Introduction
The five-year survival rate of Japanese patients
with stage II gastric cancer who receive curative resection is
relatively good (68.3%). For stage III disease, however, survival
is much lower at only 30.8% (1,2). In
total, ~30% of stage II patients suffer from recurrence/metastasis
after curative resection and this may be fatal (1,2).
According to the pathology of breast cancer, positive lymph node
metastasis indicates systemic disease with the potential for
metastasis to other organs; therefore, the presence or absence of
lymph node metastasis is considered to be one of the most important
clinical markers for breast cancer patients (3,4).
Recurrence, such as peritoneal dissemination, lymph node
metastasis, or distant metastasis in patients with lymph node
metastasis who undergo curative resection, is presumed to occur
when cancer cells that enter the circulation during the
perioperative period escape the immune system, enter the
microcirculation of target organs and tissues, and find an
appropriate microenvironment for growth and proliferation (5–7).
Various studies have investigated the close correlation between
tumor recurrence/metastasis and the detection of occult neoplastic
cells (ONCs) that are positive for cytokeratin on
immunohistochemical staining in the sinuses of lymph nodes distant
from the primary tumor (8–13). Among patients showing recurrence of
stage II/Dukes’ B colorectal cancer without lymph node metastasis,
~20–30% have ONCs. However, among patients showing recurrence of
stage III/Dukes’ C colorectal cancer with lymph node metastasis,
>70–80% have ONCs. This strongly suggests a correlation between
ONCs and recurrence/metastasis (14,15).
ONCs may be classified as single cells, clusters (2–10 cells
forming a cluster <0.2 mm in diameter) and aggregates (>10
cells). It has been reported that freely floating ONC clusters in
the lymph node sinuses are more dangerous for occult systemic
metastasis than isolated tumor cells (≤0.2 mm) or micrometastases
(0.2 to ≤2 mm) in the lymph nodes (14–16).
Residual cancer cells in the microcirculation may be eradicated by
early postoperative adjuvant chemotherapy, although tumor
susceptibility to anticancer agents and the optimal
dosage/administration schedule are factors that need to be
considered. If it was possible to identify a high-risk group of
patients with gastric cancer who are more likely to develop
recurrence/metastasis, survival could be improved by providing
potent adjuvant chemotherapy for these patients in the early
postoperative period. In addition, identifying a low-risk group of
patients who are not likely to develop recurrence/metastasis could
contribute to reducing the psychological burden on these patients
and to devising more appropriate follow-up schedules for them.
Cytokeratin is an epithelial marker that is useful
for detecting micrometastases to lymph nodes, as >99% of normal
nodes are not stained. AE1/AE3 and CAM 5.2 are anti-cytokeratin
antibodies (17–21). Since the structure of cancer cells
can be examined in detail, histological and immunohistochemical
studies are superior to tests such as polymerase chain reaction in
terms of assessing tumor cell viability and proliferative potential
(17,18). Poorly differentiated adenocarcinoma
and signet ring cell carcinoma as single cells without clusters are
common histological types of gastric cancer, whereas colorectal
cancer is usually well/moderately differentiated adenocarcinoma.
Thus, ONCs are thought to have a different significance in gastric
cancer from that of colorectal cancer with regard to morphological
and biochemical characteristics, as the concept of clusters does
not apply to gastric cancer, particularly in patients with poorly
differentiated adenocarcinoma or signet ring cell carcinoma.
However, a detailed clinicopathological examination
comparing the prevalence of ONCs with the clinical course, stage
and/or tumor histological type has not been reported to date.
Accordingly, the purpose of this study was to investigate the
correlation between the presence of ONCs and the
clinicopathological features of stage II/III gastric cancer by
cytokeratin immunohistochemical staining of the lymph nodes in
surgically resected specimens.
Patients and methods
Patients
We identified 186 patients with stage II/III gastric
cancer from April 2005 to March 2012, who could be followed up to
assess survival for more than five years on the basis of accurate
and complete medical records. Among them, we investigated 164
patients (stage II, n=89; stage III, n=75) from whom >20 lymph
nodes were collected. They included 62 patients (stage II, n=19;
stage III, n=43) who had metastasis and/or recurrence within five
years (recurrence group) and 102 patients (stage II, n=70; stage
III, n=32) without metastasis or recurrence (non-recurrence group).
We performed immunohistochemical staining for cytokeratin in the
D2-dissected lymph nodes obtained from these two groups, and then
compared the detection of ONCs with the clinical course. The study
was approved by the ethics committee of Tokai University Hachioji
Hospital (Tokyo, Japan). Informed consent was obtained from the
patient.
Methods
The routine indirect immunoperoxidase method was
used for cytokeratin staining of the resected lymph nodes (17–19).
Thin sections (3 μm) were prepared from the largest cut surface of
each formalin-fixed and paraffin-embedded lymph node. After
deparaffinization, the sections were immunostained using an
automated staining apparatus (BenchMark® XT; Roche
Diagnostics K.K., Tokyo, Japan). After enzymatic treatment with
protease 1 (0.5 U/ml; Roche Diagnostics K.K.) for 4 min at 37°C to
activate the antigen, monoclonal anti-cytokeratin antibodies
(AE1/AE3 and PCK26; Roche Diagnostics K.K.) were added as the
primary antibodies, and the iVIEW DAB Detection kit (Roche
Diagnostics K.K.) was employed for detection. Dehydration and
mounting of the sections were performed after nuclear staining with
hematoxylin. Hematoxylin and eosin staining and cytokeratin
immunostaining were performed on serial sections of each lymph node
in order to detect cancer cells. Tumor cells and/or tumor nests
associated with fibrosis in the lymph nodes were not considered to
be freely floating cells and were excluded from this study.
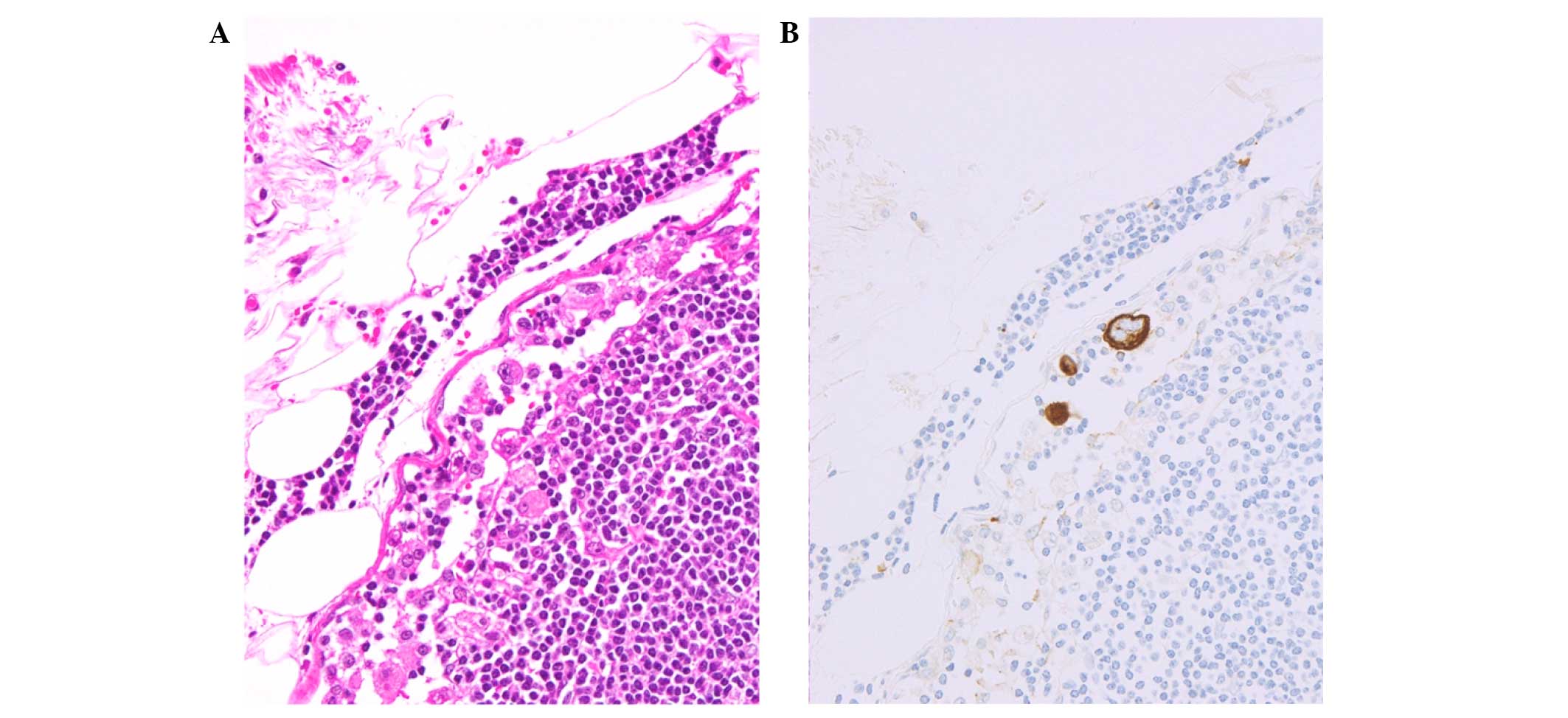
Cytokeratin-immunostained cells freely floating in the lymph node
sinuses (single cell type) were identified (Fig. 1) (5–7). We
defined patients with ≥10 freely floating single cells as ONC(+)
and patients with <10 freely floating single cells as ONC(−).
Assessment was performed by S.Y., who was blinded to the clinical
background of the patients; while M.M., Y.M. and K.K. performed
data collection and analysis. The five-year relapse-free survival
rate (5Y-RFS) and the five-year overall survival rate (5Y-OS) were
calculated for the ONC(+) and ONC(−) groups. The incidence of ONC
positivity in each stage (stages II or III) and histological type
(signet ring cell, poorly differentiated or papillary/tubular type)
was also calculated. Subsequently, the sensitivity, false positive
(FP) rate, specificity, false negative (FN) rate, positive
predictive value (PPV), negative predictive value (NPV) and
predictive accuracy for distinguishing between the recurrence group
and the non-recurrence group were determined. Additionally, the
first site of recurrence was determined in ONC(+) patients from the
recurrence group.
Statistical analysis
The 5Y-RFS and 5Y-OS were calculated by the
Kaplan-Meier method, while the log-rank test and hazard ratio (95%
CI) were employed for comparison of survival between two groups.
The χ2 test was used to compare the recurrence group
with the non-recurrence group and risk ratios (95% CI) were
calculated. P<0.05 was considered to indicate a statistically
significant difference in all analyses. SPSS 17.0 statistical
software (IBM SPSS, Statistics 17.0; International Business
Machines Corp., Armonk, NY, USA) was used for these analyses.
Results
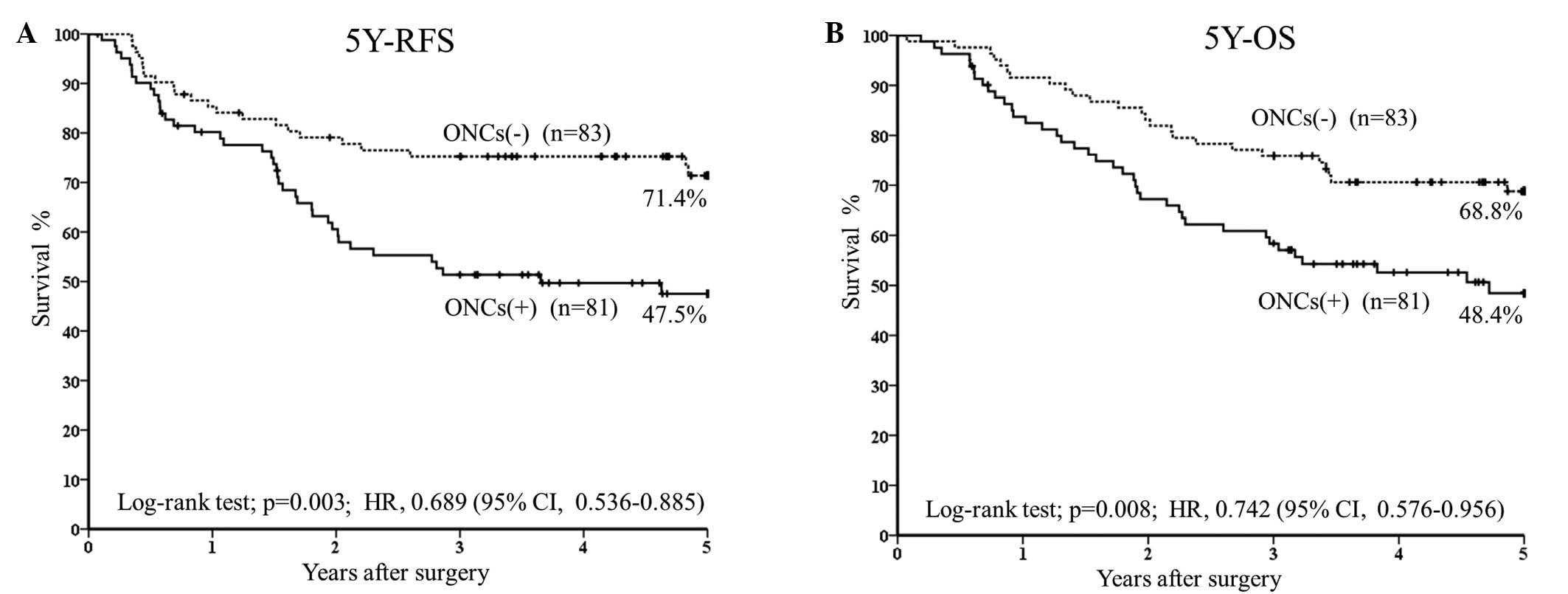
The 5Y-RFS was 71.4% (n=83) in the ONC(−) group and
47.5% (n=81) in the ONC(+) group (P=0.003; HR, 0.689; 95% CI,
0.536–0.885) (Fig. 2A), while 5Y-OS
was 68.8% (n=83) and 48.4% (n=81), respectively (P=0.008; HR,
0.742; 95% CI, 0.576–0.956) (Fig.
2B).
Among stage II patients, 34.8% (31/89) were ONC(+),
with the rate being 77.8% (7/9) in signet ring cell cancer, 51.9%
(14/27) in poorly differentiated cancer and 25.0% (9/36) in
papillary/tubular cancer (Table I).
Among stage II patients, 65.2% (58/89) were ONC(−), with the rate
being 22.2% (2/9) in signet ring cell carcinoma, 41.8% (13/27) in
poorly differentiated adenocarcinoma and 75.0% (27/36) in
papillary/tubular adenocarcinoma (Table
I). Among stage III patients, 66.7% (50/75) were ONC(+),
including 87.5% (7/8) with signet ring cell cancer, 76.2% (16/21)
with poorly differentiated cancer and 59.5% (22/37) with
papillary/tubular cancer (Table I).
Among stage III patients, 33.3% (25/75) were ONC(−), including
12.5% (1/8) with signet ring cell cancer, 23.8% (5/21) with poorly
differentiated cancer and 40.5% (15/37) with papillary/tubular
cancer (Table I).
 | Table IClinicopathological features of stage
II/III gastric cancer patients with each stage/histological type
who were ONC(+) or ONC(−) in the lymph node sinuses. |
Table I
Clinicopathological features of stage
II/III gastric cancer patients with each stage/histological type
who were ONC(+) or ONC(−) in the lymph node sinuses.
| Histological
type | ONCs(+) 81 cases, n
(%) | ONCs(−) 83 cases, n
(%) | Total cases, n |
|---|
| Stage II (89
cases) |
| Pap/tub | 9 (25.0) | 27 (75.0) | 36 |
| Poorly
differentiated | 14 (51.9) | 13 (48.1) | 27 |
| Signet ring
cell | 7 (77.8) | 2 (22.2) | 9 |
| Others/unknown | 1 (5.9) | 16 (94.1) | 17 |
| Stage III (75
cases) |
| Pap/tub | 22 (59.5) | 15 (40.5) | 37 |
| Poorly
differentiated | 16 (76.2) | 5 (23.8) | 21 |
| Signet ring
cell | 7 (87.5) | 1 (12.5) | 8 |
| Others/unknown | 5 (55.6) | 4 (44.4) | 9 |
When ONCs were assessed in the 164 patients as a
method of distinguishing between the recurrence/non-recurrence
groups, the sensitivity of ONC positivity was 64.5% (40/62;
P=0.003; HR, 0.689; 95% CI, 0.536–0.885), the FP rate was 40.2%
(41/102), the specificity was 59.8% (61/102), the FN rate was 35.5%
(22/62), the PPV was 49.4% (40/81), the NPV was 73.5% (61/83) and
the predictive accuracy was 61.6% (101/164) (Table II).
 | Table IIDetection rates of ONCs in the lymph
node sinuses (positive, ≥10 floating single cells; negative, <10
floating single cells) in the recurrence and non-recurrence
groups. |
Table II
Detection rates of ONCs in the lymph
node sinuses (positive, ≥10 floating single cells; negative, <10
floating single cells) in the recurrence and non-recurrence
groups.
| Total 164 cases
(predictive accuracy 61.6%) | Recurrence group
(n=62) | Non-recurrence group
(n=102) |
|---|
| ONCs(+) | 40 casesa | 41 cases |
| 81 cases (PPV
49.4%) | (Sensitivity
64.5%) | (FP rate 40.2%) |
| ONCs(−) | 22 cases | 61 cases |
| 83 cases (NPV
73.5%) | (FN rate 35.5%) | (Specificity
59.8%) |
In total, 40 out of 81 ONC(+) patients developed
metastasis and/or recurrence within five years. The first site of
recurrence was peritoneal dissemination in five cases (12.5%),
local/lymph nodes in 19 cases (47.5%), the liver in six cases
(15.0%), the lung in one case (2.5%), and others/unknown in nine
cases (22.5%) (Table III).
 | Table IIIONC(+) patients in the recurrence
group (n=40) and the patterns of recurrence/metastasis. |
Table III
ONC(+) patients in the recurrence
group (n=40) and the patterns of recurrence/metastasis.
| Site of
reccurrence/metastasis | n (%) |
|---|
| Peritoneal
dissemination | 5 (12.5) |
| Local/lymph node | 19 (47.5) |
| Liver | 6 (15.0) |
| Lung | 1 (2.5) |
| Others/unknown | 9 (22.5) |
Discussion
In Europe and America, it is considered that D2
lymph node dissection during surgery on primary colon cancer
contributes to the survival of stage II/Dukes’ B cases, but is not
useful in the case of stage III/Dukes’ C patients (22). In Japan, the Guideline for Gastric
Cancer Treatment recommends at least D2 lymph node dissection
(1,2). The purpose of lymph node dissection
during surgery is to achieve the complete en bloc removal of
metastatic nodes, and it also contributes to standardizing the
assessment of true node negativity by collecting a large number of
lymph nodes with/without metastasis from specific regions (23). Identification of ≥12 lymph nodes is
also recommended in the NCI guidelines for diagnosing true node
negativity, and the most important prognostic factor is considered
to be the number of metastatic foci observed in lymph nodes
retrieved by D2 dissection (22–24).
The JSCCR Guidelines for the Treatment of Colorectal Cancer include
a detailed description of the criteria for high-risk cases who
should receive postoperative adjuvant chemotherapy, which include
lymph node metastasis affecting at least four nodes (TNM; N2),
direct invasion of other organs, budding at the deepest leading
edge of the primary tumor, and the presence of vascular involvement
(25,26). The Japanese Guideline for Gastric
Cancer Treatment recommends postoperative adjuvant chemotherapy for
stage II/III patients, with the exception of those who are T1
cases, but does not provide a detailed description of the criteria
for high-risk of recurrence/metastasis (1,2).
Colorectal cancer patients with lymph node
metastases (stage IIIA or higher) are considered to have systemic
disease, similar to breast cancer patients, and ~30–40% of them are
presumed to be at high-risk of recurrence, while the remaining
60–70% belong to the low-risk group (14,15).
In patients with stage III colorectal cancer, it is presumed that
numerous free tumor cells have dispersed into the portal
circulation and reached the liver, lungs and other organs, unlike
the patients who have stage II/N0 localized tumors. The level of
nonspecific immunity was also reported to be much lower in stage
III patients than in stage II patients, which may be the result of
persistent attempts by the immune system to eradicate ONCs
(27,28).
Gastric cancers often initially recur as peritoneal
dissemination or local lymph node metastasis, although one of the
most common metastatic sites of colorectal cancer is the liver
caused by venous invasion (29,30).
It is thought that ONCs from gastric cancer enter small lymph
vessels and then reach the peritoneal space. Initial recurrence as
local lymph node metastasis is more frequent than that observed due
to peritoneal dissemination, with first recurrence in the lymph
nodes for 2/40 patients (5.0%) in N0, 7/40 patients (17.5%) in N1,
6/40 patients (5.0%) in N2, and 25/40 patients (62.5%) in N3
disease. As 77.5% of patients are N1 or N2, lymph node metastasis
tends to affect the local nodes. However, more detailed
clinicopathological examination of the characteristics of gastric
cancer recurrence, such as peritoneal dissemination or local lymph
node metastasis, is required.
In colorectal cancer, floating tumor cell clusters
are considered to be the prime cause of distant metastasis to sites
such as the liver or lungs (14,15).
In the present study of gastric cancer, certain patients had
relatively well-differentiated tumors and clusters of ONCs in their
lymph nodes sinuses similar to those observed in colorectal cancer
(data not shown). However, no significant difference was found
between the recurrence group and the non-recurrence group based on
comparison of clusters. The majority of colorectal cancers have the
histology of well/moderately differentiated adenocarcinoma and
poorly differentiated or special types are rare. By contrast, there
are various histological types of gastric cancer, such as papillary
adenocarcinoma, tubular adenocarcinoma, poorly differentiated
adenocarcinoma and signet ring cell carcinoma (31). In this study, poorly differentiated
(non-solid) and signet ring cell cancer, which are unlikely to form
cell clusters, were frequent in the ONC(+) group, including 21/31
patients (67.7%) in stage II and 23/50 patients (46.0%) in stage
III. It is thought that cells from poorly differentiated and signet
ring cell cancers do not form clusters due to the abnormal
expression of cell adhesion molecules (32,33).
Floating single cells and clusters of well-differentiated
adenocarcinoma form glandular structures as they proliferate,
whereas poorly differentiated carcinoma cells are thought to
persist as single cells or only form small nests. It is therefore
suggested that the biological characteristics of gastric cancer
mean that there is no correlation between floating clusters and
recurrence/metastasis. The correlation between single tumor cells
in the lymph node sinuses and recurrence/metastasis has not been
clarified to date, possibly as only less viable ONCs become trapped
in the lymph nodes and these cells are not involved in recurrence
or metastasis (5–7,14,15).
In patients who have <10 floating cells, ONC(−), these cells are
assumed to be eradicated by the host immune system and are unlikely
to cause recurrence or metastasis, whereas in those with ≥10
floating cells, ONC(+), certain cells may not be eliminated by the
immune system and may proliferate to form micrometastases.
Conventionally, tumors that show budding at the leading edge are
thought to have a worse prognosis, but certain stage II/III gastric
cancer patients without lymph node metastases show a relatively
good prognosis (8–13). In the present study, ONC(+) stage
II/III gastric cancer without lymph node metastasis was infrequent,
accounting for 17.8% of cases (8/45). This finding suggests that
ONC(−) stage II gastric cancer without lymph node metastasis is a
localized tumor with a low risk of recurrence, similar to stage
II/N0 colorectal cancer. In addition to factors such as host
immunity and tumor susceptibility to anticancer agents, the results
of this study suggest that ONC(+) may be a useful indicator for
selecting patients with a high risk of recurrence in the early
postoperative period. Conversely, the ONC(−) state is a useful
indicator for identifying the low-risk group, as patients without
ONCs exhibited a high NPV for recurrence. In the future,
investigations should be performed to distinguish the high-risk
group with a high sensitivity/high PPV and the low-risk group with
a high specificity/high NPV. Clinical indicators with a high
sensitivity/high PPV and a high specificity/high NPV should be
identified to more accurately select the high-risk and low-risk
groups for recurrence/metastasis, respectively.
Acknowledgements
This study was supported by grants from the Occult
Neoplastic Cells Research and Study Group (2012–5007; Tokai
University Hachioji Hospital, Hachioji, Tokyo, Japan) and the
Research and Study Program of Tokai University Educational System
General Research Organization (Tokai University Hospital, Isehara,
Kanagawa, Japan).
Abbreviations:
|
ONCs
|
occult neoplastic cells
|
|
5Y-RFS
|
five-year relapse-free survival
|
|
5Y-OS
|
five-year overall survival
|
|
PPV
|
positive predictive value
|
|
NPV
|
negative predictive value
|
|
FP
|
false positive
|
|
FN
|
false negative
|
References
|
1
|
Guideline for Gastric Cancer Treatment in
Japan. Japanese Gastric Cancer Association; 2nd edition. Tokyo:
2004
|
|
2
|
Guideline for Gastric Cancer Treatment in
Japan. Japanese Gastric Cancer Association; 3rd edition. Tokyo:
2010
|
|
3
|
National Institutes of Health Consensus
Development Conference Statement: adjuvant therapy for breast
cancer, November 1–3, 2000. J Natl Cancer Inst. 93:979–989.
2001.
|
|
4
|
Goldhirsch A, Glick JH, Gelber RD, Coates
AS and Senn HJ: Meeting highlights: International Consensus Panel
on the Treatment of Primary Breast Cancer. Seventh International
Conference on adjuvant therapy of primary breast cancer. J Clin
Oncol. 19:3817–3827. 2001.
|
|
5
|
Mukai M, Sato S, Komatsu N, Nishida T,
Shiba K, Ito I, Nakasaki H and Makuuchi H: Correlation between
occult neoplastic cells in the lymph node sinuses and recurrence in
patients with curatively resected Dukes’ B colorectal cancer. Oncol
Rep. 10:1177–1181. 2003.PubMed/NCBI
|
|
6
|
Mukai M, Sato S, Komatsu N, Nishida T,
Shiba K, Ito I, Nakasaki H and Makuuchi H: Correlation between
occult neoplastic cells in the lymph node sinuses and recurrence in
patients with Dukes’ C colorectal cancer. Oncol Rep. 10:1165–1169.
2003.
|
|
7
|
Mukai M, Sato S, Nakasaki H, Tajima T,
Saito Y, Nishiumi N, Iwasaki M, Tokuda Y, Ogoshi K, Inoue H and
Makuuchi H: Occult neoplastic cells in the lymph node sinuses and
recurrence of primary breast, lung, esophageal, and gastric cancer.
Oncol Rep. 11:81–84. 2004.PubMed/NCBI
|
|
8
|
Mukai M, Sato S, Komatsu N, Kimura T,
Ninomiya H, Kawada M, Nakasaki H, Ogoshi K and Makuuchi H: Accuracy
of criteria for predicting recurrence and metastasis in stage II
and III gastric cancer patients with lymph node metastasis. Oncol
Rep. 12:63–66. 2004.PubMed/NCBI
|
|
9
|
Mukai M, Sato S, Kimura T, Komatsu N,
Ninomiya H, Nakasaki H, Ogoshi K and Makuuchi H: Criteria for
predicting the recurrence and metastasis of stage I and II gastric
cancer without lymph node metastasis. Oncol Rep. 12:59–62.
2004.PubMed/NCBI
|
|
10
|
Mukai M, Tajima T, Sato S, Ninomiya H,
Wakui K, Komatsu N, Tsuchiya K, Nakasaki H and Makuuchi H:
Recurrence and 5-FU sensitivity of stage II/III node-positive
gastric cancer with occult neoplastic cells in lymph node sinuses.
Oncol Rep. 14:1505–1510. 2005.
|
|
11
|
Mukai M, Sato S, Tajima T, Ninomiya H,
Wakui K, Komatsu N, Tsuchiya K, Nakasaki H and Makuuchi H:
Recurrence and 5-FU sensitivity of stage I/II node-negative breast,
lung, or gastric cancer with occult neoplastic cells in lymph node
sinuses. Oncol Rep. 15:815–820. 2006.PubMed/NCBI
|
|
12
|
Mukai M, Sato S, Ninomiya H, Wakui K,
Komatsu N, Matsui N, Nakamura M, Nakasaki H and Makuuchi H:
Sensitivity to CPT-11 and platinum derivatives of stage I/II
node-negative breast, lung, and gastric cancer with occult
neoplastic cells in lymph node sinuses. Oncol Rep. 18:33–39.
2007.PubMed/NCBI
|
|
13
|
Mukai M, Sato S, Ninomiya H, Wakui K,
Komatsu N, Matsui N, Nakamura M, Ogoshi K and Makuuchi H:
Sensitivity to CPT-11 and platinum derivatives of stage II/III
node-positive gastric cancer with occult neoplastic cells in lymph
node sinuses. Ann Cancer Res Ther. 15:22–26. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sekido Y, Mukai M, Kishima K, Tajima T,
Hoshikawa T, Nakamura M, Nakamura N and Ogoshi K: Occult neoplastic
cells in the lymph node sinuses and recurrence/metastasis of stage
II/Dukes’ B colorectal cancer. Oncol Rep. 25:69–73. 2011.PubMed/NCBI
|
|
15
|
Sekido Y, Mukai M, Kishima K, Tajima T,
Hoshikawa T, Nakamura M, Nakamura N and Ogoshi K: Occult neoplastic
cells in the lymph node sinuses and recurrence/metastasis of stage
III/Dukes’ C colorectal cancer. Oncol Rep. 25:915–919.
2011.PubMed/NCBI
|
|
16
|
Sobin L, Gospodarowicz M and Wittekind C:
TNM classification of malignant tumors. 7th edition. John Wiley and
Sons, Ltd; New York: 2009
|
|
17
|
Nakane PK and Pierce GB Jr: Enzyme-labeled
antibodies: preparation and application for localization of
antigens. J Histochem Cytochem. 14:929–931. 1966. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nakane PK and Pierce GB Jr: Enzyme-labeled
antibody for the light and electron microscopic localization of
tissue antigens. J Cell Biol. 33:307–318. 1967. View Article : Google Scholar
|
|
19
|
Greenson JK, Isenhart CE, Rice R, Mojzisik
C, Houchens D and Martin EW Jr: Identification of occult
micrometastases in pericolic lymph nodes of Duke’s B colorectal
cancer patients using monoclonal antibodies against cytokeratin and
CC49. Correlation with long-term survival. Cancer. 73:563–569.
1994.PubMed/NCBI
|
|
20
|
Nicholson AG, Marks CG and Cook MG: Effect
on lymph node status of triple levelling and immunohistochemistry
with CAM 5.2 on node negative colorectal carcinomas. Gut.
35:1447–1448. 1994. View Article : Google Scholar
|
|
21
|
Isaka N, Nozue M, Doi M and Fukao K:
Prognostic significance of perirectal lymph node micrometastases in
Dukes’ B rectal carcinoma: an immunohistochemical study by CAM5.2.
Clin Cancer Res. 5:2065–2068. 1999.PubMed/NCBI
|
|
22
|
Prandi M, Lionetto R, Bini A, Francioni G,
Accarpio G, Anfossi A, Ballario E, Bacchi G, Bonilauri S, Carobbi
A, et al: Prognostic evaluation of stage B colon cancer patients is
improved by an adequate lymphadenectomy: results of a secondary
analysis of a large scale adjuvant trial. Ann Surg. 235:458–463.
2002. View Article : Google Scholar
|
|
23
|
Mukai M, Ito I, Mukoyama S, Tajima T,
Saito Y, Nakasaki H, Sato S and Makuuchi H: Improvement of 10-year
survival by Japanese radical lymph node dissection in patients with
Dukes’ B and C colorectal cancer: A 17-year retrospective study.
Oncol Rep. 10:927–934. 2003.PubMed/NCBI
|
|
24
|
Nelson H, Petrelli N, Carlin A, Couture J,
Fleshman J, Guillem J, Miedema B, Ota D and Sargent D: Guidelines
2000 for colon and rectal cancer surgery. J Natl Cancer Inst.
93:583–596. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
JSCCR Guidelines for the Treatment of
Colorectal Cancer. Japanese Society for Cancer of the Colon and
Rectum (JSCCR); Tokyo: 2009
|
|
26
|
Makuuchi M and Sugihara K: Surgery of the
Colon, Rectum and Anus. 2nd edition. Knacks and Pitfalls. Bunkoudou
Co, Ltd; Tokyo: 2004
|
|
27
|
Ito I, Mukai M, Ninomiya H, Kishima K,
Tsuchiya K, Tajima T, Oida Y, Nakamura M and Makuuchi H: Comparison
between intravenous and oral postoperative adjuvant
immunochemotherapy in patients with stage II colorectal cancer.
Oncol Rep. 20:1189–1194. 2008.
|
|
28
|
Ito I, Mukai M, Ninomiya H, Kishima K,
Tsuchiya K, Tajima T, Nakamura M and Makuuchi H: Comparison between
intravenous and oral postoperative adjuvant immunochemotherapy in
patients with stage III colorectal cancer. Oncol Rep. 20:1521–1526.
2008.
|
|
29
|
Rosai J: Rosai and Ackerman’s Surgical
Pathology. 9th edition. Mosby; St. Louis, MO: pp. 670–671. pp.
817–818. 2004
|
|
30
|
Inada K, Shimokawa K, Ikeda T, Hayashi M
and Azuma S: Development of liver metastasis in colorectal
carcinoma. With special reference to venous invasion and basement
membrane laminin. Acta Pathol Jpn. 41:240–245. 1991.
|
|
31
|
Japanese Classification of Gastric
Carcinoma. Japanese Gastric Cancer Association; 14th edition.
Tokyo, Japan: 2010
|
|
32
|
Muta H, Noguchi M, Kanai Y, Ochiai A,
Nawata H and Hirohashi S: E-cadherin gene mutations in signet ring
cell carcinoma of the stomach. Jpn J Cancer Res. 87:843–848. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mayer B, Johnson JP, Leitl F, Jauch KW,
Heiss MM, Schildberg FW, Birchmeier W and Funke I: E-cadherin
expression in primary and metastatic gastric cancer:
down-regulation correlates with cellular dedifferentiation and
glandular disintegration. Cancer Res. 53:1690–1695. 1993.
|