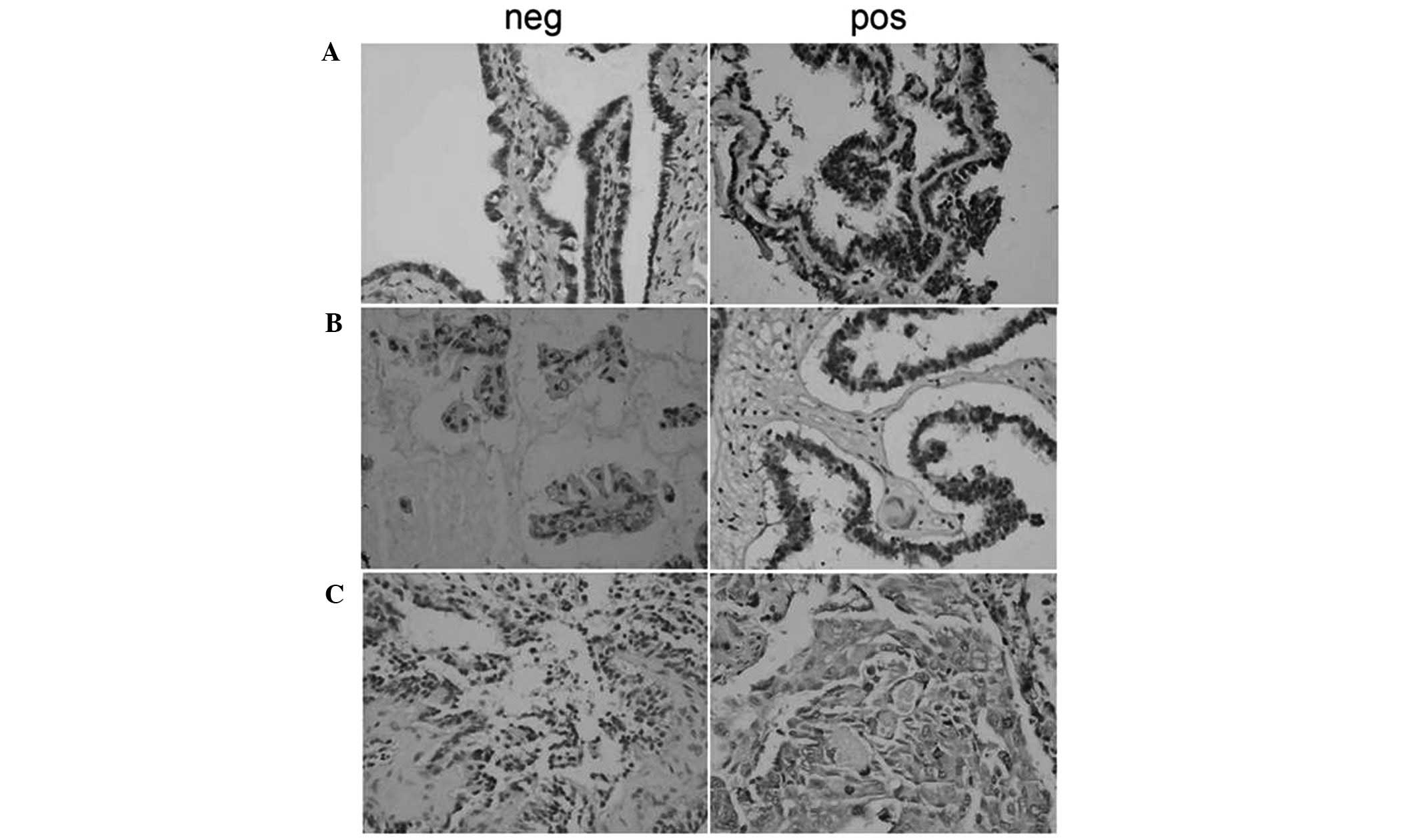
p-Akt was predominantly located in the nuclei and
cytoplasm of ovarian carcinoma cells, appearing as dark brown
sediments (Figs. 1 and 2). Based on the immunohistochemistry S-P
assay results (Tables I–III), p-Akt prevalence was significantly
different between the NOT, OSA, OS-BT and OSC groups
(χ2=19.781; P<0.01). In the OSC samples, the
prevalence of p-Akt expression was reversely associated with tumor
differentiation (P<0.01). p-Akt prevalence was positively
associated with lymphatic metastasis (r=0.334; P=0.023) and a
higher p-Akt prevalence was observed in OSC samples with lymphatic
metastasis compared with metastasis-free OSC samples
(P<0.05).
 | Table IExpression of p-Akt in different
groups. |
Table I
Expression of p-Akt in different
groups.
| | p-Akt expression, n
(%) | |
|---|
| |
| |
|---|
| Groups | n | − | + | P-value |
|---|
| NOT | 10 | 10/10 (100.00) | 0/10 (0.00) | |
| OSA | 12 | 10/12 (83.33) | 2/12 (16.67) | 0.481 |
| OS-BT | 18 | 8/18 (44.44) | 10/18 (55.56) | 0.004 |
| OSC | 46 | 16/46 (34.78) | 30/46 (65.22) | 0.000 |
 | Table IIICorrelation between the expression of
p-Akt and infiltration and metastasis of cancer tissue. |
Table III
Correlation between the expression of
p-Akt and infiltration and metastasis of cancer tissue.
| p-Akt expression |
|---|
|
|
|---|
| Infiltration or
metastasis | − | + |
|---|
| − | 12 | 12 |
| + | 4 | 18 |
Expression of cyclin D1 in ovarian
carcinoma
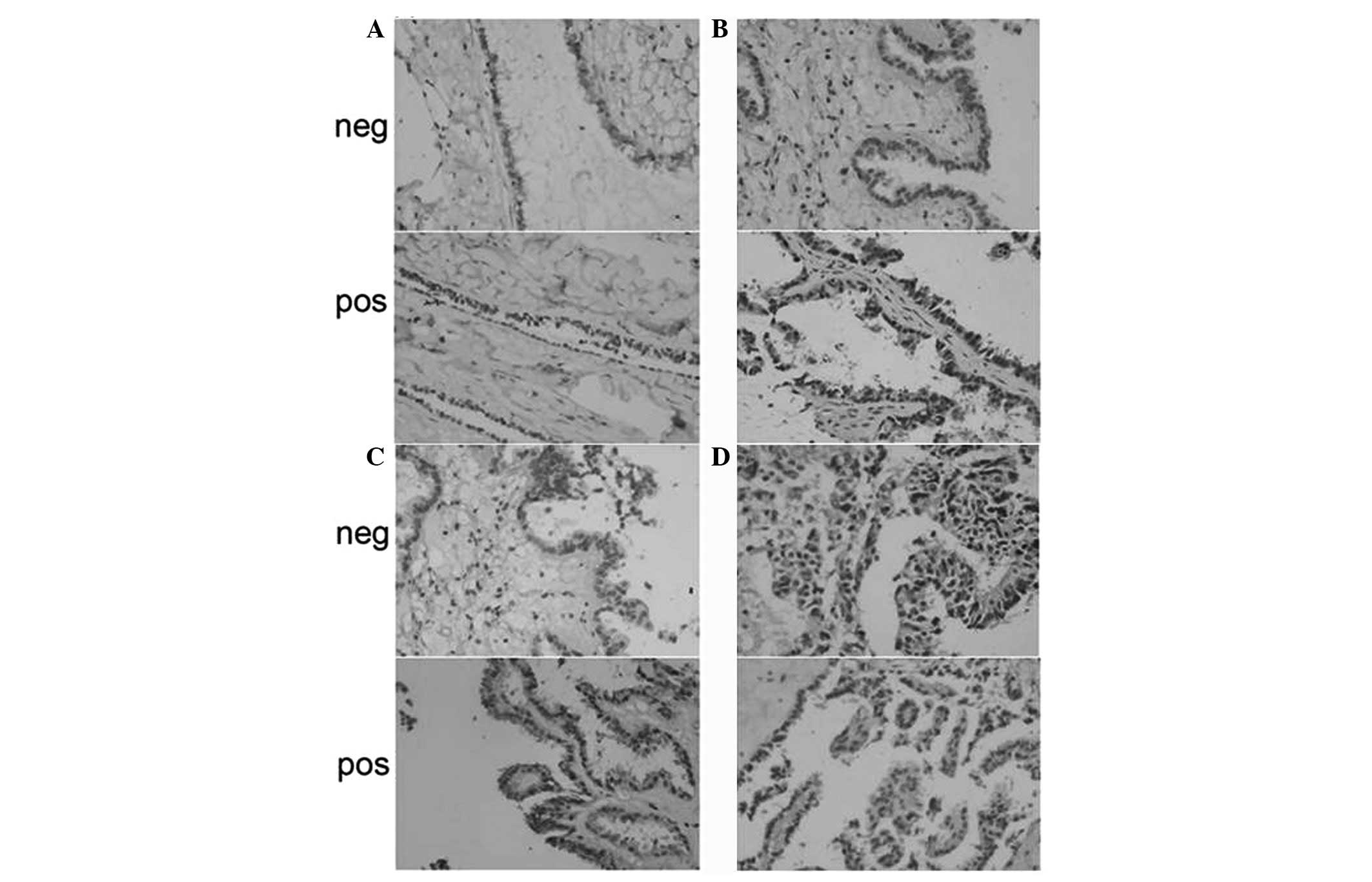
Cyclin D1 was predominantly located in the nuclei
and cytoplasm of ovarian carcinoma cells, appearing as brown nuclei
and dark brown sediments in the cytoplasm (Fig. 3). Based on the immunohistochemistry
S-P assay results (Tables
IV–VI), the prevalence of
cyclin D1 expression was significantly different among the NOT,
OSA, OS-BT and OSC groups (χ2=19.241; P<0.01). OSA
and OSC groups exhibited significantly higher cyclin D1 levels
compared with the NOT group (P<0.05 and P<0.01,
respectively). No significant difference in the prevalence of
cyclin D1 expression was observed among the three tumor
differentiation stages within the 46 OSC samples (P>0.05). The
prevalence of cyclin D1 expression was positively associated with
lymphatic metastasis (r=0.371; P=0.011), since a higher cyclin D1
prevalence was observed in OSC samples with lymphatic metastasis
compared with metastasis-free OSC samples (P<0.05).
 | Table IVExpression of cyclin D1 in different
groups. |
Table IV
Expression of cyclin D1 in different
groups.
| | Cyclin D1 expression,
n (%) | |
|---|
| |
| |
|---|
| Group | n | − | + | P-value |
|---|
| NOT | 10 | 9/10 (100.00) | 1/10 (10.00) | |
| OSA | 12 | 9/12 (75.00) | 3/12 (25.00) | 0.594 |
| OS-BT | 18 | 8/18 (44.44) | 10/18 (55.56) | 0.041 |
| OSC | 46 | 12/46 (26.09) | 34/46 (73.91) | 0.000 |
 | Table VICorrelation between the expression of
cyclin D1 and the infiltration and metastasis of OSC. |
Table VI
Correlation between the expression of
cyclin D1 and the infiltration and metastasis of OSC.
| Cyclin D1
expression |
|---|
|
|
|---|
| Infiltration or
metastasis | − | + |
|---|
| − | 10 | 14 |
| + | 2 | 20 |
Association of p-AKT, caspase-3 and
cyclin D1
The prevalence of p-Akt expression was positively
associated with the prevalence of cyclin D1 expression
(P<0.001), but negatively associated with the prevalence of
caspase-3 expression (P=0.017) (Tables VII and VIII).
 | Table VIICorrelation between the expression of
p-Akt and cyclin D1 in OSC. |
Table VII
Correlation between the expression of
p-Akt and cyclin D1 in OSC.
| Cyclin D1
expression |
|---|
|
|
|---|
| p-Akt
expression | + | − |
|---|
| + | 28 | 2 |
| − | 6 | 10 |
 | Table VIIICorrelation between the expression of
p-Akt and caspase-3 in OSC. |
Table VIII
Correlation between the expression of
p-Akt and caspase-3 in OSC.
| Caspase-3
expression |
|---|
|
|
|---|
| p-Akt
expression | + | − |
|---|
| + | 8 | 22 |
| − | 6 | 10 |
Discussion
The Akt gene, also known as protein kinase B, is an
oncogene that was identified in 1987 by Staal and recognized as a
serine/threonine-specific protein kinase (5). Akt was cloned in 1991 and is composed
of an N-terminal regulatory domain, central kinase domain and
C-terminal regulatory domain, as well as a hinge region.
Phosphorylation of Akt at Ser473 and Thr308 is essential for the
activation of p-Akt. Activated Akt relocates to the cytoplasm and
nucleus where it phosphorylates multiple substrates to activate or
inhibit downstream targets, including Bad (a Bcl-2 family member)
(11), nuclear factor κB (12), glycogen synthase kinase-3 (GSK-3)
(13), transcription regulatory
proteins and other proteins involved in the regulation of cell
proliferation, differentiation and apoptosis. Expression levels of
p-Akt indicate the bioactivity of the PI3K/Akt signaling pathway.
In the current study, immunohistochemistry S-P assays were used to
detect the expression of p-Akt in ovarian carcinoma tissues. No
p-Akt expression was identified in NOTs, but positive expression
rates of 16.7, 55.6 and 82.6% were identified in OSA, OS-BT and OSC
tissues, respectively. The positive expression rates of p-Akt in
well-, moderately and poorly differentiated ovarian carcinoma were
43.6, 65.0 and 80.0%, respectively. The prevalence of p-Akt
expression in ovarian carcinoma tissue with lymphatic metastasis
was 81.8% and in metastasis-free ovarian carcinoma tissue was
50.0%. Statistical analyses indicated that the prevalence of p-Akt
expression was significantly different among the NOT, OSA, OS-BT
and OSC groups (χ2=19.781; P<0.01), particularly,
between the NOT and OS-BT, OSA and OS-BT and OSA and OSC groups
(P<0.01). Among the 46 OSC samples, p-Akt occurrence was
negatively associated with the degree of tumor differentiation
(P<0.01) and statistical analysis also revealed that p-Akt
prevalence was positively associated with lymphatic metastasis
(r=0.334; P=0.023), since OSC tissue with lymphatic metastasis
exhibited significantly higher p-Akt levels compared with
metastasis-free OSC tissue. Previous studies have reported a
possible involvement of the PI3K/Akt signaling pathway in ovarian
carcinoma development and its clinical implications. Philp et
al found that the p85 subunit of PI3K may be a new ovarian
carcinoma oncogene and that mutations in PI3KCA may play critical
roles in the development of ovarian carcinoma (14). Based on immunohistochemistry assay
results, Noske et al found that Akt expression was 58%
higher in primary ovarian carcinoma compared with that in NOTs, and
it was significantly associated with positive lymph node rates and
International Federation of Gynecology and Obstetrics stages
(15). In addition, western blot
analyses revealed that positive Akt expression in all investigated
ovarian carcinoma cell lines and gonadal hormones increased the
invasion and metastasis of epithelial ovarian carcinoma cells via
activation of the PI3K/Akt signaling pathway, which is consistent
with the results of the current study.
Uncontrolled proliferation is a critical marker of
malignant carcinoma, as vigorous proliferative activity of
carcinoma cells is the basis and prerequisite of carcinoma invasion
and metastasis. Proliferation of carcinoma cells is regulated by
complex signaling pathways and dysfunctional cell cycle regulatory
mechanisms. The cell cycle is coregulated by CDKs and CDKIs and a
combination of cyclins with CDKs is essential for the activation of
CDKs. Cyclin D1 is one of the most important cyclins, playing a
crucial role in the transition in the G1/S cell cycle phase and
controls the initiation of the cell cycle and mitosis completion
(16,17). In the present study,
immunohistochemistry S-P assays were used to detect the expression
of cyclin D1 in ovarian carcinoma tissues and found an increasing
trend in the positive staining rates of NOT, OSA, OS-BT and OSC
samples from 10.0, 25.0, 55.6 to 73.9%, respectively. The positive
rates of cyclin D1 in well-, moderately- and poorly-differentiated
ovarian carcinoma were 68.8, 70.0 and 90.0%, respectively. Ovarian
carcinoma tissue with lymphatic metastasis showed a 90.9%
prevalence of cyclin D1 expression and a 50.0% prevalence in
metastasis-free ovarian carcinoma tissue. Statistical analyses
indicated that cyclin D1 expression was significantly different
among the NOT, OSA, OS-BT and OSC groups (χ2=19.241;
P<0.01). The prevalence of cyclin D1 expression in the OSA and
OSC groups was significantly higher compared with that in the NOT
group (P<0.05 and P<0.01, respectively). Among the 46 OSC
samples, the prevalence of cyclin D1 expression did not
significantly vary within the three tumor differentiation stages
(P>0.05). An additional statistical analysis also revealed that
cyclin D1 expression was positively associated with lymphatic
metastasis (r=0.371; P=0.011), while cyclin D1 prevalence in OSC
samples with lymphatic metastasis was significantly higher compared
with metastasis-free OSC samples (P<0.05). Lee et al
reported that the positive rates of cyclin D1 expression were
increased in the NOT, OSA, OS-BT and OSC groups (P<0.05) and
correlated with tumor differentiation, clinical stages and
lymphatic metastasis, which is consistent with the results of the
current study (18).
In the present study, the expression levels of p-Akt
and cyclin D1 were analyzed in OSC samples and the prevalence of
p-Akt expression was found to positively correlate with that of
cyclin D1, indicating an association between the PI3K/Akt signaling
pathway and OSC proliferation and apoptosis. In addition,
activation of the PI3K/Akt signaling pathway in proliferation and
apoptosis regulatory signal pathways was confirmed, which is
consistent with previous studies. Akt directly regulates endogenous
antiapoptotic effectors of the Bcl-2 family members and
phosphorylates apoptosis cascade-related regulatory proteins that
share the Bcl-2 homogenous domain 3. Bad belongs to this endogenous
antiapoptotic Bcl-2 family, and p-Akt directly phosphorylates Bad
by combining BH3 with apoptosis cascade-related regulatory proteins
to further regulate protein bioactivity, inhibit antiapoptotic
effects and induce apoptosis (19,20).
Previous studies have indicated that p-Akt directly phosphorylates
the prostate apoptosis response protein (Par-4) to inactivate
apoptosis induction effects and maintain carcinoma cell survival.
Forkhead box (Fox) proteins have conserved Akt phosphorylation
sequences. Once Fox proteins are phosphorylated by Akt, they
migrate out of the nucleus and chelate with cytoplasmic proteins,
losing their facilitating effects on the transcription of apoptosis
related genes, Fas-L and Bim, which induce apoptosis, arrest the
cell cycle and stimulate metabolism (21). The PI3K/Akt signaling pathway blocks
cyclin production or inhibits CDKI activity via multiple signaling
pathways (22). p-Akt binds
specifically to the p53 negative regulatory protein, MDM2, at
Ser166 and Ser186 and relocates MDM2 to the nuclei. MDM2 interacts
with p53, inducing its inactivation by blocking the arrest of p53
in cell cycle stage G1, thereby promoting the cell cycle. Akt
phosphorylates GSK-3, which is continuously produced in resting
cells, and induces the phosphorylation of cyclin D1 while being
degraded by the endogenous proteasome. This results in an extended
G1 stage, and p-Akt indirectly protects cyclin D1 by inactivating
GSK-3. In addition, p-Akt enhances β-catenin stability, thereby
improving the transcription efficiency of the LEF transcription
factor, which results in the improved transcription and expression
of cyclin D1 (23). To conclude,
the present study indicates that the PI3K/Akt signaling pathway
regulates the proliferation pathways of ovarian carcinoma cells,
improving the proliferation activity and further enhancing invasion
and metastasis. However, the PI3K/Akt signaling pathway also
regulates the apoptosis-related proteins of carcinoma cells, which
enhance the activation of endogenous antiapoptotic effectors and/or
inhibit the expression and activation of apoptosis-associated
proteases, thereby restraining apoptosis. The PI3K/Akt signaling
pathway may play a key regulatory role in the development of OSC
and become a primary target for gene therapy. Future in-depth
studies may further contribute to the understanding of the
mechanisms of ovarian carcinoma development and provide clinical
guidance.
Acknowledgements
The authors thank Yunshui Peng from the Chinese
Journal of Anesthesiology for writing assistance and Zhimin Zheng
from the First Hospital of Shijiazhuang for support.
References
|
1
|
Mooney SJ, Winner M, Hershman DL, et al:
Bowel obstruction in elderly ovarian cancer patients: a
population-based study. Gynecol Oncol. 129:107–112. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Oberaigner W, Minicozzi P, Bielska-Lasota
M, et al: Survival for ovarian cancer in Europe: the across-country
variation did not shrink in the past decade. Acta Oncol.
51:441–453. 2012. View Article : Google Scholar
|
|
3
|
Saldanha SN and Tollefsbol TO: Pathway
modulations and epigenetic alterations in ovarian tumorbiogenesis.
J Cell Physiol. Sep 16–2013.(Epub ahead of print).
|
|
4
|
Touma R, Kartarius S, Harlozinska A, Götz
C and Montenarh M: Growth inhibition and apoptosis induction in
ovarian cancer cells. Int J Oncol. 29:481–488. 2006.PubMed/NCBI
|
|
5
|
Staal SP: Molecular cloning of the akt
oncogene and its human homologues AKT1 and AKT2: amplification of
AKT1 in a primary human gastric adenocarcinoma. Proc Natl Acad Sci
USA. 84:5034–5037. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bellacosa A, Kumar CC, Di Cristofano A and
Testa JR: Activation of AKT kinases in cancer: implications for
therapeutic targeting. Adv Cancer Res. 94:29–86. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang Y, Helland A, Holm R, Kristensen GB
and Børresen-Dale AL: PIK3CA mutations in advanced ovarian
carcinomas. Hum Mutat. 25:3222005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Comstock CE, Revelo MP, Buncher CR and
Knudsen KE: Impact of differential cyclin D1 expression and
localisation in prostate cancer. Br J Cancer. 96:970–979. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liao DJ, Thakur A, Wu J, Biliran H and
Sarkar FH: Perspectives on c-Myc, Cyclin D1, and their interaction
in cancer formation, progression and response to chemotherapy. Crit
Rev Oncog. 13:93–158. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Meng Q, Xia C, Fang J, Rojanasakul Y and
Jiang BH: Role of PI3K and AKT specific isoforms in ovarian cancer
cell migration, invasion and proliferation through the p70S6K1
pathway. Cell Signal. 18:2262–2271. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Datta SR, Dudek H, Tao X, et al: Akt
phosphorylation of BAD couples survival signals to the
cell-intrinsic death machinery. Cell. 91:231–241. 1997. View Article : Google Scholar
|
|
12
|
Kane LP, Shapiro VS, Stokoe D and Weiss A:
Induction of NF-kappaB by the Akt/PKB kinase. Curr Biol. 9:601–604.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fang X, Yu SX, Lu Y, et al:
Phosphorylation and inactivation of glycogen synthase kinase 3 by
protein kinase A. Proc Natl Acad Sci USA. 97:11960–11965. 2000.
View Article : Google Scholar
|
|
14
|
Philp AJ, Campbell IG, Leet C, et al: The
phosphatidylinositol 3′-kinase p85alpha gene is an oncogene in
human ovarian and colon tumors. Cancer Res. 61:7426–7429. 2001.
|
|
15
|
Noske A, Kaszubiak A, Weichert W, et al:
Specific inhibition of AKT2 by RNA interference results in
reduction of ovarian cancer cell proliferation: increased
expression of AKT in advanced ovarian cancer. Cancer Lett.
246:190–200. 2007. View Article : Google Scholar
|
|
16
|
Malumbres M and Barbacid M: Cell cycle,
CDKs and cancer: a changing paradigm. Nat Rev Cancer. 9:153–166.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sherr CJ: Mammalian G1 cyclins. Cell.
73:1059–1065. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee SH, Lee JK, Jin SM, et al: Expression
of cell-cycle regulators (cyclin D1, cyclin E, p27kip1, p57kip2) in
papillary thyroid carcinoma. Otolaryngol Head Neck Surg.
142:332–337. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Maurer U, Charvet C, Wagman AS, Dejardin E
and Green DR: Glycogen synthase kinase-3 regulates mitochondrial
outer membrane permeabilization and apoptosis by destabilization of
MCL-1. Mol Cell. 21:749–760. 2006. View Article : Google Scholar
|
|
20
|
Robey RB and Hay N: Mitochondrial
hexokinases, novel mediators of the antiapoptotic effects of growth
factors and Akt. Oncogene. 25:4683–4696. 2006. View Article : Google Scholar
|
|
21
|
Dummler B and Hemmings BA: Physiological
roles of PKB/Akt isoforms in development and disease. Biochem Soc
Trans. 35:231–235. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Manning BD and Cantley LC: AKT/PKB
signaling: navigating downstream. Cell. 129:1261–1274. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dey A, Tergaonkar V and Lane DP:
Double-edged swords as cancer therapeutics: simultaneously
targeting p53 and NF-kappaB pathways. Nat Rev Drug Discov.
7:1031–1040. 2008. View
Article : Google Scholar : PubMed/NCBI
|