Introduction
Triple-negative breast cancer (TNBC) is a
heterogeneous clinicopathological entity defined as an estrogen
receptor (ER)-, progesterone receptor (PR)- and HER2/neu-negative
breast cancer (1). TNBC has been
proposed to be an immunohistochemistry (IHC)-based surrogate of the
basal-like breast cancer subtype; however, there is no complete
overlap between the two groups (2).
TNBC accounts for 10–20% of all breast cancer subtypes (2,3). As
TNBC is hormone receptor- and HER2/neu-negative, there is no
targeted treatment available for this cancer subtype, and a
standard chemotherapy regimen remains a basic systemic treatment
option, with no optimal cytotoxic regimen recommended. In spite of
the relative chemosensitivity of this cancer subtype, it is
characterized by aggressive clinical behavior with a high
recurrence and mortality rate, particularly in the first 5 years
following diagnosis (4). A further
subclassification of TNBC is thus required to develop a new
targeted treatment to improve prognosis in this unfavorable cancer
subtype. In previous studies, a strong correlation between
BRCA1 mutation-associated tumors and TNBC has been
identified; 57–88% of all BRCA1-associated tumors are TNBC
and/or basal-like (5,6). The prevalence/incidence of germline
BRCA1/2 mutations in the TNBC subtype is relatively
high, accounting for 10.6–19.5% in consecutive patient groups
(7,8). BRCA1-mutated tumors carry a
dysfunctional DNA double-strand break repair mechanism and,
therefore, are considered to be sensitive to platinum-based
chemotherapy regimens and inhibitors of the poly(ADP-ribose)
polymerase (PARP) (9).
Theoretically, these agents may also be a new treatment option for
TNBC and, at present, several clinical trials are underway to
investigate a therapeutic benefit of DNA-damaging agents and PARP
inhibitors in this breast cancer subtype (10,11).
Understanding the role of carrying a BRCA1 mutation may be
crucial to guide treatment strategies and conduct clinical trials.
Therefore, several previous studies have focused their attention on
the prognostic role of positive BRCA mutation status in the TNBC
subtype and have demonstrated similar outcomes in BRCA mutation
carriers and non-carriers (7,12,13).
However, these studies are associated with the following
limitations: The cut-off levels for ER and PR negativity were not
specified or defined as nuclear staining of ≤10% (12,13),
neither group was homogenized by received chemotherapy regimens
(7), missing information with
regard to accompanying cancers or patients with previous ovarian
cancer were not included in the study (7,13),
breast cancer-specific survival (BCS) rates were not evaluated
(7) and the prognostic significance
of separate BRCA1 mutations were also not evaluated
(7,12,13).
BRCA1 germline mutation variants result in various changes
in the structure of the BRCA1 proteins that impact breast or/and
ovarian cancer risk and clinical outcomes. For example, a poorer
overall survival of breast cancer BRCA1 4153delA mutation
carriers compared with 5382insC, has been reported (14,15).
Therefore, the aim of the present study was to
investigate the prognostic significance of carrying two germline
BRCA1 founder mutations (4153delA and 5382insC) in patients
with TNBC in the Latvian population.
Materials and methods
Study population
A total of 2,943 patients with invasive breast
cancer between 2005 and 2011 (~50% of all breast cancer cases
registered in Latvia during this time period) underwent genetic
testing for BRCA1/2 mutations, at the Oncology
Institute of Riga Stradins University (Riga, Latvia). In the
present study, only patients who met all inclusion and exclusion
criteria were included. Inclusion criteria were as follows: i)
invasive TNBC in stage I–IV; ii) TNBC defined as ER/PR, 0%; HER2, 0
or 1+ (16); iii) had undergone
definitive surgery between 2005 and 2011; iv) tested for
BRCA1/2 mutations; v) signed informed consent forms
to participate in the study; and vi) had available clinical data.
Exclusion criteria were as follows: i) inflammatory breast cancers;
ii) a history of ovarian or other advanced cancers; and iii)
BRCA2 mutation carriers. A total of 78 consecutive
BRCA1 mutation-negative TNBCs treated at Pauls Stradins
Clinical University Hospital and 38 BRCA1 mutation-positive
TNBCs were deemed eligible for study. The study was approved by the
Ethical Committee of Riga Stradins University.
Pathological examination and IHC
Histological parameters of all cases were reviewed
by breast pathologists. Histological type and grade of ductal
breast cancers were determined for each case according to the
Bloom-Richardson system modified by Elston and Ellis (17).
ER and PR status were determined using IHC. For ER
and PR, monoclonal antibodies were obtained from DakoCytomation
(Glostrup, Denmark).
HER2 was also assessed through IHC. The assessment
of HER-2/neu expression was carried out using the HercepTest kit
(Dako, Glostrup, Denmark) according to the manufacturer’s
instructions. IHC was scored on a quantitative scale between 0 and
3, in accordance with the Dako HerceptTest™ (Dako).
Genetic testing
Patients in Latvia were tested for the two common
founder mutations in BRCA1 (4153delA and 5382insC) using a
multiplex-specific polymerase chain reaction assay.
Statistical methods
The outcomes were analyzed in all 116 patients.
Locoregional recurrence (LRR) was defined as clinical and
histological documented recurrence in the ipsilateral breast, chest
wall or regional lymph nodes (axillary, supraclavicular and
internal mammary). LRR-free survival (LRFS) was defined as the time
from diagnosis to clinical and histological documented evidence of
local recurrence. Distant recurrence was defined as clinical and
radiographical evidence of distant relapse. Distant recurrence-free
survival (DRFS) was defined as the time from diagnosis to first
evidence of distant recurrence. The DRFS was censored at the date
of the last follow-up if no distant recurrence was observed. The
BCS was calculated from the date of diagnosis until the patient
succumbed due to breast cancer. Routine follow-up was performed
every 3–6 months for 3 years, every 6–12 months for 4–5 years and
annually thereafter. The median follow-up from the original
diagnosis until analysis was 36 months (range, 8–85 months) in the
BRCA1 mutation non-carriers and 41 months (range, 8–86
months) in the BRCA1 mutation carriers. Clinicopathological
characteristics of BRCA1 mutation carriers and non-carriers
were compared using a χ2 and Fisher’s exact test.
Univariate and multivariate Cox proportional-hazards models were
used to compute independent predictors of BCS and DRFS. The
following prognostic variables were analyzed: Age, T stage, nodal
status, clinical stage, BRCA1 status, type of surgery
performed, radiation and chemotherapy. BCS was estimated using the
Kaplan-Meier method and compared by a long-rank test. P≤0.05 was
considered to indicate a statistically significant difference.
Statistical analysis was performed using the SPSS software, version
16.0 (SPSS, Inc., Chicago, IL, USA).
Results
Patient characteristics
Of the 116 TNBC patients, 38 patients (32.8%) were
BRCA1 mutation carriers and 78 patients (67.2%) were
BRCA1 mutation non-carriers.
Surgery
All patients underwent definitive surgery. The type
of chemotherapy and postoperative radiotherapy received were at the
discretion of the multidisciplinary treating team. BRCA1
mutation carriers were significantly younger at diagnosis than
non-carriers (median age, 48.8 vs. 54.4 years, respectively;
P<0.034). No statistically significant difference was identified
in relation to tumor size, T stage, stage, Ki-67 status and tumor
differentiation grade between the two groups. Invasive ductal
carcinoma was the most common histological type in the two groups,
but BRCA1 mutation non-carriers were more likely to have
invasive lobular carcinomas. There was a higher proportion of lymph
node-negative patients in the BRCA1 mutation carriers group
(P<0.004), however, there was no difference in performed
lymphadenectomy and sentinel node biopsy between the two groups. A
higher proportion of BRCA1 mutation carriers experienced
mastectomy (P<0.001). No statistically significant difference
was identified between the two groups in terms of received
chemotherapy. BRCA1 mutation non-carriers were more likely
to have received radiation therapy (P<0.027; Table I). A total of three patients (3.9%)
from the BRCA1 carrier group and two patients (5.3%) from
the BRCA1 non-carrier group underwent bilateral
salpingo-oophorectomy under the age of 50 years. Prophylactic
mastectomy was performed in three BRCA1 mutation carriers
(7.7%). Patients with positive BRCA1 mutation experienced
more bilateral breast cancers than non-carriers [6 (15.8%) vs. 2
(2.6%), respectively].
 | Table IClinicopathlogical characteristics of
BRCA1 mutation carriers (n=38) and non-carriers (n= 78). |
Table I
Clinicopathlogical characteristics of
BRCA1 mutation carriers (n=38) and non-carriers (n= 78).
|
Characteristics | BRCA1
mutation carriers, n (%) | BRCA1
mutation non-carriers, n (%) | P-value |
|---|
| Age at diagnosis,
years | | | <0.034 |
| Median | 48.8 | 54.4 | |
| Range | 27–75 | 31–82 | |
| Histology |
| Ductal
carcinoma | 26 (68.4) | 53 (67.9) | 0.9584 |
| Lobular
carcinoma | 0 (0) | 12 (15.4) | <0.006 |
| Medullary
carcinoma | 5 (13.2) | 4 (5.1) | 0.16 |
| Tumor grade |
|
Well-differentiated | 0 (0) | 0 (0) | |
| Moderately
differentiated | 7 (26.9) | 10 (18.9) | 0.4364 |
| Poorly
differentiated | 19 (73.1) | 43 (81.1) | 0.6098 |
| Tumor size, mm | 36.2 | 32.9 | 0.467 |
| T stage |
| T1 | 7 (18.4) | 21 (26.9) | 0.3283 |
| T2 | 24 (63.2) | 38 (48.7) | 0.1503 |
| T3 | 3 (7.9) | 12 (15.4) | 0.2772 |
| T4 | 4 (10.5) | 7 (18.4) | 0.7810 |
| Nodal status |
| N0 | 25 (65.8) | 29 (37.2) | <0.004 |
| N1 | 5 (13.2) | 23 (29.5) | 0.1145 |
| N2 | 5 (13.2) | 15 (19.2) | 0.2482 |
| N3 | 3 (7.9) | 8 (10.2) | 0.8776 |
| Ki-67 | 59.8 | 52.2 | 0.271 |
| Stage |
| I | 7 (18.4) | 15 (19.2) | 0.9329 |
| II | 21 (55.3) | 33 (42.3) | 0.1979 |
| III | 8 (21) | 30 (38.5) | 0.0627 |
| IV | 1 (2.6) | 0 (0) | 0.3276 |
| Surgery |
|
Breast-conserving | 6 (15.8) | 36 (46.1) | <0.001 |
| Mastectomy | 32 (84.2) | 42 (53.9) | |
| Axillary
lymphadenectomy |
| No | 7 (18.4) | 13 (16.7) | 0.8075 |
| Yes | 31 (81.6) | 64 (82) | 0.9384 |
| Sentinel node
biopsy |
| No | 31 (79.5) | 64 (80) | 0.4759 |
| Yes | 8 (20.5) | 14 (17.5) | 0.6882 |
| Chemotherapy |
| Yes | 34 (89.5) | 67 (85.9) | 0.1954 |
|
Anthracycline-based | 19 (50) | 45 (57.7) | 0.4429 |
| CMF | 4 (10.6) | 6 (7.7) | 0.6162 |
|
Platine-based | 3 (7.9) | 3 (3.8) | 0.3940 |
| Anthracycline +
taxane | 6 (15.8) | 12 (15.4) | 0.9408 |
| Unknown
chemotherapy regimen | 2 (10.6) | 6 (7.7) | 0.6741 |
| None | 2 (10.6) | 6 (7.7) | 0.6741 |
| Radiation |
| Yes | 22 (57.9) | 61 (78.2) | <0.027 |
| No | 15 (39.5) | 10 (2.6) | <0.001 |
| Bilateral breast
cancer | 6 (15.8) | 2 (2.6) | <0.016 |
Estimates of survival outcomes
No statistically significant difference was
identified in the LRR rate between BRCA1 mutation
non-carriers and carriers [3 (3.9%) vs. 1 (2.6%), respectively;
P=0.8022]. A total of two patients with LRR in the BRCA1
mutation non-carriers group underwent mastectomy and one patient
underwent breast-conserving surgery; in the BRCA1 mutation
group, one patient with LRR in the right axillary lymph nodes
underwent breast-conserving surgery. The LRFS was 5.7 months
(range, 4–8 months) in the BRCA1 mutation non-carriers group
and 20 months in the BRCA1 mutation carriers group.
A higher proportion of BRCA1 mutation
non-carriers experienced distant recurrence compared with mutation
carriers [22 (28.2%) vs. 4 (10.5%), respectively; P<0.03]. The
DRFS was 32.2 months (range, 6–85 months) in the BRCA1
mutation non-carriers group and 39 months (range, 9–85 months) in
the BRCA1 mutation carriers group. BRCA1 mutation
non-carriers were more likely to succumb to breast cancer than
BRCA1 mutation carriers [18 (23.1%) vs. 2 (5.3%),
respectively; P<0.014]. BRCA1 mutation carriers had a
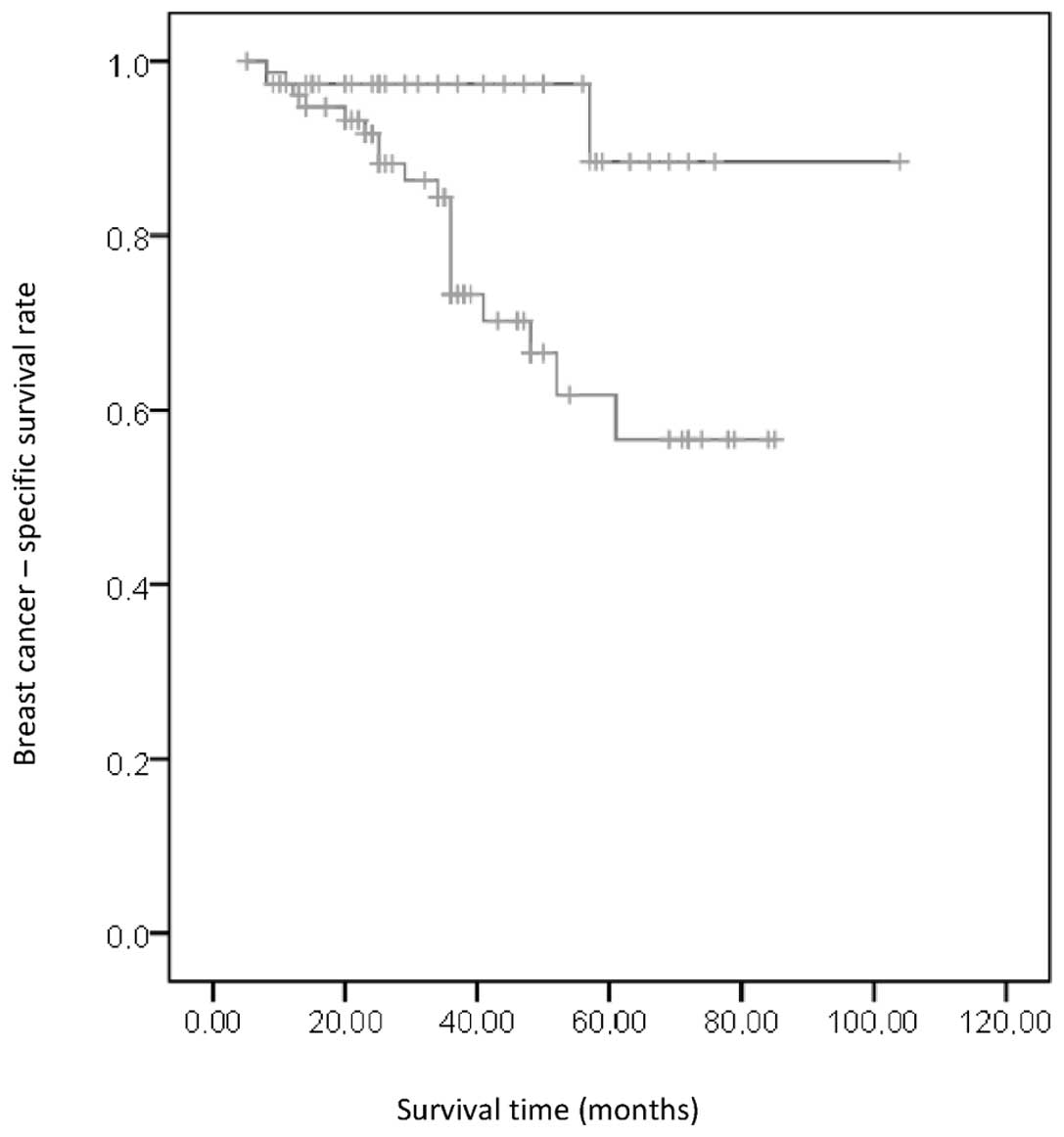
statistically significant higher BCS than non-carriers (94.9% in
the BRCA1 mutation carriers and 76.9% in the BRCA1
mutation non-carriers; P<0.02; Fig.
1). The development of bilateral breast cancer did not
significantly impact the survival outcomes (HR, 0.040; 95% Cl,
0.001–4.804; P=0.590).
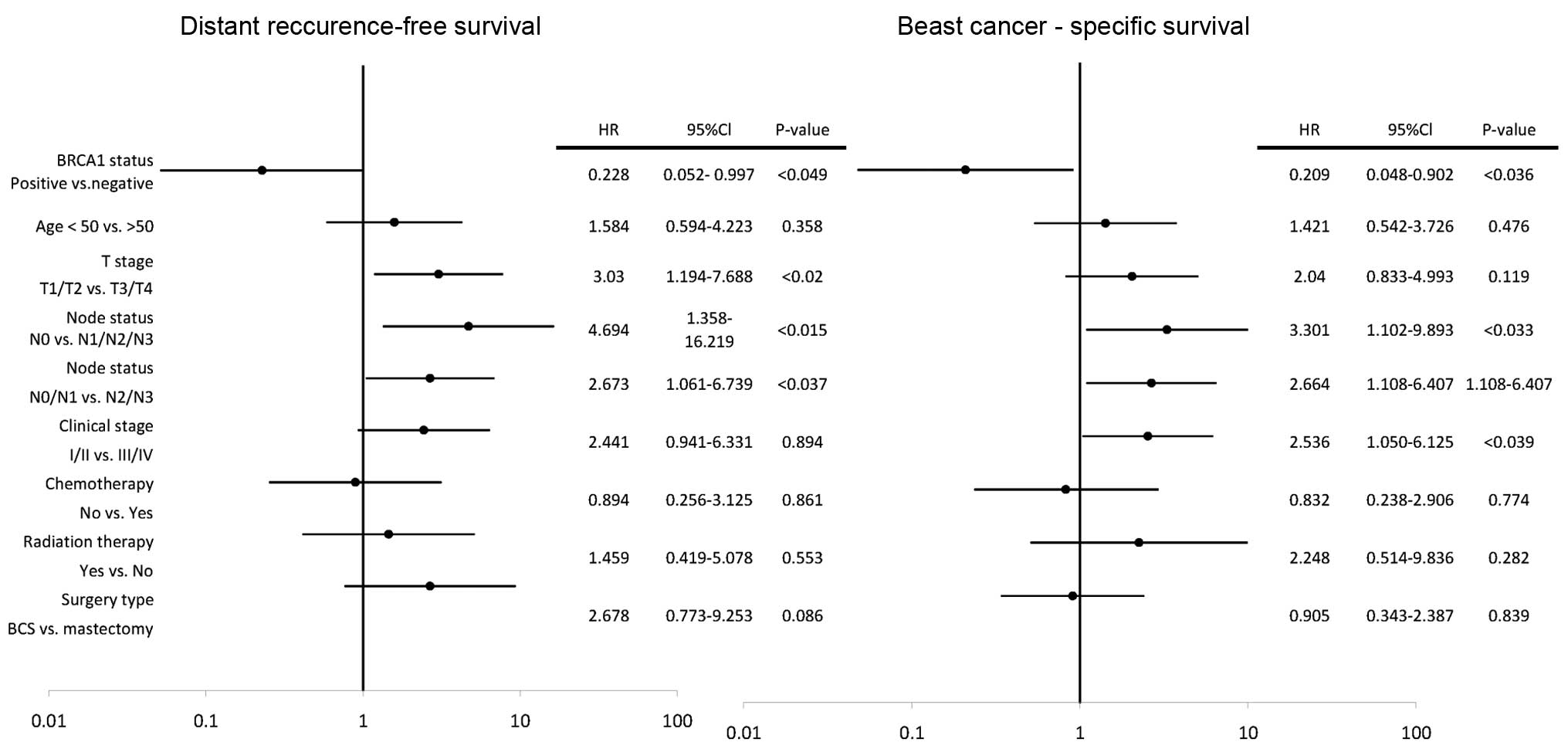
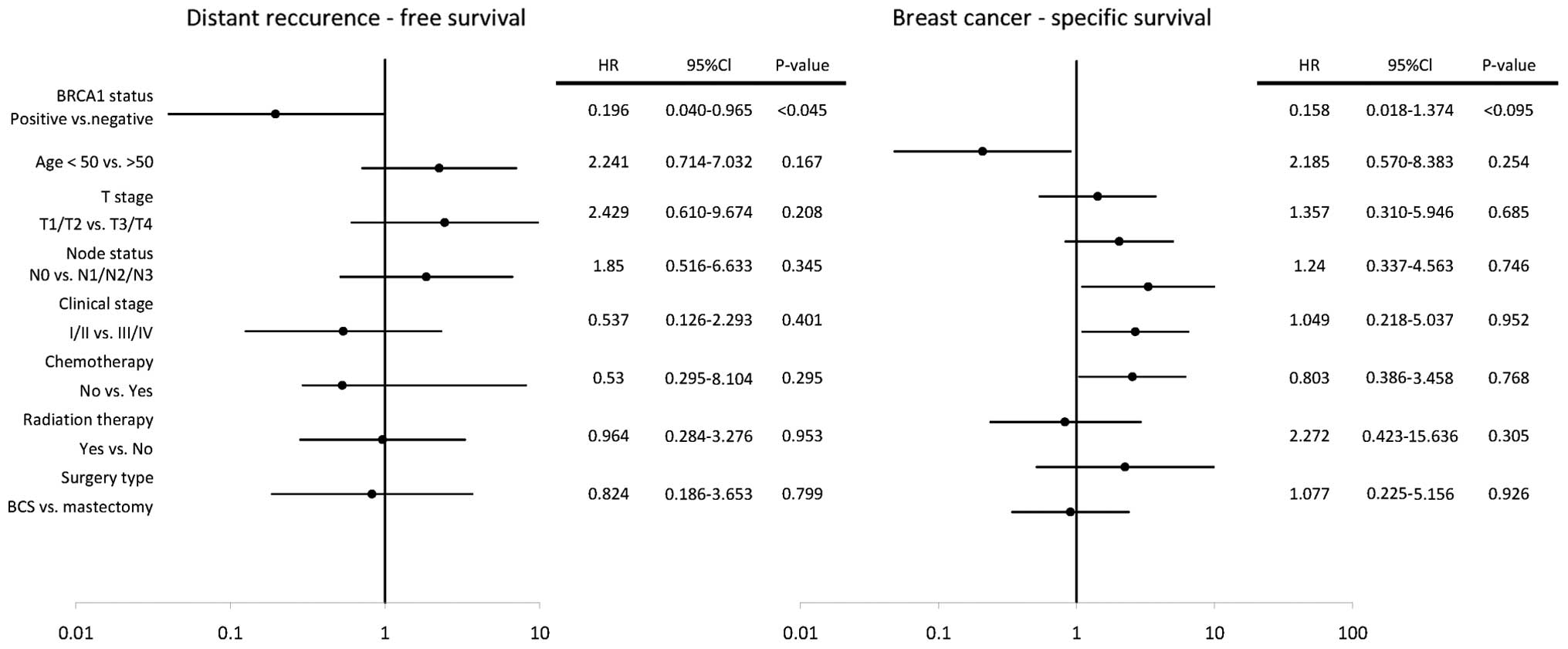
In the univariate analyses, clinical T stage 3 and 4
(HR, 3.030; 95% Cl, 1.194–7.688; P<0.02) and positive lymph node
status (HR, 4.694; 95% Cl, 1.358–16.219; P<0.015) were
associated with a higher risk of distant recurrence, however,
BRCA1-positive status (HR, 0.228; 95% Cl, 0.052–0.997;
P<0.049) was associated with a decreased risk of distant
recurrence (Fig. 2). In the
multivariate analyses Cox proportional-hazards model,
BRCA1-positive status was an independent favorable
prognostic factor for DFRS (HR, 0.196; 95% Cl, 1.040–0.965;
P<0.045).
In the univariate analyses, clinical stages III and
IV (HR, 2.536; 95% Cl, 1.050–6.125; P<0.039) and positive lymph
node status (HR, 3.301; 95% Cl, 1.102–9.893; P<0.033) were
associated with an increased risk of breast cancer-specific
mortality, however, BRCA-1 positive status (HR, 0.209; 95%
Cl, 0.048–0.902; P<0.036) was associated with a decreased risk
of breast cancer-specific mortality (Fig. 3). In the multivariate analysis Cox
proportional-hazards model, no statistically significant effect of
evaluated risk factors on BCS was found.
Discussion
Evidence from the present study indicates that
germline BRCA1 founder mutation (4153delA and 5382insC)
carriers, with no evidence of ovarian cancer or other cancers in
advanced stage, have significantly improved prognosis, relative to
non-carriers. The study demonstrated that positive BRCA1
mutation status reduces the risk of distant recurrence and breast
cancer-specific mortality with statistical significance. Following
adjustment for age, T stage, nodal status, stage, surgery,
radiation therapy and chemotherapy, positive BRCA1 mutation
status was an independent prognostic factor for lower distant
recurrence risk.
Several previous studies have reported no difference
or poorer survival outcomes in the BRCA1 mutation carriers
compared with non-carriers (18–20).
An equal or improved prognosis for BRCA1 mutation carriers
compared with wild-type was demonstrated; however, this difference
was not statistically significant (21). These data were supported by Cortesi
et al, who identified a statistically significant overall
survival advantage in BRCA1-positive patients compared with
BRCA1 mutation-negative and sporadic breast cancers
(22). None of these studies
evaluated the prognostic significance of BRCA1 mutations in
the context of breast cancer subtypes, histological types, tumor
grade or received chemotherapy regimens. Several previous studies
have focused their attention on the prognostic role of positive
BRCA1 mutation status in the TNBC subtype; however they
failed to show a statistically significant improvement in survival
for BRCA1 mutation carriers.
In a study by Lee et al(12), the authors reported similar 5-year
BCS and overall survival rates in BRCA1 mutation carriers
and non-carriers. In this study, the two groups were well balanced,
as all patients received alkylating chemotherapy; however, the
definition of TNBC and positivity of ER and PR cut-off levels were
not specified. Furthermore, 8% of patients received hormonal
treatment.
Gonzalez-Angulo et al showed improved
recurrence-free survival for BRCA1 mutation-positive
patients treated with surgery and anthracycline-taxane chemotherapy
when compared with BRCA1 mutation non-carriers; however,
these patients failed to demonstrate a significant difference in
overall survival. The main limitation of this study was that there
was a statistically significant difference in received chemotherapy
between two groups and, in addition, missing information with
regard to other primary cancers and BCS were not evaluated
(7). In the study by Bayraktar
et al, 227 patients with TNBC were included; of 114 BRCA
mutation carriers, 94 had a BRCA1 mutation and 20 had a
BRCA2 mutation. Patients with bilateral and/or metastatic
breast cancer and previous breast cancer were not included in the
study population. No statistically significant difference was
identified in 5-year overall survival rates between
BRCA1/2 mutation carriers and non-carriers. Following
adjustment for patient age and disease stage, no association with
BRCA1/2 mutation status and overall survival was
found. In this study, no separate effect of BRCA1 mutation
status on overall prognosis of TNBC was evaluated, negative ER and
PR status was defined as nuclear staining of ≤10% and patients with
previous ovarian cancer were included in the study (13).
In the present study, a strict criteria of ASCO/CAP
guideline recommendations for IHC-based testing of ER and PR was
adopted (ER or PR are considered negative if <1% of tumor cell
nuclei are immunoreactive) to identify the TNBC phenotype (16), which significantly diminished the
number of TNBC cases included in the study. Study data were based
on a relatively small number of cases; however, the two groups were
homogeneous by tumor grade, the median tumor size, T stage, stage
of the disease and received chemotherapy (Table I) and only patients with two common
germline founder BRCA1 mutations (4153delA and 5382insC)
were included in the study.
Another difference of the present study was that
patients with ovarian cancer and other cancers in advanced stages
were not included in the study population. In spite of a
significantly improved prognosis for BRCA1 mutation carriers
with ovarian cancer reported by Bolton et al, 5-year overall
survival for these patients was only 46% (23). In each patient excluded from the
study, ovarian cancer was diagnosed in advanced stages (IIIC or IV)
and all patients succumbed to disseminated ovarian cancer within a
median period of 28.5 months (range, 6–45 months) from the time of
diagnosis. The risk of ovarian cancer is ~3% by 40 years old and
54% by 60 years old (24). Several
studies have shown a significant heterogeneity of breast and/or
ovarian cancer prevalence among various mutations of BRCA1
gene (14,15,24).
Prophylactic salpingo-oophorectomy reduces the penetrance of
ovarian/fallopian tube cancer by 75–96% and breast cancer by 56%
(25) in patients with the
BRCA1 mutation. In addition, Bayraktar et al found
that bilateral prophylactic oophorectomy significantly reduces the
risk for mortality in patients with TNBC (HR, 0.01; 95% CI,
0.01–0.69; P<0.02) (13).
Improved breast-cancer specific survival in TNBC
BRCA1 mutation carriers compared with non-carriers may be
explained by biological differences and/or a higher sensitivity to
chemotherapy. In the BRCA1 carriers group, when compared
with the non-carriers group, a higher proportion of node-negative
breast cancers were observed (65.8 vs. 37.2%; P<0.004) with no
statistically significant difference identified between the T stage
of the two groups. A number of studies reported similar data with
regard to the prevailing node-negativity in BRCA1 mutation
carriers, even in patients with large tumor size. These may be
characterized as one of the main biological features of
BRCA1 carriers. Tumor size and nodal status are independent
prognostic factors for survival outcomes. In the univariate
analysis, T stage and nodal status, as well as clinical stage, were
strong predictors of outcomes. In the multivariate analyses, the
factors failed to predict outcomes in BRCA1 mutation carrier
and non-carrier TNBC, perhaps due to a relatively small study
population. However, according to Foulkes et al, there was
no association between increasing tumor size and lymph node
positivity in BRCA1 mutation-positive breast cancers; tumor
size and nodal status were weak predictors of outcomes in
BRCA1 mutation carriers (26).
A higher chemosensitivity for BRCA1 mutation
carriers has been proposed in previous studies. Rennert et
al reported a significantly improved 10-year survival rate for
BRCA1 mutation carriers when compared with non-carriers in
patients treated with chemotherapy and no difference in survival
rates among patients who did not receive chemotherapy (18). Results of the present study were
similar with 89.5% of patients in the BRCA1 mutation group
and 85.9% of patients in the BRCA1 mutation non-carriers
group who received chemotherapy. The heterogeneity of the TNBC
phenotype may explain the phenomenon that, regardless of high
chemosensitivity, TNBC showed poorer survival outcomes compared
with other cancer subtypes.
TNBC is an extremely heterogeneous
clinicopathological entity with various prognostic implications and
clinical features for pathological and molecular subgroups. The
majority of TNBCs are presented by ductal carcinomas (27); however, several other histological
breast cancer types may also lack expression of ER/PR and HER2/neu
IHC-based staining (medullary, apocrine, pleomorphic lobular,
metaplastic and adenoid cystic carcinomas). Apocrine, adenoid
cystic and classical medullary carcinomas are associated with
favorable prognosis. By contrast, metaplastic TNBC displayed a
similarly poor prognosis as high grade adenocarcinomas, but was
less sensitive to conventional chemotherapy (28–31).
According to gene expression profile studies, TNBC may be divided
into several distinct subgroups: Basal-like breast cancer (40–80%),
normal-like, claudin-low, interferon-rich, molecular apocrine and
HER2-enriched TNBC (32). However,
this subclassification of TNBC requires further investigation. A
significantly poorer prognosis has been reported for basal-like
TNBC when compared with non-basal-like breast cancers. There is an
overlap between BRCA1-associated cancers, TNBC and
basal-like breast cancer. BRCA1-mutated tumor cells have a
defective homologous-recombination repair pathway that predisposes
a high sensitivity to DNA-damaging agents (10). Sporadic TNBC or basal-like breast
cancers may also have a dysfunctional BRCA1 pathway that is
caused by epigenetic mechanisms, for example, upregulation of
inhibitor of DNA binding 4 (33) or
BRCA1 promoter hypermethylation (34). In studies on an experimental cell
system, BRCA1-defective cell lines demonstrated higher
sensitivity to platinum agents compared with BRCA1-competent
cell lines and resistance to taxanes (35). Therefore, several clinical trials
are currently underway to investigate the role of DNA-damaging
agents (platinum-based regimens) and PARP-inhibitors in the
treatment of BRCA1-associated TNBC (36,37).
In conclusion, the present study demonstrates that
positive BRCA1 founder mutation status in TNBC significantly
improves prognosis and may be useful for counseling patients with
regard to life expectancy, affecting the choice of chemotherapy
regimens and providing the potential for treatment with
molecular-targeted therapy. Results of the present study indicate
that BRCA1-associated TNBC should be considered as a
biologically and prognostically distinct subtype of TNBC that
displays higher sensitivity to chemotherapy.
Abbreviations:
|
TNBC
|
triple-negative breast cancer
|
|
ER
|
estrogen receptor
|
|
PR
|
progesterone receptor
|
|
HER2/neu
|
human epidermal growth factor receptor
2
|
|
PARP inhibitors
|
poly(ADP-ribose)-polymerase
inhibitors
|
|
IHC
|
immunohistochemistry
|
|
LRR
|
locoregional recurrence
|
|
LRFS
|
locoregional recurrence free
survival
|
|
DRFS
|
distant recurrence-free survival
|
|
BCS
|
breast cancer-specific survival
|
|
ASCO/CAP
|
American Society of Clinical
Oncology/College of American Pathologists
|
|
BRCA1
|
breast cancer 1
|
References
|
1
|
Bauer KR, Brown M, Cress RD, et al:
Descriptive analysis of estrogen receptor(ER)-negative,
progesterone receptor (PR)-negative, and HER2-negative invasive
breast cancer, the so-called triple negative phenotype: A
population-based study from the California cancer rRegistry.
Cancer. 109:1721–1728. 2007. View Article : Google Scholar
|
|
2
|
Rakha EA, Elsheikh SE, Aleksandarany MA,
et al: Triple-negative breast cancer: distinguishing between basal
and nonbasal subtypes. Clin Cancer Res. 15:2302–2310. 2009.
View Article : Google Scholar
|
|
3
|
Kaplan HG and Malmgren JA: Impact of
triple negative phenotype on breast cancer prognosis. Breast J.
14:456–463. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Carey LA, Dees EC, Sawyer L, et al: The
triple negative paradox: primary tumor chemosensitivity of breast
cancer subtypes. Clin Cancer Res. 13:2329–2334. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Atchley DP, Albarracin CT, Lopez A, et al:
Clinical and pathologic characteristics of patients with
BRCA-positive and BRCA-negative breast cancer. J Clin Oncol.
26:4282–4288. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Reis-Filho JS and Tutt AN: Triple negative
tumours: a critical review. Histopathology. 52:108–118. 2008.
View Article : Google Scholar
|
|
7
|
Gonzalez-Angulo AM, Timms KM, Liu S, et
al: Incidence and outcome of BRCA mutations in unselected patients
with triple receptor-negative breast cancer. Clin Cancer Res.
17:1082–1089. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hartman AR, Kaldate RR, Sailer LM, et al:
Prevalence of BRCA mutations in an unselected population of
triple-negative breast cancer. Cancer. 118:2787–2789. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Farmer H, McCabe N, Lord CJ, et al:
Targeting the DNA repair defect in BRCA mutant cells as a
therapeutic strategy. Nature. 434:917–921. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Silver DP, Richardson AL, Eklund AC, et
al: Efficacy of neoadjuvant Cisplatin in triple-negative breast
cancer. J Clin Oncol. 28:1145–1153. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tutt A, Robson M, Garber JE, et al: Oral
poly(ADP-ribose) polymerase inhibitor olaparib in patients with
BRCA1 or BRCA2 mutations and advanced breast cancer: a
proof-of-concept trial. Lancet. 376:235–244. 2010. View Article : Google Scholar
|
|
12
|
Lee LJ, Alexander B, Schnitt SJ, et al:
Clinical outcome of triple-negative breast cancer in BRCA1
mutation carriers and noncarriers. Cancer. 117:3093–100. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bayraktar S, Gutierrez-Barrera AM, Liu D,
et al: Outcome of triple-negative breast cancer in patients with
and without deleterious BRCA mutations. Breast Cancer Res Treat.
130:145–153. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Plakhins G, Iremjs A, Gardovskis A, et al:
Genotype-phenotype correlations among BRCA1 4153delA and 5382insC
mutation carriers form Latvia. BMC Med Genet. 12:1472011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Thompson D and Easton D: Breast cancer
linkage consortium: variation in BRCA1 cancer risks by mutation
position. Cancer Epidemiol Biomarkers Prev. 11:329–336.
2002.PubMed/NCBI
|
|
16
|
Hammond ME, Hayes DF, Dawsett W, et al:
American Society of Clinical Oncology/College of American
Pathologists guideline recommendations for immunohistochemical
testing of estrogen and progesterone receptors in breast cancer. J
Clin Oncol. 28:2784–2795. 2010. View Article : Google Scholar
|
|
17
|
Elston CW and Ellis IO: Pathological
prognostic factors in breast cancer. I The value of histological
grade in breast cancer: experience from a large study with
long-term follow-up. Histopathology. 19:403–410. 1991. View Article : Google Scholar
|
|
18
|
Rennert G, Bisland-Naggan S,
Barnett-Griness O, et al: Clinical outcomes of breast cancer in
carriers of BRCA1 and BRCA2 mutations. N Engl J Med. 357:115–123.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bordeleau L, Panchal S and Goodwin P:
Prognosis of BRCA-associated breast cancer: a summary of evidence.
Breast Cancer Res Treat. 119:13–24. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Moller P, Evans DG, Reis MM, et al:
Surveilance for familial breast cancer: differences in outcome
according to BRCA mutation status. Int J Cancer. 121:1017–1020.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Veronesi A, de Giacomi C, Magri MD, et al:
Famialial breast cancer: characteristics and outcome of BRCA 1–2
positive and negative cases. BMC Cancer. 5:702005.
|
|
22
|
Cortesi L, Masini C, Cirilli C, et al:
Favourable ten-year overall survival in a Caucasian population with
high probability of hereditary breast cancer. BMC Cancer.
10:902010.
|
|
23
|
Bolton KL, Chenefix-Trench G, et al;
EMBRACE; kConFab Investigators; Cancer Genome Atlas Research
Network. Association between BRCA1 and BRCA2
mutations and survival in women with invasive epithelial ovarian
cancer. JAMA. 307:382–390. 2012.
|
|
24
|
King MC, Marks JH, Mandell JB, et al:
Breast and ovarian cancer risks due to inherited mutations in BRCA1
and BRCA2. Science. 302:643–646. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Finch A, Evans G, Narod SA, et al: BRCA
carriers, prophylactic salpingo-oophorectomy and menopause:
clinical management considerations and recommendations. Womens
Health (Lond Engl). 8:543–555. 2012. View Article : Google Scholar
|
|
26
|
Foulkes WD, Metcalfe K, Hanna W, et al:
Disruption of the expected positive correlation between breast
tumor size and lymph node status in BRCA-1 related breast
carcinoma. Cancer. 98:1569–1577. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Carey LA, Perou CM, Livasy CA, et al:
Race, breast cancer subtypes, and survival in the Carolina Breast
Cancer Study. JAMA. 295:2492–2502. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Vincent-Salomon A, Gruel N, Lucchesi R, et
al: Identification of typical medullary breast carcinoma as a
genomic sub-group of basal-like carcinomas, a heterogeneous new
molecular entity. Breast Cancer Res. 9:R242007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Azoulay S, Laé M, Fréneaux P, et al: KIT
is highly expressed in adenoid cystic carcinoma of the breast, a
basal-like carcinoma associated wtih favorable outcome. Mod Pathol.
18:1623–1631. 2005.
|
|
30
|
Marchiò C, Irivani M, Natrajan R, et al:
Mixed micropapillary-ductal carcinomas of the breast: a genomic and
immunohistochemical analysis of morphological distinct components.
J Pathol. 218:301–315. 2009.
|
|
31
|
Hennessy BT, Giordano S, Broglio K, et al:
Biphasic metaplastic sarcomoid carcinoma of the breast. Ann Oncol.
17:605–613. 2009. View Article : Google Scholar
|
|
32
|
Perou CM: Molecular stratification of
triple-negative breast cancers. Oncologist. 16:61–70. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Beger C, Pierce LN, Kruger M, et al:
Identification of Id4 as a regulator of BRCA1 expression by using a
ribozyme-library-based inverse genomics approach. Proc Natl Acad
Sci USA. 98:130–135. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Grushko TA, Nwachukwu N,
Charoenthammaraksa S, et al: Evaluation of BRCA1 inactivation by
promoter methylation as a marker of triple-negative and basal-like
breast cancers. J Clin Oncol. 28(Suppl; abstract 10510):
1552010.
|
|
35
|
Tassone P, Tagliafferi P, Perricelli, et
al: BRCA1 expression modulates chemosensitivity of BRCA1-defective
HCC1937 human breast cancer cells. Br J Cancer. 88:1285–1291. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Byrski T, Huzarski T, Dent R, et al:
Response to neoadjuvant therapy with cisplatin in BRCA1-positive
breast cancer patients. Breast Cancer Res Treat. 115:359–363. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
O’Shaughnessy J, Schwartaberg LS, Danso
MA, et al: A randomized phase III study of iniparib (BSI-201) in
combination with gemcitabine/carboplatin (G/C) in metaplastic
triple-negative breast cancer (TNBC). J Clin Oncol. 29.(Suppl;
abstract 1007)2011.
|