Introduction
Gastrointestinal symptoms including heartburn,
flatulence and changes in bowel habits are extremely common
complaints. In the majority of cases, a benign aetiology is
responsible, but symptoms may also be associated with colorectal
cancer (CRC). The mean time from symptom onset to diagnosis of CRC
in Finnish patients is 10 months (1). The diagnostic delay is partly caused
by patients being ashamed of their symptoms (91% of Finnish
people), but most commonly due to the nonspecific and vague
character of CRC symptoms (2–3).
Work-up for abdominal complaints is often terminated at the
diagnosis of common findings, including lactose intolerance,
irritable bowel syndrome and Helicobacter pylori (H.
pylori) infection.
H. pylori is a gram-negative bacterium, which
colonizes the gastric epithelium and induces chronic inflammation
of the gastric mucosa (4). H.
pylori is the most important single risk factor for peptic
ulcer disease (5). Infection is
usually acquired in childhood and adolescence, (6–7) and
tends to persist unless eradicated (7–8).
Although prevalence of the infection in the western world has
rapidly decreased during the past decades (7,9), at
least half of the world’s population remains infected today
(5). The majority of patients are
asymptomatic, but in approximately 8% of H. pylori-positive
dyspepsia patients, eradication of the bacterium leads to long-term
relief of dyspepsia. Eradication is more effective than any other
treatment and, therefore, dyspepsia remains an accepted indication
for H. pylori eradication treatment (5).
Since 1994, H. pylori has been classified as
a class I carcinogen (10). H.
pylori is the most common proven risk factor for human
non-cardiac gastric cancer, and eradication of H. pylori is
the first-line therapy for low-grade gastric mucosa-associated
lymphoid tissue lymphoma (5).
Previous studies have evaluated the possible role of H.
pylori in the development of CRC. However, large
epidemiological studies remain missing and associations remain
controversial (11–14). It has been proposed that H.
pylori infection increases the secretion of gastrin, which may
lead to mucosal cell alteration in the colorectum (12,14–15).
We hypothesised that H. pylori infection may
influence tolerability of chemotherapy, and that eradication of the
bacterium may be required to minimise chemotherapy-associated
adverse events in CRC patients. As a secondary aim, we investigated
whether H. pylori-associated symptoms were able to cause a
delay in the diagnosis of CRC, and whether H. pylori status
correlates with long-term outcome in CRC patients treated in a
randomised prospective trial.
Material and methods
This was an open, prospective, randomised single
institution study in radically operated CRC patients. The LIPSYT
trial is registered on http://www.controlled-trials.com/ISRCTN98405441.
According to the study protocol, 80 patients with histologically
proven CRC were included in the study. One patient did not start
treatment due to a non-healing recto-vaginal fistula, leaving 79
patients for evaluation. The patients were treated at the
Department of Oncology at Helsinki University Central Hospital
(Helsinki, Finland), between November 1997 and October 1999. The
minimum follow-up period of the surviving patients was 120
months.
Patients were eligible for inclusion if they were
aged between 18 to 75 years, had histologically confirmed radically
operated stage II–IV CRC (amendment to include radically operated
stage IV patients in the tolerability part of the study was
performed in December 1997), WHO performance status 0–2 and
adequate bone marrow, kidney and liver function. Exclusion criteria
included history of invasive cancer other than CRC; metabolic,
neurological or psychiatric illness that was incompatible with
chemotherapy; serious thromboembolic event currently under
treatment; pregnancy, lactation or absence of adequate
contraception in fertile patients.
The Ethical Review Board at Helsinki University
Hospital approved the protocol and a written informed consent was
obtained from all patients. No patients were lost to follow-up.
Treatment regimens
Patients were randomised to receive postoperative
adjuvant chemotherapy in CRC, combined with radiotherapy in rectal
cancer if the distal margin of tumour was below the peritoneal
fold. Randomisation was performed by minimization technique, with
one out of six chances. Computer-based randomisation was performed
by the oncologist (Pia Österlund) with gender, primary tumour and
stage as factors. Adjuvant chemotherapy consisted of 5-FU and
leucovorin (LV) as bolus injection (Mayo regimen) or continuous
infusion (simplified de Gramont regimen) (16) according to randomisation. The Mayo
regimen consisted of a 3- to 5-min intravenous bolus of 370–425
mg/m2 5-FU and infusion of 10–20 mg/m2 LV on
days 1–5 of a 4-week cycle, repeated six times. In rectal cancer
during 5.5-week pelvic chemoradiation (50.4/1.8 Gy), starting on
day 56, a single 500-mg/m2 5-FU bolus was administered
intravenously during days 1–3 on the first and fifth weeks.
Radiation dose was based on CT planning and administered in three
to four fields with high-energy photons. Following penetration of
the preoperative Swedish radiotherapy results (17), certain patients received 25/5 Gy
over 5 days preoperatively without concomitant chemotherapy. In
these cases, postoperative adjuvant chemotherapy was provided. The
de Gramont regimen consisted of a 2-h infusion of 200–400
mg/m2 LV followed by a 400-mg/m2 5-FU bolus
and continuous 3.0–3.6-g/m2 5-FU infusion for 48 h,
repeated every 14 days for 12 cycles. Rectal cancer patients
received continuous infusion of 225 mg/m2 5-FU during
the same radiation treatment.
Assessment of H. pylori status, lactose
intolerance and treatment toxicity
Patients were evaluated at baseline prior to
chemotherapy, every 4 weeks throughout chemotherapy and
radiotherapy, and at 2–6 month intervals post-treatment up to five
years and at 10 years. A complete medical history and physical
examination were performed, including WHO performance status,
height and weight, and laboratory assessment with tumour markers
and blood cell counts.
Serum samples were collected prior to treatment,
during the cancer therapy (at 2, 4 and 6 months) and after
chemotherapy (at 8 and 12 months from initiation). Serum samples
were stored at −20°C until analysis for H. pylori antibodies
of the IgG and IgA classes using a locally validated in-house
enzyme immunoassay with high sensitivity and specificity (18). H. pylori eradication was
defined by ≥40% decline of the IgG titre within 6–12 months
(9).
Seventy-seven patients were evaluated for lactose
intolerance using an oral lactose tolerance test at baseline, and
at 4 and 8 months after initiation of chemotherapy. The test was
performed after a 12-h fast using an oral load of 50 g lactose, and
blood glucose levels were measured three times at 20 min intervals
and categorised as hypolactasia (a blood glucose level increase of
<1.1 mmol/l), borderline finding (1.1–1.6 mmol/l) and
normolactasia (>1.6 mmol/l).
Chemotherapy-associated toxicities were recorded in
a patient diary and graded according to the NCI-C CTC version 2
(the Common Toxicity Criteria of the National Cancer Institute of
Canada). The worst toxicity grade during adjuvant chemotherapy was
taken into account in the analysis.
Presenting symptoms of CRC were assessed from
hospital charts and by patient recall, and recorded into the
patient diary at baseline. The severity of the symptoms at baseline
as well as during the adjuvant treatment was evaluated.
All 79 patients were included in toxicity analysis.
Six patients were excluded (n=73) in the analysis of diagnostic
delay. In two patients it was impossible to determine the time from
the onset of symptoms to diagnosis, one with gradually worsening
anaemia and persistent constipation, the other with stomach pain
and rectal bleeding from nonsymptomatic haemorrhoids. Four
radically operated stage IV patients (local relapse or distant
metastases resected) were included in the toxicity assessment, but
excluded from diagnostic delay and survival analysis. Only stage
II–III patients were included (n=73) in the efficacy analysis of
disease-free survival and overall survival.
Statistical analysis
The sample size calculation was based on the
expected worst grade 3–4 oro-gastrointestinal toxicity. It was
assumed that the toxicity was 60% in H. pylori-seropositive
vs. 30% in H. pylori-seronegative patients. It was also
assumed that there is no interaction between H. pylori
positivity and chemotherapy treatment. With a 0.05 two-sided
significance level and 80% power, the required sample size in each
group is 40.
The gastrointestinal and dyspeptic symptoms during
the chemotherapy treatment were the primary variables. The worst
symptoms of grade 3–4 or symptoms of any grade, when appropriate,
were compared between H. pylori-seropositive and
-seronegative patients using the binary logistic regression
analysis. As the patients were randomised to receive adjuvant
chemotherapy (simplified de Gramont vs. Mayo regimen), the
chemotherapy regimen was included as a categorical covariate. The
interaction between H. pylori positivity and chemotherapy
treatment was tested first, and if existence of interaction was not
evident (P>0.10), the interaction term was omitted. The results
are expressed as adjusted odds ratios with 95% confidence intervals
(CIs). In case of interaction, the association between H.
pylori and a symptom was assessed in both treatment groups
separately.
Disease-free survival, overall survival and the
diagnostic delay from onset of symptoms to surgery were the
secondary variables. Disease-free survival was defined as the time
from the date of initiation of the chemotherapy to the day of
relapse, new CRC or mortality due to any cause. Overall survival
was defined from the date of initiation of the chemotherapy to the
date of mortality from any cause, or censored at the time of last
follow-up, which was ≥10 years later. Cox regression analysis was
applied to compare disease-free and overall survival between H.
pylori-seropositive and -seronegative patients. The
chemotherapy treatment was included as a categorical covariate and
the interaction between H. pylori positivity. The results
are given as adjusted hazard ratios with 95% CIs. The log-rank test
was used to compare the diagnostic delay between H.
pylori-seropositive and -seronegative patients.
The log-rank test was also used to compare the
diagnostic delay between groups with lactose intolerance.
Kaplan-Meier curves were drawn to describe the survival curves and
diagnostic delay for H. pylori-seropositive and
-seronegative patients. The χ2 and Mann-Whitney U tests
were used for patient characteristics between H.
pylori-seropositive vs. -seronegative patients. All tests were
two-tailed and P<0.05 was considered to indicate a statistically
significant result. IBM SPSS Statistics for Windows, version 20.0
(IBM Corp., Armonk, NY, USA) was used in calculations.
Results
Patient characteristics are presented in Table I. The treatment arms were
well-balanced by age, gender, and tumour stage and site. The median
age of patients was 60 years (range, 31–76). At baseline 37
patients were H. Pylori-seropositive and 42 patients were
H. Pylori-seronegative, of which two had received
eradication treatment successfully years earlier. The 42 patients
who were seronegative prior to chemotherapy remained negative for
the next 12 months, and after chemotherapy. A total of 30 patients
had positive H. pylori status before and after chemotherapy.
Seven patients were seropositive before chemotherapy but became
seronegative within 12 months. These seven patients had received
antimicrobial therapy for bacterial infections during the treatment
course and one also for H. pylori in the diagnostic
phase.
 | Table IPatient characteristics in
Helicobacter pylori-seronegative and -seropositive. |
Table I
Patient characteristics in
Helicobacter pylori-seronegative and -seropositive.
| Characteristic | Total | Helicobacter
pylori- seronegative | Helicobacter
pylori-seropositive | P-value |
|---|
| Number, n (%) | 79 (100) | 42 (53) | 37 (47) | |
| Median age, years
(range) | 60 (31–76) | 58 (31–73) | 60 (36–75) | 0.89 |
| Gender, n (%) |
| Male | 40 (51) | 18 (43) | 15 (41) | 0.14 |
| Female | 39 (49) | 24 (57) | 22 (59) | |
| Tumour site, n
(%) |
| Colon | 42 (53) | 26 (62) | 21 (57) | 0.10 |
| Rectum | 37 (47) | 16 (38) | 16 (43) | |
| Tumour stage, n
(%) |
| II | 19 (24) | 9 (21) | 10 (27) | 0.68 |
| III | 54 (68) | 31 (74) | 23 (62) | |
| IV | 6 (8) | 2 (5) | 4 (11) | |
| Chemotherapy, n
(%) |
| Bolus regimen | 41 (52) | 24 (57) | 17 (46) | 0.32 |
| Infusion
regimen | 38 (48) | 18 (43) | 20 (54) | |
| Rectal
radiotherapy, n (%) |
| No | 48 (61) | 29 (69) | 19 (51) | 0.30 |
| 25/5 Gy | 5 (6) | 1 (2) | 4 (11) | |
| 45.0–50.4/1.8
Gy | 26 (33) | 12 (29) | 14 (38) | |
| Lactose
intolerance, n | 77 | 40 | 37 | |
| Hypolactasia, n
(%) | 14 (18) | 6 (15) | 8 (22) | 0.20 |
| Borderline, n
(%) | 12 (16) | 9 (22) | 3 (8) | |
| Normolactasia, n
(%) | 51 (66) | 25 (63) | 26 (70) | |
Oro-gastrointestinal symptoms during
chemotherapy
Adverse events during 5-FU treatment were compared
between patients who were H. pylori-seronegative and those
who were H. pylori-seropositive at baseline and no
statistically significant differences were observed (Table II). The worst oro-gastrointestinal
toxicity was grade 3 or 4 in 54% of the seropositive and 62% in the
seronegative patients (P=0.68). Functional dyspeptic symptoms
(postprandial fullness, nausea, belching, early satiety, epigastric
pain and burning) were present in 46% of the seropositive and in
48% of the seronegative patients (P=0.91). Digestive symptoms,
including nausea, constipation and flatulence, were equally common
(present in 43–86% of the patients) in both H. pylori
groups. Severe mucositis, characterised as stomatitis or diarrhoea,
was common (present in 81–93% of patients), but no differences
between groups were observed.
 | Table IIToxicity during chemotherapy in
Helicobacter pylori-seronegative and -seropositive
patients. |
Table II
Toxicity during chemotherapy in
Helicobacter pylori-seronegative and -seropositive
patients.
| | | Helicobacter
pylori-seropositive vs. -seronegative patients |
|---|
| | |
|
|---|
| Toxicity/grade | Seronegative
patients (n=42), n (%) | Seropositive
patients (n=37), n (%) | ORa | 95% CI | P-value |
|---|
| Worst
oro-gastro-intestinal toxicity |
| None | 0 (0) | 0 (0) | 0.82 | 0.32–2.11 | 0.68 |
| Grade 1–2 | 16 (38) | 17 (46) | | | |
| Grade 3–4 | 26 (62) | 20 (54) | | | |
| Stomatitis |
| None | 3 (7) | 6 (16) | 0.65 | 0.23–1.86 | 0.43 |
| Grade 1–2 | 24 (57) | 22 (60) | | | |
| Grade 3–4 | 15 (36) | 9 (24) | | | |
| Functional
dyspepsia |
| None | 22 (52) | 20 (54) | 0.95 | 0.39–2.32 | 0.91 |
| Grade 1–2 | 19 (45) | 16 (43) | | | |
| Grade 3–4 | 1 (2) | 1 (3) | | | |
| Diarrhoeab |
| None | 8 (19) | 9 (24) | 0.61 | 0.21–1.73 | 0.35 |
| Grade 1–2 | 20 (48) | 20 (54) | | | |
| Grade 3–4 | 14 (33) | 8 (22) | | | |
| Constipation |
| None | 23 (55) | 21 (57) | 0.94 | 0.38–2.30 | 0.89 |
| Grade 1–2 | 19 (45) | 16 (43) | | | |
| Grade 3–4 | 0 (0) | 0 (0) | | | |
| Flatulence |
| None | 14 (33) | 17 (46) | 0.55 | 0.22–1.40 | 0.21 |
| Grade 1–2 | 27 (64) | 20 (54) | | | |
| Grade 3–4 | 1 (2) | 0 (0) | | | |
| Nausea |
| None | 6 (14) | 12 (32) | 1.52 | 0.37–6.21 | 0.56 |
| Grade 1–2 | 32 (76) | 20 (54) | | | |
| Grade 3–4 | 4 (10) | 5 (14) | | | |
Interaction between H. pylori status and
chemotherapy regimen-related adverse events was observed only for
diarrhoea of any grade (P=0.006), but no significant interaction
was observed separately for grade 3–4 diarrhoea (P=0.110). In H.
pylori-seropositive patients there was no significant
difference between chemotherapy groups, as 65% in the bolus group
vs. 85% in the infusion group had any grade of diarrhoea (P=0.16)
and 24 vs. 20% had grade 3–4 diarrhoea (P=0.80). However, in
seronegative patients any grade of diarrhoea was significantly more
common in the bolus group (96 vs. 61%, P=0.018) and diarrhoea of
grade 3–4 was more common in the bolus group (50 vs. 11%,
P=0.015).
Diagnostic delay from onset of symptoms
to surgery for CRC
The most common symptoms of CRC present at diagnosis
were bowel symptoms (including diarrhoea, constipation, alternating
function or mucous faeces) in 53 (73%), blood in the stool in 36
(49%), functional dyspepsia in 26 (35%), anaemia in 13 (18%),
occlusion/perforation in 10 (14%) and infectious symptoms in 10
(14%) patients.
The median (inter quartile range, IQR) time from the
onset of symptoms to CRC operation was 6 months (range, 4–11) in
all 73 evaluable patients. The longest time from symptoms to
surgery was 42 months. In the 35 H. pylori-seropositive
patients, the delay was significantly longer than in the 38
seronegative patients; median 6 months (range, 4–12) vs. 5 months
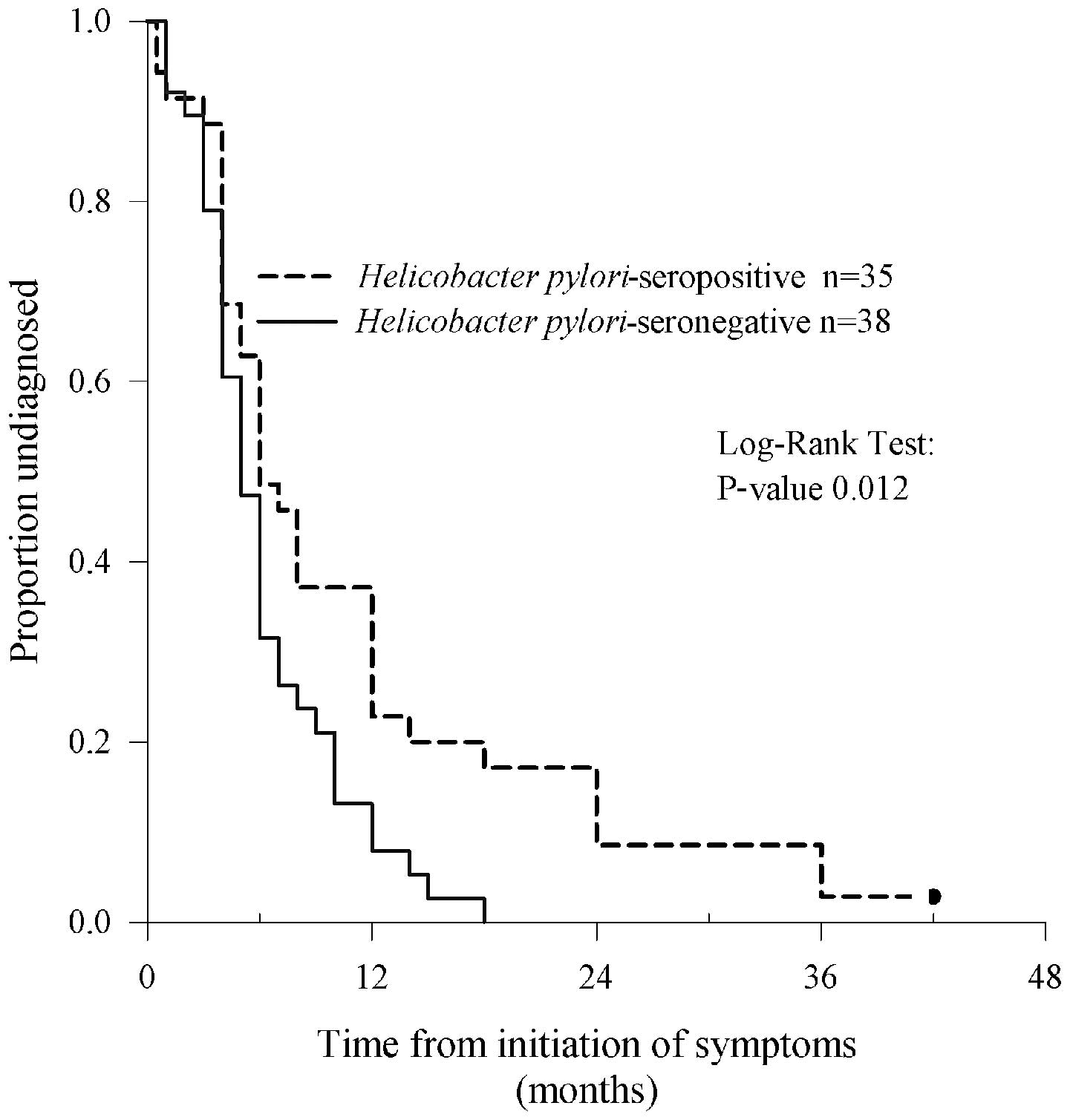
(range, 4–8) (P=0.012, Fig. 1). In
six (17%) seropositive patients, but in none of the seronegative,
the diagnostic delay was greater than 18 months.
Twenty six (35%) out of 73 patients who had
functional dyspepsia at presentation (postprandial fullness,
nausea, belching, early satiety, epigastric pain and burning) had
significantly delayed diagnosis; median 7.5 months (range, 4–14)
vs. 5 months (range, 2–8) (P=0.035). By comparison, in patients
presenting with anaemia (H. pylori-seropositive, 26% vs.
H. pylori-seronegative, 11%), bowel symptoms (77 vs. 68%),
occlusion (11 vs. 16%), blood in the stool (43 vs. 55%) or
infectious symptoms (14 vs. 13%) had no statistically significant
diagnostic delays.
Lactose intolerance with oral lactose test was
assessed in 77 (97%) of the patients. Fourteen (18%) patients, out
of the 77 tested, were diagnosed with hypolactasia and 12 (16%)
with borderline finding, whereas normolactasia was found in 51
(66%) patients. There was no correlation between hypolactasia and
H. pylori seropositivity (P=0.20). Patients with
hypolactasia, borderline or normolactasia did not differ
significantly with respect to median diagnostic delay; 5 months
(range, 4–10) vs. 5 months (range, 4–14) vs. 6 months (range,
4–10), respectively (log-rank test P=0.99).
Disease-free and overall survival
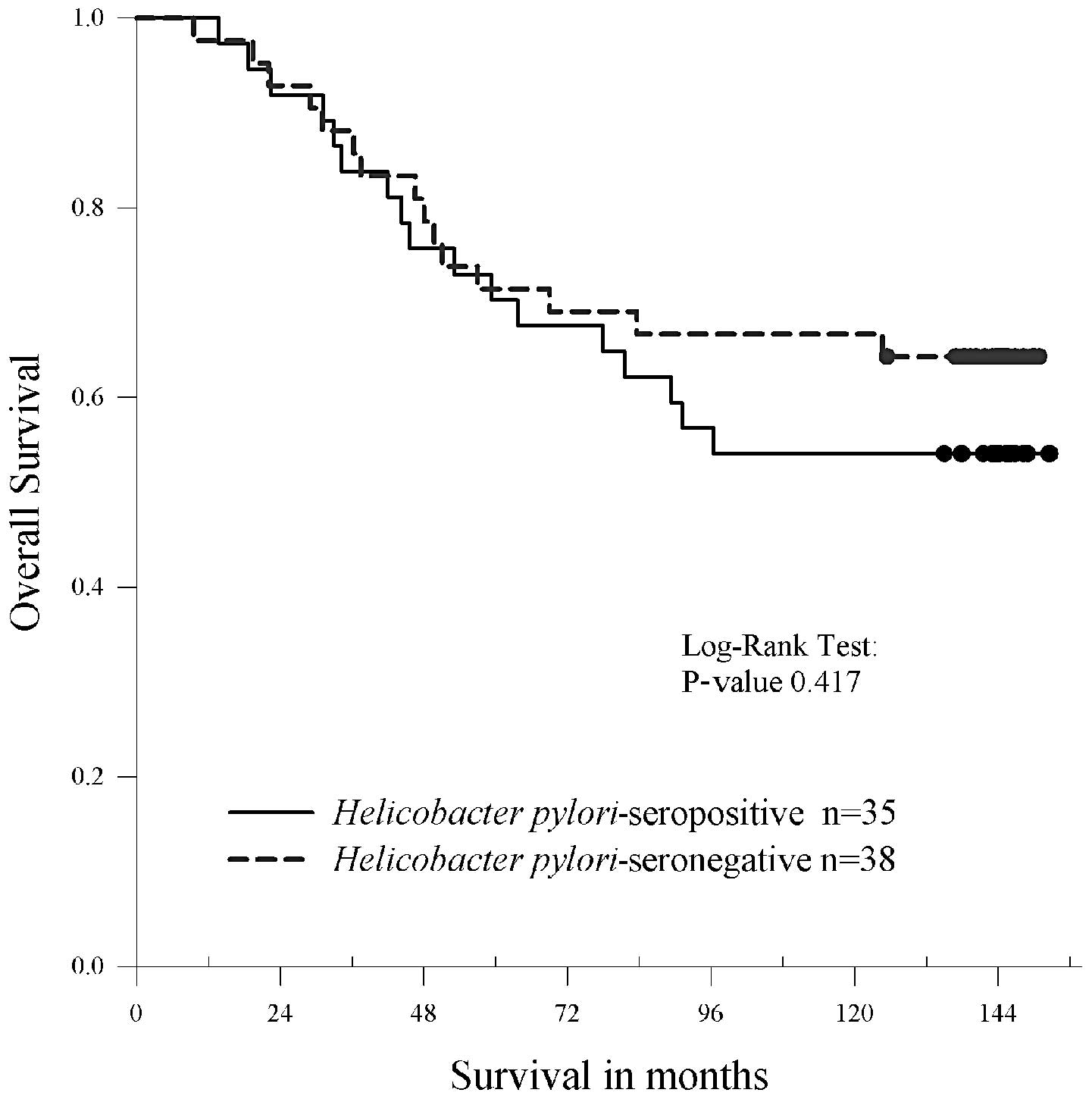
The minimum follow-up time for the surviving 73
stage II and III patients was 120 months. At this 10-year time
point the disease-free survival rate was 64% and the overall
survival rate was 65%. Between groups, the disease free survival
rate was 61% for H. pylori-seropositive patients vs. 67% for
seronegative patients, and the overall survival rate was 61 vs.
69%. The differences in survival curves between seropositive and
seronegative patients were statistically nonsignificant. Adjusted
hazard ratios were 1.32 (95% CI, 0.64–2.72; P=0.453) and 1.34 (95%
CI, 0.61–2.92; P=0.461) for disease-free and overall survival,
respectively. The Kaplan-Meier survival curve for overall survival
is shown in Fig. 2.
Discussion
While the incidence of gastric cancer in western
countries has declined along with the decreasing prevalence of
H. pylori infection (6,19), CRC
has become the third most common type of cancer in the western
world and the second leading cause of cancer-associated mortality
(20). This makes the impact of CRC
and its treatment even more significant. In the present study, we
investigated the possible role of H. pylori infection in CRC
patients undergoing adjuvant chemotherapy. Although H.
pylori-seropositive patients were not shown to develop
gastrointestinal adverse events more often than the seronegative
patients during treatment and there were no significant differences
between the two groups in disease-free and overall survival rates,
we demonstrated a significant delay in the diagnosis of CRC among
the H. pylori-positive patients as compared with the H.
pylori-negative patients. Of the different symptoms studied,
functional dyspepsia was significantly associated with a delayed
diagnosis of CRC.
Functional dyspepsia is a common complaint and often
represents with a wide spectrum of upper abdominal symptoms,
including postprandial fullness, nausea, belching, early satiety,
epigastric pain and burning. Although the majority of H.
pylori infected patients are asymptomatic, H. pylori is
considered to be significant in functional dyspepsia. H.
pylori eradication is thus far the best therapy available, and
it provides long-term relief of symptoms in approximately one of 12
H. pylori-positive dyspepsia patients treated (5). Therefore screening of H. pylori
and eradication treatment in infected dyspepsia patients are often
considered as routine (4,21). In this study, patients with
functional dyspepsia at diagnostic work-up and those who were H.
pylori-seropositive at initiation of adjuvant chemotherapy, had
a statistically significant delay in their diagnosis of CRC.
Dyspeptic symptoms may first lead to gastroscopy or other
interventions associated with H. pylori and, thus, result in
postponed colonoscopy and delayed diagnosis of CRC. Colon cancer
has previously been observed to mimic functional dyspepsia in one
small retrospective patient series (22), which is in accordance with our
present prospective study with functional dyspeptic symptoms
misleading diagnostic work-up in CRC patients. The investigation of
nonspecific symptoms, including functional dyspepsia and the
subsequent diagnosis of H. pylori, may even postpone the
diagnosis of malignancies other than CRC. We recently detected, in
a large cohort of Finnish subjects who had received reimbursement
for drugs used for H. pylori eradication, a significantly
higher incidence of numerous different malignancies (including
colon and rectum cancers) as compared with the average population
(23).
Symptoms typically associated with CRC include
changes in bowel habits, gastrointestinal bleeding leading to
anaemia or blood in the stool, abdominal pain, weight loss and
obstructive symptoms. In the diagnosis of CRC, the highest positive
predictive values have been observed for rectal bleeding in
combination with change in bowel habit or with perianal symptoms
(3). In this study, when different
symptoms were analysed in association with diagnostic delay, no
significant differences were observed between H.
pylori-seropositive and -seronegative patients.
Diagnostic CRC work-up is occasionally stopped when
common complaints other than those likely associated with H.
pylori have been diagnosed. Although H. pylori infection
was the most common finding in our patient material, lactose
intolerance was also frequent. The majority of the world’s
population has hypolactasia but not all have symptoms and, thus,
genetic and nutritional factors influence lactose tolerance.
Although numerous typical symptoms of lactose intolerance,
including abdominal pain, bloating, flatulence, diarrhoea,
borborygmi, and in certain occasions nausea and vomiting (24), are the same as those in functional
dyspepsia, there was no statistically significant diagnostic delay
of CRC in patients with hypolactasia at baseline in our study.
The most common chemotherapy-associated side-effects
are oro-gastrointestinal, including stomatitis, diarrhoea and
nausea. These symptoms may have an extremely negative impact on
quality of life during the treatment and may lead to dose
reductions or, in the worst case, to early cessation of
chemotherapy. We aimed to study whether H. pylori infection
influences development of chemotherapy-associated side-effects in
CRC patients. According to our results, H.
pylori-seropositive patients tolerated the treatment equally
well compared with H. pylori-seronegative patients. This is
also in accordance with the findings that not all H.
pylori-infected individuals possess symptoms (4). Although the number of patients in our
study was relatively small, it appears unlikely that H.
pylori infection would exaggerate oro-gastrointestinal toxicity
during chemotherapy and, therefore, the screening or eradication of
H. pylori in CRC patients prior to adjuvant treatment is
unnecessary.
Among gastric cancer patients treated with surgery
and adjuvant therapy, H. pylori-negative patients have an
inferior outcome compared with H. pylori-positive
individuals (25,26). The reason for this remains
unexplained, but it may be associated with immunological aspects
(25). Although the role of H.
pylori in the carcinogenesis of CRC remains unclear, we
evaluated the possible prognostic influence of H. pylori
status in the survival of CRC patients treated with adjuvant
chemotherapy. There were no significant differences in disease-free
or overall survival between H. pylori-seropositive and H.
pylori-seronegative CRC cohorts. Thus, the diagnostic delay
observed in H. pylori positive patients did not become a
significantly inferior curative outcome in this small series.
We conclude that H. pylori infection does not
appear to increase toxicity during 5-FU-based adjuvant chemotherapy
in operated CRC patients and, therefore, screening of H.
pylori is not recommended in these patients prior to the start
of the adjuvant chemotherapy. However, the most important finding
in this study is that dyspeptic symptoms or the presence of H.
pylori may lead to diagnostic delay of CRC. Although in this
study we could not show inferior disease-free or overall survival
in H. pylori-positive patients as compared with H.
pylori-negative patients during the follow-up period of ≥10
years, it is important to emphasize that diagnostic CRC work-up
should not be terminated due to diagnosis of chronic H.
pylori infection.
Acknowledgements
The authors would like to thank all the patients
participating in this prospective study and Professor Inkeri Elomaa
and Professor Heikki Joensuu for their important contribution to
the design of this study. The study was funded in part by the
Cancer Society of Finland, the Finnish Medical Association (Finska
Läkaresällskapet) and Valio Ltd. Research Centre that provided
Lactobacillus rhamnosus GG free of charge and support for
sampling.
References
|
1
|
Turunen MJ and Peltokallio P: Delay in the
diagnosis of colorectal cancer. Ann Chir Gynaecol. 71:277–282.
1982.PubMed/NCBI
|
|
2
|
Keighley MR, O’Morain C, Giacosa A, Ashorn
M, Burroughs A, Crespi M, Delvaux M, Faivre J, Hagenmuller F, Lamy
V, et al; United European Gastroenterology Federation Public
Affairs Committee. Public awareness of risk factors and screening
for colorectal cancer in Europe. Eur J Cancer Prev. 13:257–262.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
John SK, George S, Primrose JN and Fozard
JB: Symptoms and signs in patients with colorectal cancer.
Colorectal Dis. 13:17–25. 2011. View Article : Google Scholar
|
|
4
|
McColl KE: Clinical practice.
Helicobacter pylori infection. N Engl J Med. 362:1597–1604.
2010.PubMed/NCBI
|
|
5
|
Malfertheiner P, Megraud F, O’Morain CA,
Atherton J, Axon AT, Bazzoli F, Gensini GF, Gisbert JP, Graham DY,
Rokkas T, et al; European Helicobacter Study Group. Management of
Helicobacter pylori infection - the Maastricht IV/Florence
Consensus Report. Gut. 61:646–664. 2012.
|
|
6
|
Rehnberg-Laiho L, Rautelin H, Koskela P,
Sarna S, Pukkala E, Aromaa A, Knekt P and Kosunen TU: Decreasing
prevalence of helicobacter antibodies in Finland, with reference to
the decreasing incidence of gastric cancer. Epidemiol Infect.
126:37–42. 2001.
|
|
7
|
Malaty HM: Epidemiology of Helicobacter
pylori infection. Best Pract Res Clin Gastroenterol.
21:205–214. 2007.
|
|
8
|
Kosunen TU, Aromaa A, Knekt P, Salomaa A,
Rautelin H, Lohi P and Heinonen OP: Helicobacter antibodies in 1973
and 1994 in the adult population of Vammala, Finland. Epidemiol
Infect. 119:29–34. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rautelin H and Kosunen TU: Helicobacter
pylori infection in Finland. Ann Med. 36:82–88. 2004.
View Article : Google Scholar
|
|
10
|
No authors listed. Schistosomes, liver
flukes and Helicobacter pylori. In: IARC Working Group on
the Evaluation of Carcinogenic Risks to Humans; Lyon. 7–14 June
1994; IARC Monogr Eval Carcinog Risks Hum. 61. pp. 1–241. 1994
|
|
11
|
Collins D, Hogan AM and Winter DC:
Microbial and viral pathogens in colorectal cancer. Lancet Oncol.
12:504–512. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Buso AG, Rocha HL, Diogo DM, Diogo PM and
Diogo-Filho A: Seroprevalence of Helicobacter pylori in
patients with colon adenomas in a Brazilian university hospital.
Arq Gastroenterol. 46:97–101. 2009.
|
|
13
|
Burnett-Hartman AN, Newcomb PA and Potter
JD: Infectious agents and colorectal cancer: a review of
Helicobacter pylori, Streptococcus bovis, JC virus,
and human papillomavirus. Cancer Epidemiol Biomarkers Prev.
17:2970–2979. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Machida-Montani A, Sasazuki S, Inoue M,
Natsukawa S, Shaura K, Koizumi Y, Kasuga Y, Hanaoka T and Tsugane
S: Atrophic gastritis, Helicobacter pylori, and colorectal
cancer risk: a case-control study. Helicobacter. 12:328–332.
2007.
|
|
15
|
Inoue I, Mukoubayashi C, Yoshimura N, Niwa
T, Deguchi H, Watanabe M, Enomoto S, Maekita T, Ueda K, Iguchi M,
et al: Elevated risk of colorectal adenoma with Helicobacter
pylori-related chronic gastritis: a population-based
case-control study. Int J Cancer. 129:2704–2711. 2011.PubMed/NCBI
|
|
16
|
Osterlund P, Ruotsalainen T, Korpela R,
Saxelin M, Ollus A, Valta P, Kouri M, Elomaa I and Joensuu H:
Lactobacillus supplementation for diarrhoea related to chemotherapy
of colorectal cancer: a randomised study. Br J Cancer.
97:1028–1034. 2007. View Article : Google Scholar
|
|
17
|
No authors listed. Preoperative short-term
radiation therapy in operable rectal carcinoma. A prospective
randomized trial. Stockholm Rectal Cancer Study Group. Cancer.
66:49–55. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Oksanen A, Veijola L, Sipponen P, Schauman
KO and Rautelin H: Evaluation of Pyloriset Screen, a rapid
whole-blood diagnostic test for Helicobacter pylori
infection. J Clin Microbiol. 36:955–957. 1998.PubMed/NCBI
|
|
19
|
Houghton J and Wang TC: Helicobacter
pylori and gastric cancer: a new paradigm for
inflammation-associated epithelial cancers. Gastroenterology.
128:1567–1578. 2005. View Article : Google Scholar
|
|
20
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar
|
|
21
|
Lan L, Yu J, Chen YL, Zhong YL, Zhang H,
Jia CH, Yuan Y and Liu BW: Symptom-based tendencies of
Helicobacter pylori eradication in patients with functional
dyspepsia. World J Gastroenterol. 17:3242–3247. 2011.PubMed/NCBI
|
|
22
|
O’Reilly D and Long RG: Carcinoma of the
colon presenting with dyspepsia. Postgrad Med J. 63:215–216.
1987.
|
|
23
|
Kosunen TU, Pukkala E, Sarna S and
Rautelin H: Eradication therapy and subsequent gastric and other
common malignancies. Helicobacter. 15:327(Abstract). 2010.
|
|
24
|
Lomer MC, Parkes GC and Sanderson JD:
Review article: lactose intolerance in clinical practice - myths
and realities. Aliment Pharmacol Ther. 27:93–103. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kang SY, Han JH, Ahn MS, Lee HW, Jeong SH,
Park JS, Cho YK, Han SU, Kim YB, Kim JH, et al: Helicobacter
pylori infection as an independent prognostic factor for
locally advanced gastric cancer patients treated with adjuvant
chemotherapy after curative resection. Int J Cancer. 130:948–958.
2012. View Article : Google Scholar
|
|
26
|
Marrelli D, Pedrazzani C, Berardi A, Corso
G, Neri A, Garosi L, Vindigni C, Santucci A, Figura N and Roviello
F: Negative Helicobacter pylori status is associated with
poor prognosis in patients with gastric cancer. Cancer.
115:2071–2080. 2009.PubMed/NCBI
|