Introduction
Breast non-Hodgkin’s lymphoma (NHL) is a rare
entity, comprising <0.5% of all breast malignancies and ~0.7% of
all NHL cases, in which secondary forms are more common than
primary (1,2). Breast lymphomas are most commonly
B-cell and occasionally T-cell types (3). T-cell lymphomas have a poorer
prognosis than the B-cell type (4).
Primary breast lymphoma commonly presents as a painless breast
mass, which is similar to breast carcinoma (5). The right breast has been previously
reported to be most frequently involved, however, the cause of this
remains unknown (6). In the present
case report, a rare case of primary T-cell breast lymphoma (PTBL)
is described, including the histopathological and
immunohistochemical observations of the primary and recurrent
tumors and the clinical observations.
Case report
Patient presentation
A 27-year-old Chinese female presented with a left
mammary mass to the First Affiliated Hospital, Hangzhou, China, in
April 2010. There was no history of fever, weight loss, night
sweats or other symptoms. The patient’s medical and family
histories were unremarkable. Upon physical examination, a single
non-tender mass was palpable at the upper outer quadrant of the
left breast and an enlarged lymph node was palpable in the
ipsilateral axilla. There was no cervical or inguinal
lymphadenopathy. An ultrasound examination revealed a hypoechoic,
non-cystic mass with ill-defined borders in the upper outer
quadrant of the left breast, which measured 3.3×2.6 cm. Computed
tomography (CT) scans of the abdomen, chest X-rays, a bone marrow
aspiration and a biopsy revealed no significant observations.
Laboratory data, including the total white cell count, lactate
dehydrogenase (LDH), β2-microglobulin and immunoglobulin levels,
were normal.
Histopathological and immunohistochemical
observations
A segmental resection was performed. Examination of
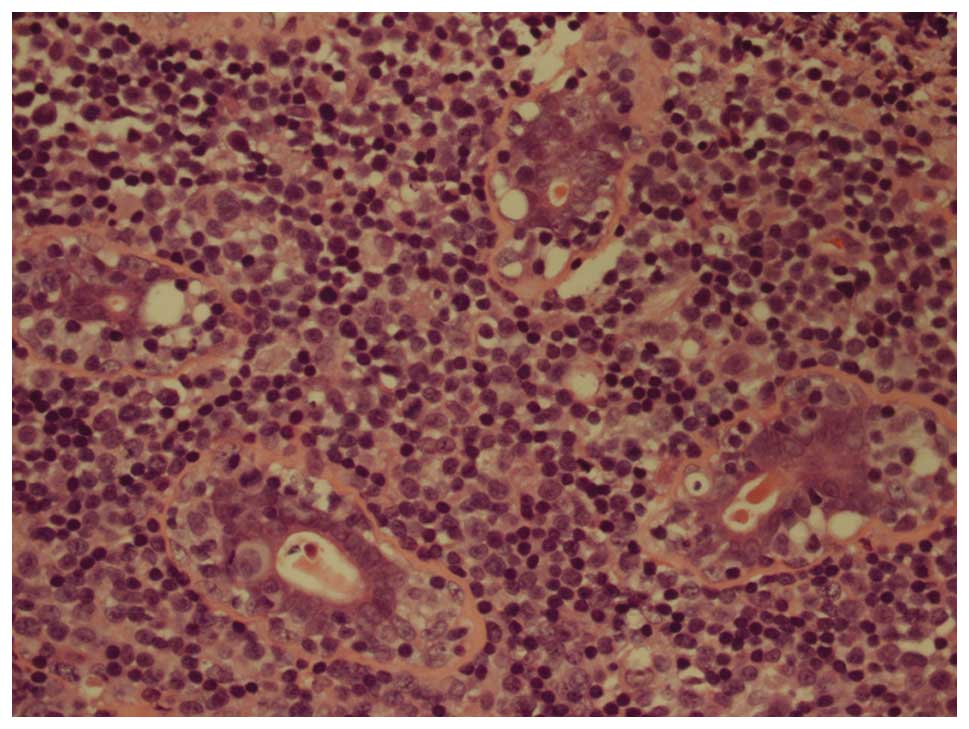
the hematoxylin and eosin staining revealed sheets of uniform,
small and medium-sized, round, blue cells, and infiltration into
ducts and blood vessels in certain regions was observed. Mitotic
figures and a large number of eosinophils were observed (Fig. 1). A panel of antibodies was used for
an immunohistochemical study, including CD2, CD4, CD43, CD3, CD5,
CD8, p53, cyclin D1, cytokeratin, vimentin, desmin, myeloperoxidase
(MPO), CD79a, CD7, CD30, anaplastic lymphoma kinase (ALK), CD34,
CD10, CD56, granzyme B, B-cell lymphoma 2 (BCL2), BCL6, S100,
terminal deoxynucleotidyl transferase (TDT), estrogen receptor
(ER), progesterone receptor (PR) and paired box (pax)5. The tumor
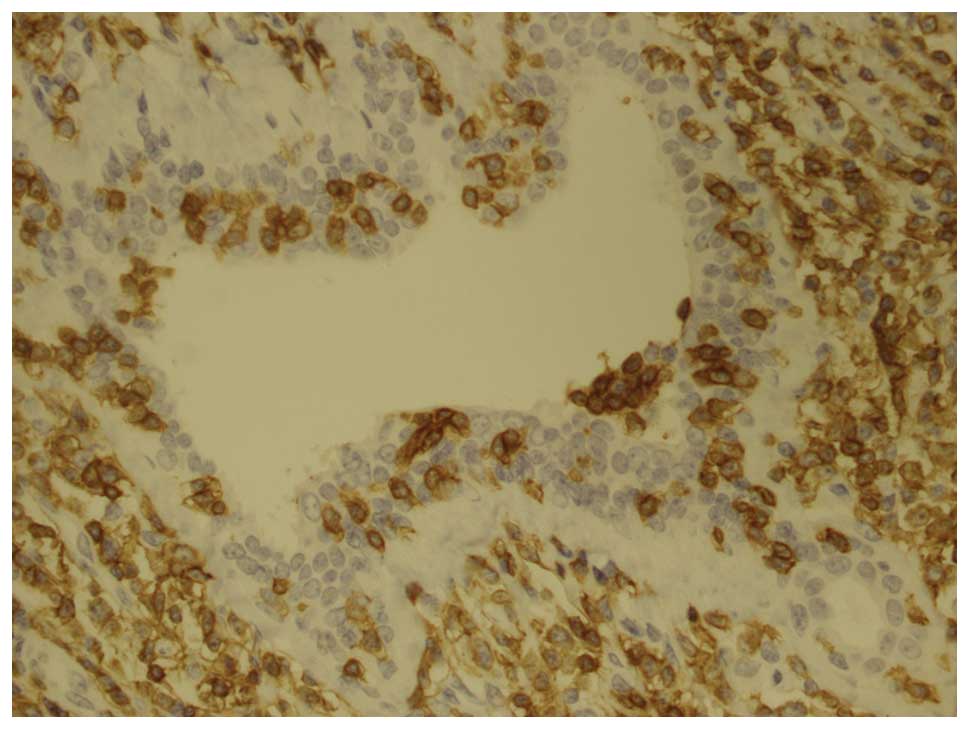
cells were markedly positive for CD2, CD4, CD43 and CD3 (Fig. 2) and weakly positive for CD5, CD8
and p53. Certain tumor cells were cyclin D1-positive. Reactivity
for cytokeratin, vimentin, desmin, MPO, CD79a, CD7, CD30, ALK,
CD34, CD10, CD56, granzyme B, BCL2, BCL6, S100, TDT, ER, PR and
pax5 was negative. Based on these observations, the patient was
diagnosed with PTBL, unspecified. A subsequent positron emission
tomography (PET)/CT scan demonstrated multiple hypermetabolic foci
in the left breast and the ipsilateral axillary lymph nodes.
Drug administration
The patient was administered one cycle of CHOP (750
mg/m2 cyclophosphamide on day 1, 60 mg/m2
epirubicin on day 1, 1.4 mg/m2 vindesine on day 1 and
100 mg prednisolone on days 1–5, every 3–4 weeks). A chest CT scan
revealed that the left breast was enlarged with multiple soft
tissue masses. Subsequently, the patient received six cycles of
ECHOP (120 mg/m2 VP-16 on days 1–3 plus CHOP every 3–4
weeks). Marked tumor regression was observed following the first
cycle of ECHOP and a complete clinical response was achieved
following the third cycle. Six months after diagnosis and two
months after chemotherapy, the patient received an autologous
peripheral blood stem cell transplantation. Following
transplantation, a CT scan showed no evidence of recurrence.
Recurrence
Six months following transplantation, two left
mammary masses were identified by the patient. A PET/CT scan
indicated an abnormal accumulation of fluorodeoxyglucose in the
left breast, the left axilla and the right ilium, indicative of
recurrence and metastasis. LDH levels were elevated to 315 U/l
(normal limit, 91–250 U/l). β2-microglobulin and immunoglobulin
levels remained within the normal limits. The patient underwent an
excisional biopsy of the left breast. Histopathology showed that
the recurrent tumor increased levels of karyopyknosis and nuclear
fragmentation compared with the primary tumor. The positive
expression rates of Ki67 in the recurrent tumor (~80%) were higher
than in the primary tumor (~40%). The immunohistochemical results
were consistent with those of the primary tumor. Subsequently, the
patient received two cycles of ECHOP and one cycle of GDP (1000
mg/m2 gemcitabine on days 1 and 8, 40 mg dexamethasone
on days 1–4 and 25 mg/m2 cisplatin on days 1–3, every
3–4 weeks). The recurrent tumor was resistant to the ECHOP and GDP
regimen. The individual succumbed to PTBL, unspecified, 18 months
following the diagnosis.
This study was approved by the institutional ethics
committee of The First Affiliated Hospital, Zhejiang University.
Informed consent was obtained from the patient’s family.
Discussion
PTBL is an extremely rare and aggressive disease.
The age range of patients with this disease is between 13 and 77
years old (3,7). The most common subtype of peripheral
T-cell lymphoma is unspecified, accounting for ~50% of all cases.
The standard diagnostic criteria to distinguish between primary
breast lymphoma and a secondary form was outlined by Wiseman and
Liao in 1972 (8). These include the
following characteristics: i) Adequate pathological specimens; ii)
mammary tissue and lymphomatous infiltration in close association;
iii) no evidence of lymphomatous infiltrate with other lymphoma
focus at the time of diagnosis, except for compromised ipsilateral
axillary lymph node; and iv) no prior diagnosis of extra-mammary
lymphoma. In the present case, the clinical features and
histopathological and imaging observations fulfilled these four
criteria. Thus, a diagnosis of unspecified PTBL was made.
To date, no clear clinical or radiological features
have been described to distinguish primary breast lymphoma from any
other type of infiltrating breast carcinoma, however, prominent
lymph vessels in a patient with a breast mass and B symptoms (i.e.
fever, night sweats and weight loss) must raise the suspicion of
breast lymphoma, and the diagnosis may be excluded if
calcifications or a desmoplastic reaction are present (9,10).
Lymphoma must be included in the differential diagnosis of breast
masses, since no pathognomonic radiological observations exist for
its diagnosis (11). The key to the
diagnosis of these cases remains as the requirement for adequate
tissue from biopsy for histopathological evaluation and
immunophenotyping. A core needle biopsy of the breast mass is
sufficient to differentiate breast lymphoma from breast carcinoma.
However, a segmental resection was performed on the patient. We
consider that this was a pitfall in the treatment process of the
patient in the present study.
The treatment modalities for PBTL have not been
clearly defined (12). Bhele and
Gujral described the case of a 26-year-old pregnant female with
bilateral peripheral T-cell breast lymphoma successfully treated
with CHOP chemotherapy (13). By
contrast, in other cases, chemotherapy alone has been reported to
be an inadequate treatment for this disease (14). In the current case, the patient
exhibited a complete clinical response to the ECHOP regimen and
subsequently received an autologous peripheral blood stem cell
transplant. To the best of our knowledge, this is the first
instance where a PTBL patient has been treated with this modality.
However, the patient rapidly exhibited recurrence and metastasis
following transplantation.
The recurrent tumor exhibited increased
karyopyknosis and nuclear fragmentation compared with the primary
tumor. Furthermore, the Ki67 index was higher. These changes are
likely to imply that the recurrent tumor was more progressive than
the primary one. Indeed, the relapsed tumor was not responsive to
ECHOP and GDP.
Ann Arbor stage, International Prognostic Index
(IPI), LDH and radiotherapy are important factors for the
relapse-free survival of patients with lymphoma (14). According to the IPI, the current
patient belonged to the moderate risk group. However, the patient
relapsed after 12 months and succumbed to PTBL 18 months following
diagnosis. The survival of the patient was shorter than the
majority of previously reported PTBL cases. Aquino et al
hypothesized that the overexpression of cyclin D1 may be an
unfavorable prognostic factor in primary breast T-cell lymphoma,
unspecified (15). In the current
case, cyclin D1 was positive in certain regions, indicating that
cyclin D1 may be a marker of poor prognosis. However, this must be
confirmed in additional cases.
In conclusion, the optimal treatment for PTBL
remains unknown. Autologous peripheral blood stem cell
transplantation was ineffective to cure PTBL in the present case.
The literature review indicated that a biopsy of any recurrent
tumors is important and re-examination of the Ki-67 index may be
useful for the prediction of response prior to the initiation of
chemotherapy.
Acknowledgements
The authors thank the Professors at the Department
of Pathology (The First Affiliated Hospital of Zhejiang University,
Hangzhou, China) for their consultation on this case.
References
|
1
|
Uesato M, Miyazawa Y, Gunji Y and Ochiai
T: Primary non-Hodgkin’s lymphoma of the breast: report of a case
with special reference to 380 cases in the Japanese literature.
Breast Cancer. 12:154–158. 2005.
|
|
2
|
Lim H, Cho KR, Kim I, et al: Primary
Peripheral T-cell lymphoma of the breast: radiologic and pathologic
findings. J Breast Cancer. 13:318–322. 2010. View Article : Google Scholar
|
|
3
|
Aguilera NS, Tavassoli FA, Chu WS and
Abbondanzo SL: T-cell lymphoma presenting in the breast: a
histologic, immunophenotypic and molecular genetic study of four
cases. Mod Pathol. 13:599–605. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jaffe ES: Pathobiology of peripheral
T-cell lymphomas. Hematology. 2006:317–322. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Duncan VE, Reddy VV, Jhala NC, Chhieng DC
and Jhala DN: Non-Hodgkin’s lymphoma of the breast: a review of 18
primary and secondary cases. Ann Diagn Pathol. 10:144–148.
2006.
|
|
6
|
Loughrey MB, Windrum P, Catherwood MA, et
al: WHO reclassification of breast lymphomas. J Clin Pathol.
57:1213–1214. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gualco G, Chioato L, Harrington WJ Jr,
Weiss LM and Bacchi CE: Primary and secondary T-cell lymphomas of
the breast: clinico-pathologic features of 11 cases. Appl
Immunohistochem Mol Morphol. 17:301–306. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wiseman C and Liao KT: Primary lymphoma of
the breast. Cancer. 29:1705–1712. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mason HS, Johari V, March DE and Crisi GM:
Primary breast lymphoma: radiologic and pathologic findings. Breast
J. 11:495–496. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Roldán-Valadez E, del García-Blanco MC,
Rojas-Marín C, Sánchez-Avila F, León-Rodrìguez E and
Hernández-Ortiz J: Secondary non-Hodgkin’s B-cell lymphoma
involving the breast: radiologic imaging. Gac Med Mex. 141:63–67.
2005.(In Spanish).
|
|
11
|
Zagouri F, Sergentanis TN, Nonni A, et al:
Secondary breast lymphoma diagnosed by vacuum-assisted breast
biopsy: a case report. J Med Case Rep. 1:1132007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ozkan K, Sehsuvar G, Erdem C, Mustafa C,
Ibrahim S and Fatih O: A rare occurrence of primary T-cell lymphoma
of the breast in pregnancy. Acta Oncol. 50:1262–1263. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bhele S and Gujral S: Bilateral peripheral
T-cell lymphoma of breast: a case report. Indian J Pathol
Microbiol. 50:816–818. 2007.PubMed/NCBI
|
|
14
|
Lin Y, Guo XM, Shen KW, Wang JL and Jiang
GL: Primary breast lymphoma: Long-term treatment outcome and
prognosis. Leuk Lymphoma. 47:2102–2109. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Aquino G, Franco R, Ronconi F, et al:
Peripheral T-cell Lymphoma with Cyclin D1 overexpression: a case
report. Diagn Pathol. 7:792012. View Article : Google Scholar : PubMed/NCBI
|