Introduction
Hepatocellular carcinoma (HCC) is the third most
common cause of cancer-associated mortality worldwide (1). Local treatments, including surgical
resection and radiofrequency ablation (RFA) for early-stage HCC,
give favorable outcomes, but no effective treatment has been
established for advanced HCC that is not amenable to surgical
resection, and the prognosis of advanced HCC is poor.
Sorafenib (Nexvar; Bayer Healthcare pharmaceuticals;
Leverkusen, Germany) is an oral multi-targeted tyrosine kinase
inhibitor that is indicated for unresectable advanced HCC and
significantly improves progression-free survival (PFS) and overall
survival (OS) (2,3). In the SHARP (Sorafenib HCC Assessment
Randomized Protocol) trial (2),
survival time was significantly prolonged from 7.9 months in the
placebo group to 10.7 months in the sorafenib group, but a complete
response (CR) was not achieved in any of the 299 patients in the
sorafenib group. Similarly, a CR did not occur in any of the 150
patients in the Asia-Pacific trial (conducted in the Asia-Pacific
region) (3), indicating that
achieving a CR is infrequent in treatment with sorafenib.
The acquisition of a CR following sorafenib
treatment has occasionally been reported, and the discontinuation
of medication subsequent to acquiring a CR in these cases would be
beneficial, as sorafenib is an expensive drug and has adverse
effects (4). However, it is unclear
whether CR is maintained following discontinuation. The present
study describes a case of recurrent HCC with a portal vein tumor
thrombus (PVTT) of the third portal vein after resection in a
patient who was treated with sorafenib and achieved a CR, which was
then maintained for more than one year following the
discontinuation of the medication. A literature review is also
presented. Written informed consent was obtained from the
patient.
Case report
The patient was a 68-year-old male with hepatitis C
virus-related liver cirrhosis. A giant HCC was detected and an
S7/S8 segmentectomy of the liver was performed at another hospital.
Recurrence in the residual liver, PVTT in the right portal branch
and right abdominal disseminated lesions were noted four months
after the surgery, although only the disseminated lesions were
surgically excised at the request of the patient. The patient was
referred to Toho University Medical Center, Omori Hospital (Tokyo,
Japan) to continue treatment for the intrahepatic recurrence. In
the initial blood tests at the hospital, liver function was graded
as Child-Pugh A and tumor marker levels were high: α-fetoprotein
(AFP), 4,773 ng/ml; AFP-L3, 60.5%; and des-γ carboxyprothrombin
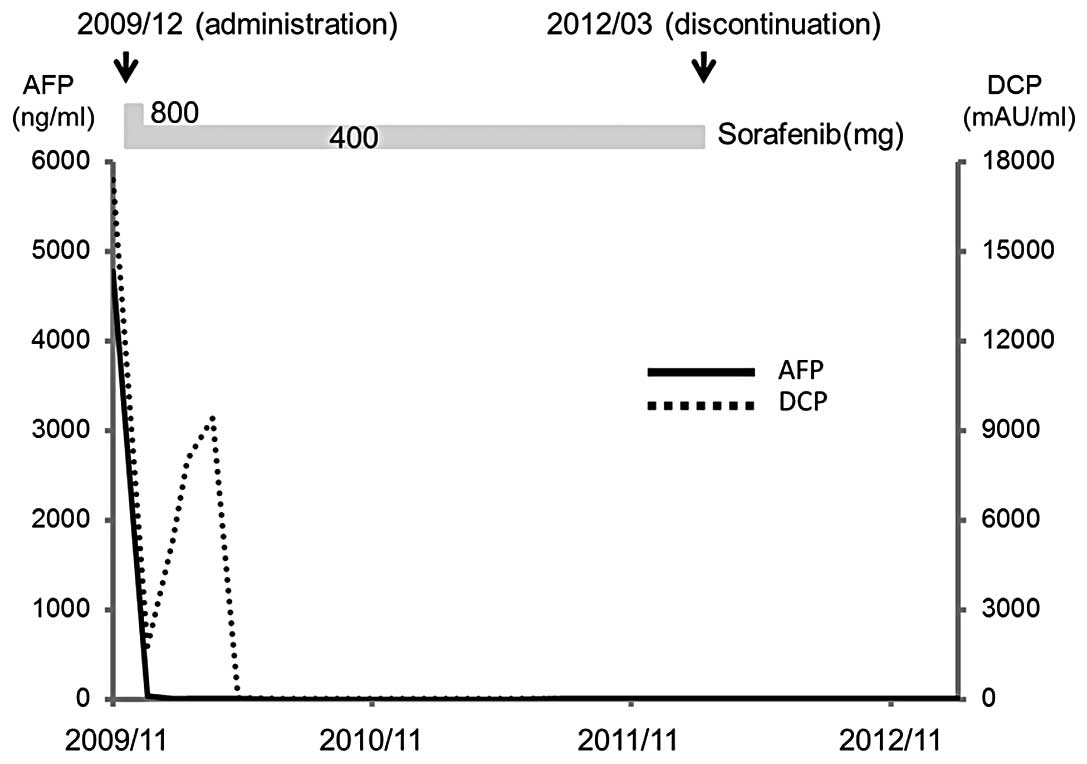
(DCP), 17,400 mAU/ml (Fig. 1).
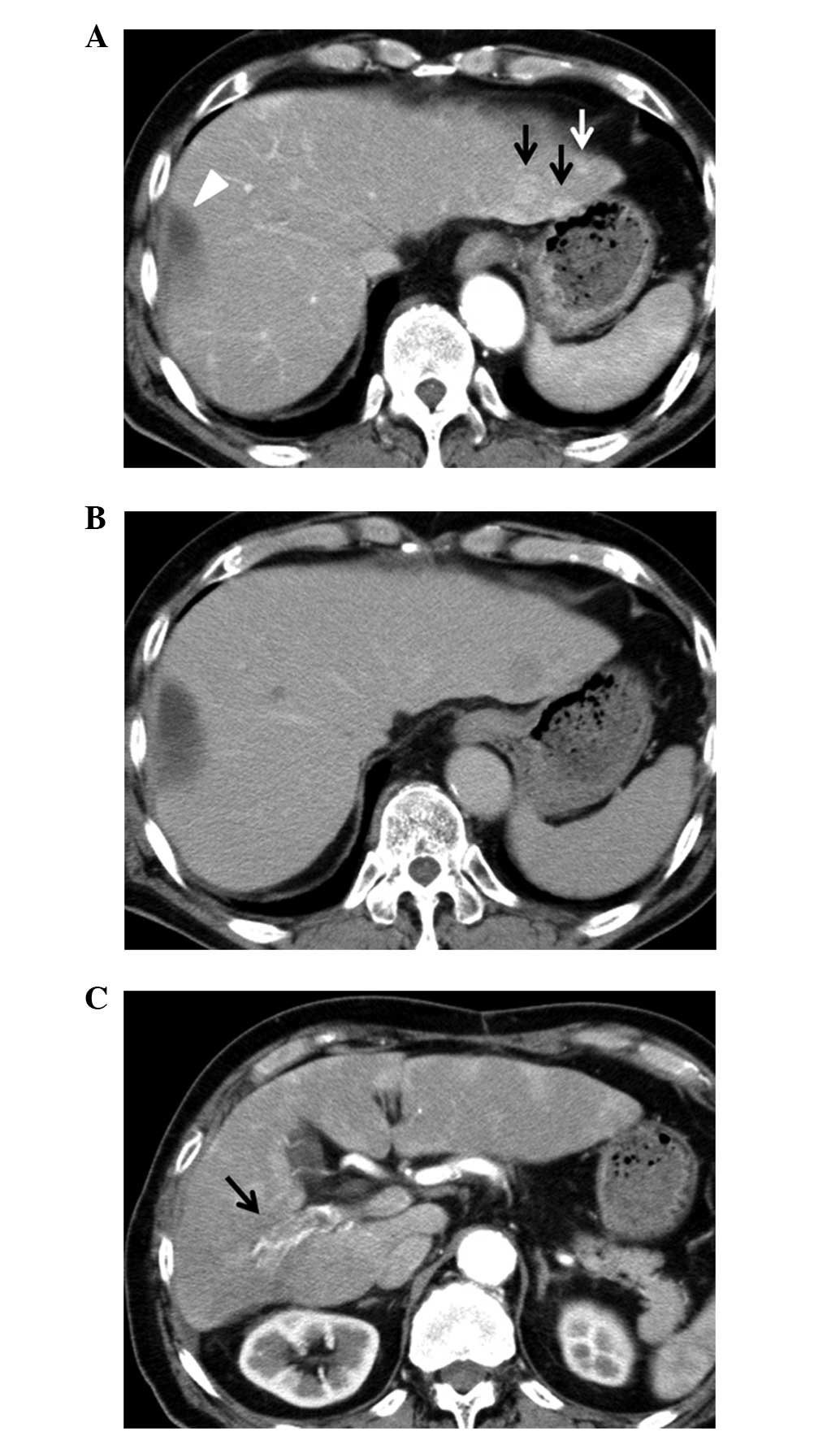
Abdominal computed tomography (CT) showed numerous tumors in the
bilateral lobes and a PVTT in the right portal branch (Fig. 2). Oral sorafenib therapy was
initiated at the recommended dose of 800 mg/day. Grade 3 hand-foot
syndrome (Common Terminology Criteria for Adverse Events version
4.0) (5) developed 7 days after the
initiation of sorafenib treatment, and the dose was reduced to 400
mg/day on day 10.
After one month of administration, the AFP level was
decreased to 45.7 ng/ml, but there were no changes in PVTT or in
the multiple tumors in the bilateral lobes on abdominal CT. The
condition was judged to be of a stable disease based on the
modified Response Evaluation Criteria in Solid Tumors (mRECIST)
(6). A partial response was
achieved after six months. On abdominal CT after two years of
sorafenib administration, multiple tumors in the bilateral lobes
had shrunk and the intense staining due to the PVTT had been
resolved, based on which the condition was judged to have achieved
a CR. Sorafenib at 400 mg/day was continued thereafter, but mild
cerebellar infarction developed at two years and four months after
the initiation of administration, and sorafenib was withdrawn at
the request of the patient. A CR was maintained for approximately
one year after the discontinuation based on abdominal CT findings
and normal tumor marker levels.
Discussion
Sorafenib is a multikinase inhibitor with reported
activity against Raf-1, B-Raf, vascular endothelial growth factor
receptor 2 (VEGFR2), platelet-derived growth factor receptor
(PDGFR) and c-Kit receptors, as well as other receptor tyrosine
kinases and serine threonine kinases (7). Sorafenib is a molecular-targeted drug
that exerts an antitumor effect by inhibiting tumor growth and
vascularization. The efficacy of sorafenib has been shown in the
SHARP (2) and Asia-Pacific trials
(3). Survival was significantly
prolonged in the sorafenib group compared with the placebo group in
all these studies, although none of the patients (449 in total)
achieved a CR in a RECIST-based judgment of the effect. An
evaluation of tumor hemodynamics is now considered to be important
for the judgment of therapeutic effect based on the characteristics
of the antitumor effect of sorafenib, and the utility of
hemodynamic evaluation using mRECIST and contrast-enhanced
ultrasonography (CEUS) has previously been described (8). Therefore, the judgment of the
therapeutic effect of sorafenib using RECIST in previous clinical
studies may not be completely reliable, although it is clear that a
CR is rarely achieved with sorafenib treatment.
Certain HCC patients worldwide have been observed to
achieve a CR with sorafenib, such as the present case (4,9–12). In
this present case, administration was started at 800 mg/day, but
the dose was reduced to 400 mg/day soon after initiation due to
adverse effects. The recommended dose of sorafenib is 800 mg/day
and most reported CR cases have received oral administration at
this dose (9,11,12),
although Wang et al(10) and
Inuzuka et al(4) have
described cases treated with 400 mg/day in which a CR was achieved.
These results indicate that further investigation of the usefulness
of a low-dose administration of sorafenib may be necessary. It is
also of note that the present case had PVTT, since it is considered
that an effect with sorafenib is not readily obtained in cases with
PVTT. However, Wang et al(10) and Sacco et al(12) have reported CR in cases with PVTT
following treatment with sorafenib. VEGF is important in the
vascularization and progression of PVTT in HCC, and sorafenib may
have a favorable therapeutic effect on PVTT through the inhibition
of the VEGF pathway (13). More
detailed investigations of VEGF levels in individual patients may
enable a prediction of the efficacy of sorafenib for cases with
PVTT prior to treatment.
The most important point in the present case is the
maintenance of a CR following the discontinuation of sorafenib.
Four cases with the maintenance of a CR subsequent to
discontinuation have been reported, including that of the present
patient (4,9,10).
Wang et al(10) described a
case with PVTT in which a CR was achieved at a low dose of
sorafenib, similar to the present case. A CR was acquired at eight
months after the initiation of oral administration and the drug was
withdrawn subsequent to achieving a CR, with no recurrence for 16
months after discontinuation. So et al(9) reported a case in which sorafenib was
used at the recommended dose for HCC with lung metastasis. A CR was
achieved following five months of oral administration and there was
no recurrence for six months after discontinuation. Inuzuka et
al(4) also reported achieving a
CR in a case of HCC with lung metastasis treated with a low dose of
sorafenib. A CR was obtained following eight months of oral
administration and there was no recurrence for a further eight
months following discontinuation. In the present case, a CR was
achieved after two years of oral administration and no recurrence
has been detected for one year since discontinuation.
Several hypotheses concerning the maintenance of a
CR following the discontinuation of sorafenib have been discussed.
Wang et al(10) considered
it most likely due to the uniqueness of the tumor biopsy, i.e.,
activated by a single or few pathway(s) that was/were completely
blocked by sorafenib.
Alternatively, So et al(9) suggested that the tumor was highly
dependent for survival on one or more of the receptor tyrosine
kinases that are inhibited by sorafenib. The mechanism is unclear,
but there may be specific molecular level features of HCC cases in
which CR is maintained following the discontinuation of sorafenib
that differ from those of other cases.
In the present patient, sorafenib was discontinued
four months after the judgment of a CR, whereas the drug was
withdrawn at almost the same time as the diagnosis of a CR in two
of the previous cases (4,10) and after one month in one case
(9). In patients with renal cell
carcinoma (RCC) treated with sorafenib, Johannsen et
al(14) observed that recurrent
or new metastatic lesions developed following discontinuation of
the drug in five out of 12 patients who achieved a CR. A further
accumulation of cases is required to understand the appropriate
timing of the discontinuation of sorafenib after a CR is
achieved.
In conclusion, the present study described a case of
advanced HCC with PVTT that showed a CR following treatment with
low-dose sorafenib (400 mg once daily) and in which this CR was
maintained for approximately one year after treatment was
discontinued. Tumors may recur due to the discontinuation of
treatment, and the appropriate timing of sorafenib discontinuation
requires further investigation.
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar
|
|
2
|
Llovet JM, Ricci S, Mazzaferro V, et al:
Sorafenib in advanced hepatocellular carcinoma. N Engl J Med.
359:378–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cheng AL, Kang YK, Chen Z, et al: Efficacy
and safety of sorafenib in patients in the Asia-Pacific region with
advanced hepatocellular carcinoma: a phase III randomized,
double-blind, placebo-controlled trial. Lancet Oncol. 10:25–34.
2009. View Article : Google Scholar
|
|
4
|
Inuzuka T, Nishikawa H, Sekikawa A, et al:
Complete response of advanced hepatocellular carcinoma with
multiple lung metastases treated with sorafenib: a case report.
Oncology. 81(Suppl 1): S152–S157. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chan A and Tan EH: How well does the MESTT
correlate with CTCAE scale for the grading of dermatological
toxicities associated with oral tyrosine kinase inhibitors? Support
Care Cancer. 19:1667–1674. 2011. View Article : Google Scholar
|
|
6
|
Edeline J, Boucher E, Rolland Y, et al:
Comparison of tumor response by Response Evaluation Criteria in
Solid Tumors (RECIST) and modified RECIST in patients treated with
sorafenib for hepatocellular carcinoma. Cancer. 118:147–156. 2012.
View Article : Google Scholar
|
|
7
|
Wilhelms S, Carter C, Lynch M, et al:
Discovery and development of sorafenib: a multikinase inhibitor for
treating cancer. Nat Rev Drug Discov. 5:835–844. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shiozawa K, Watanabe M, Kikuchi Y, Kudo T,
Maruyama K and Sumino Y: Evaluation of sorafenib for hepatocellular
carcinoma by contrast-enhanced ultrasonography: a pilot study.
World J Gastroenterol. 18:5753–5758. 2012. View Article : Google Scholar
|
|
9
|
So BJ, Bekaii-Saab T, Bloomston MA and
Patel T: Complete clinical response of metastatic hepatocellular
carcinoma to sorafenib in a patient with hemochromatosis: a case
report. J Hematol Oncol. 1:182008. View Article : Google Scholar
|
|
10
|
Wang SX, Byrnes A, Verma S, Pancoast JR
and Rixe O: Complete remission of unresectable hepatocellular
carcinoma treated with reduced dose of sorafenib: a case report.
Target Oncol. 5:59–63. 2010. View Article : Google Scholar
|
|
11
|
Kudo M and Ueshima K: Positioning of a
molecular-targeted agent, sorafenib, in the treatment algorithm for
hepatocellular carcinoma and implication of many complete remission
cases in Japan. Oncology. 78:154–166. 2010. View Article : Google Scholar
|
|
12
|
Sacco R, Bargellini I, Gianluigi G, et al:
Complete response for advanced liver cancer during sorafenib
therapy: case report. BMC Gastroenterol. 11:42011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li Q, Xu B, Fu L and Hao XS: Correlation
of four vascular specific growth factors with carcinogenesis and
portal vein tumor thrombus formation in human hepatocellular
carcinoma. J Exp Clin Cancer Res. 25:403–409. 2006.
|
|
14
|
Johannsen M, Flörcken A, Bex A, et al: Can
tyrosine kinase inhibitors be discontinued in patients with
metastatic renal cell carcinoma and a complete response to
treatment? A multicenter, retrospective analysis. Eur Urol.
55:1430–1438. 2009. View Article : Google Scholar
|