Introduction
Glioblastoma multiforme (GBM) is the most common
primary brain tumor in adults and is associated with the highest
rate of mortality (1). Numerous
biological processes occur in tumorigenesis. CpG island
hypermethylation silences tumor suppressor genes, whereas
hypomethylation promotes the transcriptional activation of
oncogenes and induces chromosomal instability (2,3).
Several aberrantly methylated genes have been reported in gliomas.
For instance, the p53/Mdm2/p14ARF cell cycle control pathway genes
are affected by CpG island promoter hypermethylation (4).
Gene methylation profiling is able to simultaneously
evaluate thousands of individual gene methylation levels and reveal
molecular signatures that reflect potential pathogenic mechanisms
and are associated with survival. In the present study, we
performed genome-wide gene methylation level analysis and
identified SPRR3 as a candidate gene, which showed aberrant levels
between GBM patients and healthy individuals.
The small proline-rich proteins (SPRRs) are encoded
by a multigene family clustered within the epidermal
differentiation complex on human chromosome 1q21 (5–7). SPRR
proteins are known to be markers for terminal squamous cell
differentiation; however, they also function in nonsquamous tissues
(8). SPRR3 (also named esophagin)
is abundantly expressed in oral and esophageal epithelia (9,10).
SPRR3 has been considered as a differentiation marker of squamous
epithelium, since its expression is strictly correlated with
keratinocyte terminal differentiation (5,11).
SPRR3 is frequently downregulated in esophageal squamous cell
carcinoma (ESCC) and it has been demonstrated to suppress the
tumorigenicity of ESCC cells (4,7,12).
However, it has previously been reported that SPRR3 is upregulated
in colorectal and breast cancer (13,14),
suggesting that SPRR3 is associated with malignant
tumorigenesis.
In the present study, we found that SPRR3
hypomethylation was associated with the clinical outcome in GBM
patients. U251 cells demonstrated a significant decrease in
proliferation and invasion following specific knockdown of SPRR3.
Immunohistochemical staining demonstrated that SPRR3 was highly
expressed in tumors compared with normal tissue.
Materials and methods
Patients, tissue samples and cell
lines
All glioma samples included in the present study
were obtained from the Chinese Glioma Genome Atlas (http://www.cgga.org.cn). The patients underwent
surgical resection between January 2006 and December 2010, and
subsequently received radiation therapy or concomitant and adjuvant
temozolomide chemotherapy. Tumor tissue samples were obtained by
surgical resection provided that the diagnosis of glioma was
established according to the 2007 WHO classification, and eight
normal brain tissues were included. The present study was approved
by the institutional review boards of Beijing Tiantan Hospital
(Beijing, China) and written informed consent was obtained from all
patients. U251 glioma cells were purchased from the Chinese Academy
of Sciences Cell Bank (Kunming, Yunnan, China). U251 cells were
cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented
with 10% fetal bovine serum (FBS). All cells were maintained in a
37°C, 5% CO2 incubator and routinely passaged at 2–3 day
intervals.
Genomic DNA extraction and DNA
methylation profiling
All the tissue samples were immediately snap-frozen
in liquid nitrogen following surgery. Genomic DNA from frozen tumor
tissues was extracted using the QIAamp DNA Mini kit (Qiagen,
Hilden, Germany) according to the manufacturer’s instructions. DNA
concentrations were measured using a NanoDrop ND-1000
spectrophotometer (NanoDrop Technologies, Houston, TX, USA).
Illumina Infinium HumanMethylation27 BeadChips (Illumina Inc., San
Diego, CA, USA) were used as previously described (15). Methylation of 27,578 CpG sites at
14,475 consensus coding sequencing sites was performed following
the manufacturer’s instructions at the Wellcome Trust Centre for
the Human Genetics Genomics Lab (Oxford, UK). The array results
were analyzed with the BeadStudio software (Illumina Inc.).
SPRR3 gene knockdown by siRNA
Logarithmically growing cells were seeded at a
density of 105 cells per 6-cm dish. Oligonucleotide
transfection was performed using the siRNA and siRNA negative
control (NC; Table I) that were
chemically synthesized by the Shanghai GenePharma Company
(Shanghai, China). Cells were transfected using Lipofectamine 2000
reagent (Invitrogen Life Technologies, Carlsbad, CA, USA).
Transfection complexes were prepared according to the
manufacturer’s instructions and added directly to the glioma cells,
resulting in a final oligonucleotide concentration of 10 nmol/l.
The transfection medium was replaced 6 h post-transfection. Cells
were used for in vitro functional assay.
 | Table IsiRNA and negative control strand
sequences. |
Table I
siRNA and negative control strand
sequences.
| Sense | Antisense |
|---|
| siRNA |
GCCAUAGUCUCUCUCUUAUTT |
AUAAGAGAGAGACUAUGGCTT |
| Negative control |
UUCUCCGAACGUGUCACGUTT |
ACGUGACACGUUCGGAGAATT |
Western blot analysis
Following cell treatment, in order to determine the
levels of SPRR3 expression, total protein was isolated in lysis
buffer. Equal amounts of protein (15 μg) were loaded into the
sample wells and separated on a 10% SDS-polyacrylamide gel, and
transferred onto polyvinylidene difluoride membranes. Immunoblot
analysis was performed with the mouse antibodies against SPRR3
(ab58233; Abcam, Hong Kong, China; 1:1,000 dilution). β-actin
(anti-β-actin antibody was obtained from Proteintech Group,
Chicago, IL, USA; 1:4,000 dilution) was reblotted to check for
equal loading of the gel.
MTT and colony formation assay
The MTT assay was used to determine relative cell
growth. U251 cells were plated at a density of 5,000 cells per well
24 h after transfection with siRNA in 96-well plates with six
replicate wells for each condition. For quantitation of cell
viability, cultures were stained after 5 days. A cell growth assay
was performed using MTT (thiazolyl blue). In brief, 20 μl of 5
mg/ml MTT solution was added to each well and incubated for 4 h at
37°C. The cell viability was determined at an absorbance of 490 nm
following solubilization in 150 μl DMSO. All data points represent
the mean of a minimum of six wells. A colony formation assay was
performed and briefly U251 cells were transfected with siRNA or the
NC for 48 h, and seeded into the individual wells of a six-orifice
plate (2,000 per orifice). Following culture for 14 days, all
orifices were washed with PBS and stained with crystal violet. The
number of colonies with >30 cells was counted. The colonies were
manually counted using a microscope (Leica DM6000 B; Upright
Microscopes, Wetzlar, Germany).
Transwell invasion assay
Cell culture chambers (24-well) with Transwell
inserts (Corning Life Sciences, Corning, NY, USA) with an 8-μm pore
membrane precoated with Matrigel (BD Biosciences, San Jose, CA,
USA) were used in the Transwell invasion assay. U251 cells were
plated at a density of 1×104 per upper well in 200 μl
culture medium (DMEM, without FBS) and the lower chamber was filled
with 500 μl of medium (DMEM, 10% FBS) in the set control group,
siRNA control group and siRNA group. The cells were incubated at
37°C and allowed to invade for 24 h, following which, the upper
surface of the membrane of the non-invading cells was removed by
scrubbing with a cotton-tipped swab. Cells on the lower surface of
the filter were fixed for 20 min in absolute ethyl alcohol and
stained with crystal violet after being air-dried briefly. The mean
number of invaded cells was counted from five preselected
microscopic fields at magnification ×200 and all experiments were
performed in triplicate.
Immunohistochemistry
Surgical specimens were fixed in formalin, routinely
processed and paraffin embedded, then cut into 4-μm sections,
deparaffinized with xylene and rehydrated. Sections were submerged
in EDTA (pH 8.0), autoclaved for antigen retrieval and then treated
with 3% hydrogen peroxide, followed by incubation with 1% FBS.
Anti-SPRR3 antibody (Abcam; mouse monoclonal; 1:200 dilution) was
added as a primary antibody and incubated at 4°C for 2 h. Normal
mouse serum was used for the NC and horseradish peroxidase-labeled
secondary antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA) was applied and incubated for 45 min at 37°C, followed by 5
min incubation at room temperature with DAB for color development.
Finally, the sections were counterstained with hematoxylin and
mounted with Permount (BIOS, Beijing, China). The
immunohistochemical staining results were visualized and images
were captured under a bright-field microscope (Olympus BX-51;
Olympus Optical Co., Ltd., Tokyo, Japan). The SPRR3 cytoplasmic
expression was classified into two categories determined by
combining the proportion of positively stained tumor cells and the
intensity of staining. The intensity of staining was scored by two
investigators without knowledge of clinical information on a scale
of 0 to 3 (0, negative; 1, slightly positive; 2, moderately
positive; 3, intensely positive). A score of 0 and 1 or 2 and 3
indicated low or high expression, respectively.
Statistical and bioinformatics
analysis
Kaplan-Meier survival analysis was used to estimate
the survival distributions and the overall survival (OS) time was
calculated from the date of diagnosis until mortality or the last
follow-up contact. Significant differences among the groups were
determined using Student’s t-test. All analyses were two-tailed.
P<0.05 was considered to indicate a statistically significant
difference. Analyses were performed using Matlab 2009b (MathWorks,
Natick, MA, USA), GraphPad Prism (GraphPad Software Inc., La Jolla,
CA, USA) and SPSS version 13.0 (SPSS, Inc., Chicago, IL, USA).
Results
DNA methylation profile analysis
To investigate the molecular changes associated with
GBM tumorigenesis, we analyzed the gene methylation level in 42
patients with GBM and eight healthy individuals using a DNA
methylation profile (Table II).
The methylation levels of 14,475 genes were analyzed using t-tests.
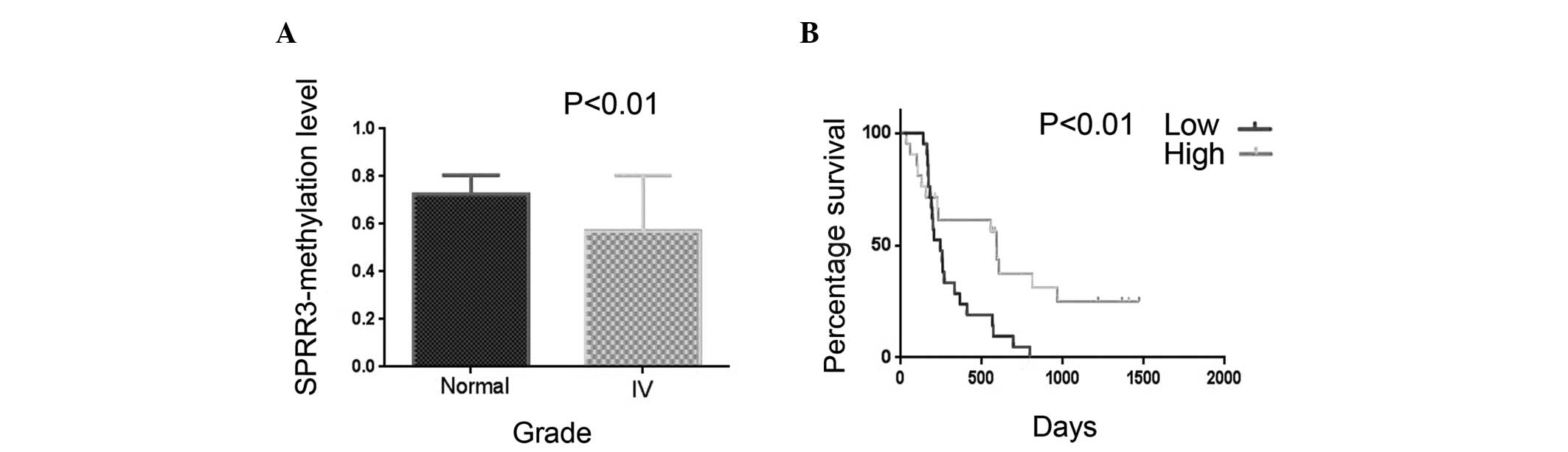
Among these genes, SPRR3 was demonstrated to be associated with
molecular changes and its methylation level was significantly lower
in GBM patients compared with healthy individuals (Fig. 1). The OS time was measured through
Kaplan-Meier survival curve analysis and the results revealed that
glioma patients with a low methylation level of SPRR3 had
significantly lower progression-free survival rates (P=0.010) and
OS times (P=0.007) than patients with a high methylation level.
Kaplan-Meier survival curves, according to the methylation level of
SPRR3 in 42 frozen glioma tissues, demonstrated that SPRR3
hypomethylation was associated with a poor clinical outcome in GBM
patients (Fig. 1). These results
indicated that the methylation level of SPRR3 is an independent
prognostic marker in glioma patients.
 | Table IIVariables associated with the
methylation level of SPRR3 in 42 glioma samples. |
Table II
Variables associated with the
methylation level of SPRR3 in 42 glioma samples.
| | | SPRR3 methylation
level | |
|---|
| | |
| |
|---|
| Variable | No. of patients | Median OS (days) | Low | High | P-value |
|---|
| Gender | | | | | 0.617 |
| Male | 26 | 261 | 4 | 22 | |
| Female | 16 | 241 | 3 | 13 | |
| Age (years) | | | | | 0.545 |
| ≤50 | 28 | 348 | 7 | 21 | |
| >50 | 14 | 211 | 0 | 14 | |
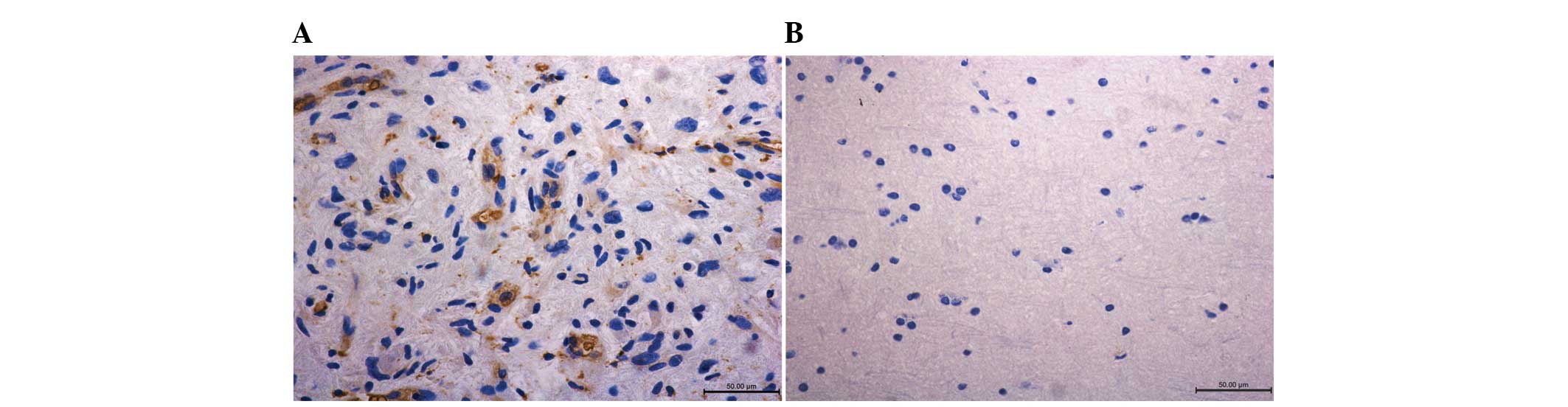
SPRR3 is upregulated in glioblastoma
Samples of the tumor immunohistochemical staining
demonstrated that SPRR3 was highly expressed in tumors compared
with the normal tissue (Fig. 2).
The quantification of immunohistochemical signals for 33 GBM
samples revealed that 24 of the samples demonstrated positive
staining for SPRR3 (72.7%) while only 11.1% of the normal tissue
stained positively for SPRR3 (one in nine samples). No significant
differences in patient age and gender were identified (Table III). We also measured the OS time
according to SPRR3 expression levels in glioma patients through
Kaplan-Meier survival estimates; however, the results revealed that
there was no significant correlation between the two subgroups with
high or low SPRR3 expression (high expression, ≥2; low expression,
<2).
 | Table IIIVariables associated with the
expression of SPRR3 in 34 glioma samples. |
Table III
Variables associated with the
expression of SPRR3 in 34 glioma samples.
| | | SPRR3 expression
level | |
|---|
| | |
| |
|---|
| Variable | No. of patients | Median OS (days) | Low | High | P-value |
|---|
| Gender | | | | | 0.935 |
| Male | 23 | 447 | 5 | 18 | |
| Female | 11 | 315 | 2 | 9 | |
| Age (years) | | | | | 0.798 |
| ≤50 | 14 | 532.5 | 2 | 12 | |
| >50 | 20 | 315 | 5 | 15 | |
Identification of SPRR3-associated
proliferation and invasion signature in GBM
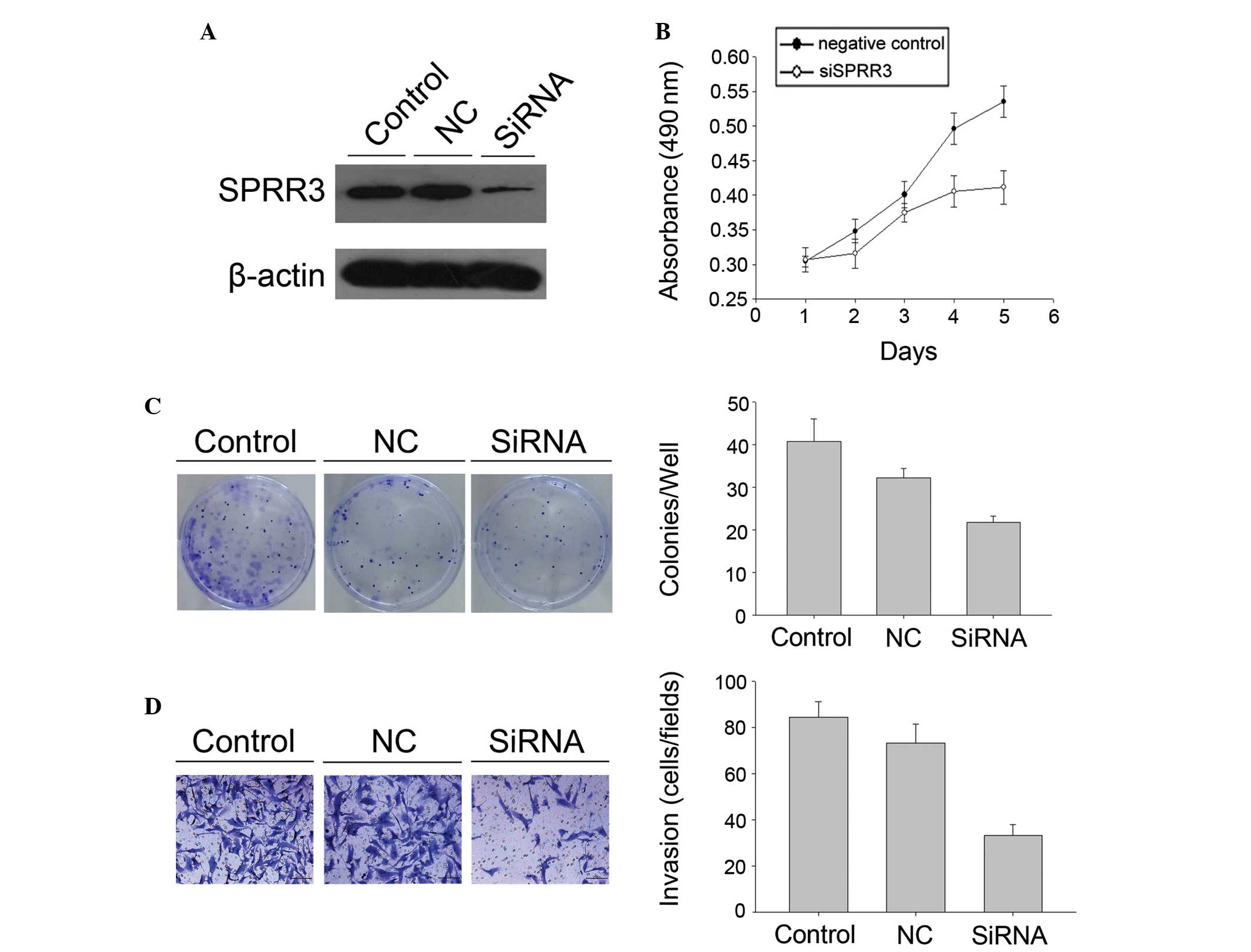
As the results of the immunohistochemical staining
demonstrated that SPRR3 was highly expressed in GBM samples, we
investigated the effect of SPRR3 on the proliferation and invasion
of GBM cells. SPRR3 siRNA was used to specifically knock down SPRR3
expression in U251 cell lines (Fig.
3). The MTT assay demonstrated a significant decrease in
proliferation in siRNA U251 cell lines compared with the cells
transfected with the control (Fig.
3). The same result was found in the colony formation assay and
the siRNA U251 cell lines demonstrated a decrease in focus numbers
compared with the control (P<0.05; Fig. 3). Cell invasion was measured using
the Transwell invasion assay and the results demonstrated that U251
cell lines significantly attenuated invasiveness following SPRR3
knockdown, as indicated by a marked decrease in the number of
invaded cells (P<0.05; Fig.
3).
Discussion
SPRR3 promoted GBM cell (U251) proliferation and
invasion. The results of the immunohistochemical staining
demonstrated that SPRR3 was upregulated in the tissue samples of
more than half of the GBM patients (72.7%) and the methylation
level of SPRR3 was correlated with the clinical outcome in glioma
patients validated by DNA methylation profile analysis. The present
study, to the best of our knowledge, is the first to examine the
functions and methylation level of SPRR3 in GBM.
GBM is the most common and aggressive primary brain
tumor in adults. Its prognosis remains extremely poor, despite
multimodal treatment by surgery, radiotherapy and chemotherapy
(16). Aberrations in DNA
methylation patterns may have critical effects on tumor initiation
and progression (17). DNA
hypermethylation of specific genes and DNA hypomethylation commonly
affecting repetitive DNA are observed in brain cancers (18–24).
In order to investigate the potential molecular
mechanisms underlying GBM tumorigenesis, we analyzed gene
methylation levels in 227 patients with glioma (141 low-grade
gliomas, 44 anaplastic gliomas and 42 GBMs) and eight healthy
individuals by using a DNA methylation profile. We found that the
methylation levels of several genes were significantly altered
(SPRR3, TES, RGN, FAM12B, AJAP1, PDE4C and SPRR2D). Among these
genes, we found that SPRR3 hypomethylation was a common event in
the majority of cases, regardless of the sex and age of the
patients, and SPRR3 hypomethylation was also demonstrated to be
associated with an adverse prognosis. In the present study, we
found that SPRR3 was hypomethylated in GBM. Previously, Ammerpohl
et al(25) demonstrated the
same result in cirrhotic liver and hepatocellular carcinoma. Mayol
et al also found that SPRR3 hypomethylation affected
cancer-related biological functions and genes relevant to
neuroblastoma pathogenesis (26).
SPRR3 is frequently downregulated in ESCC, where it
is known to inhibit tumorigenesis. Notably, in contrast to the
findings in ESCC, in the present study, SPRR3 promoted GBM cell
(U251) proliferation and invasion, and samples from the tumor
immunohistochemical staining demonstrated that SPRR3 was highly
expressed in the majority of tumors compared with the normal
tissue. Previously, SPRR3 was demonstrated to be upregulated in
colorectal and breast cancer, and these results were in accordance
with our findings. Our study suggests that upregulation of SPRR3
occurs in GBM tumorigenesis.
Considering the vital role of cellular proliferation
and invasion in GBM pathogenesis, we investigated the effect of the
overexpression of SPRR3 on GBM cell lines. SPRR3 siRNA was used to
specifically knock down SPRR3 expression. The MTT assay
demonstrated a significant decrease in proliferation and was
consistent with the colony formation assay results in the U251
glioma cell line following transfection (Fig. 3). Cellular proliferation is one of
the most important biological processes in tumorigenesis due to its
role in growth and in the maintenance of tissue homeostasis
(27,28). Each type of human cancer often has
specific signaling pathways to rely on for cellular proliferation
(29). In colorectal cancer, in
which SPRR3 was upregulated and accelerated cell proliferation, it
was proposed that the effect of SPRR3 in promoting colorectal
tumorigenesis is associated with the degradation of p53 caused by
AKT activation (13). The same
results were also found in breast cancer. SPRR3 has been
demonstrated to promote breast cancer cell proliferation by
enhancing p53 degradation via the AKT and MAPK pathways (14). We used a similar experiment and
demonstrated the same results in the U251 glioma cell line. AKT
signaling has been implicated in angiogenesis, the promotion of
tumor invasion as well as in tumor cell survival (30,31).
In particular, a higher proliferation index of the tumor cells has
been associated with shorter survival rates (32,33).
As AKT signaling promotes tumor invasion, we used a Transwell
invasion assay to measure cell invasiveness and the results
demonstrated that the invasiveness of U251 cell lines significantly
decreased following the knock down of SPRR3 expression.
The present study demonstrated that SPRR3 is
hypomethylated and upregulated in glioblastoma, and the integrated
microarray analysis revealed that SPRR3 hypomethylation was
associated with an adverse prognosis. We also demonstrated that the
overexpression of SPRR3 may be associated with cell proliferation
and invasion in GBM, and that it may be a candidate biomarker for
GBM.
Acknowledgements
We would like to thank Professor Zhang (Capital
Medical University, Beijing, China) for the technical support and
Beijing Tiantan Hospital (Beijing, China). This study was supported
by grants from the National High Technology Research and
Development Program (no. 2012AA02A508) and the International
Science and Technology Cooperation Program (no. 2012DFA30470).
References
|
1
|
Parsons DW, Jones S, Zhang X, et al: An
integrated genomic analysis of human glioblastoma multiforme.
Science. 321:1807–1812. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Herman JG and Baylin SB: Gene silencing in
cancer in association with promoter hypermethylation. N Engl J Med.
349:2042–2054. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Karpf AR and Matsui S: Genetic disruption
of cytosine DNA methyltransferase enzymes induces chromosomal
instability in human cancer cells. Cancer Res. 65:8635–8639. 2005.
View Article : Google Scholar
|
|
4
|
Bello MJ and Rey JA: The p53/Mdm2/p14ARF
cell cycle control pathway genes may be inactivated by genetic and
epigenetic mechanisms in gliomas. Cancer Genet Cytogenet.
164:172–173. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gibbs S, Fijneman R, Wiegant J, van Kessel
AG, van De Putte P and Backendorf C: Molecular characterization and
evolution of the SPRR family of keratinocyte differentiation
markers encoding small proline-rich proteins. Genomics. 16:630–637.
1993. View Article : Google Scholar
|
|
6
|
Steinert PM, Candi E, Kartasova T and
Marekov L: Small proline-rich proteins are cross-bridging proteins
in the cornified cell envelopes of stratified squamous epithelia. J
Struct Biol. 122:76–85. 1998. View Article : Google Scholar
|
|
7
|
Zhang Y, Feng YB, Shen XM, et al:
Exogenous expression of Esophagin/SPRR3 attenuates the
tumorigenicity of esophageal squamous cell carcinoma cells via
promoting apoptosis. Int J Cancer. 122:260–266. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tesfaigzi J, Th’ng J, Hotchkiss JA,
Harkema JR and Wright PS: A small proline-rich protein, SPRR1, is
upregulated early during tobacco smoke-induced squamous metaplasia
in rat nasal epithelia. Am J Respir Cell Mol Biol. 14:478–486.
1996. View Article : Google Scholar
|
|
9
|
Fischer DF, Sark MW, Lehtola MM, Gibbs S,
van de Putte P and Backendorf C: Structure and evolution of the
human SPRR3 gene: implications for function and regulation.
Genomics. 55:88–99. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cabral A, Sayin A, de Winter S, Fischer
DF, Pavel S and Backendorf C: SPRR4, a novel cornified envelope
precursor: UV-dependent epidermal expression and selective
incorporation into fragile envelopes. J Cell Sci. 114:3837–3843.
2001.
|
|
11
|
Abraham JM, Wang S, Suzuki H, et al:
Esophagin cDNA cloning and characterization: a tissue-specific
member of the small proline-rich protein family that is not
expressed in esophageal tumors. Cell Growth Differ. 7:855–860.
1996.
|
|
12
|
Chen BS, Wang MR, Cai Y, et al: Decreased
expression of SPRR3 in Chinese human oesophageal cancer.
Carcinogenesis. 21:2147–2150. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cho DH, Jo YK, Roh SA, et al: Upregulation
of SPRR3 promotes colorectal tumorigenesis. Mol Med. 16:271–277.
2010.PubMed/NCBI
|
|
14
|
Kim JC, Yu JH, Cho YK, et al: Expression
of SPRR3 is associated with tumor cell proliferation in less
advanced stages of breast cancer. Breast Cancer Res Treat.
133:909–916. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hill VK, Ricketts C, Bieche I, et al:
Genome-wide DNA methylation profiling of CpG islands in breast
cancer identifies novel genes associated with tumorigenicity.
Cancer Res. 71:2988–2999. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wen PY and Kesari S: Malignant gliomas in
adults. N Engl J Med. 359:492–507. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nagarajan RP and Costello JF: Epigenetic
mechanisms in glioblastoma multiforme. Semin Cancer Biol.
19:188–197. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fanelli M, Caprodossi S, Ricci-Vitiani L,
et al: Loss of pericentromeric DNA methylation pattern in human
glioblastoma is associated with altered DNA methyltransferases
expression and involves the stem cell compartment. Oncogene.
27:358–365. 2008. View Article : Google Scholar
|
|
19
|
Kim TY, Zhong S, Fields CR, Kim JH and
Robertson KD: Epigenomic profiling reveals novel and frequent
targets of aberrant DNA methylation-mediated silencing in malignant
glioma. Cancer Res. 66:7490–7501. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Foltz G, Ryu GY, Yoon JG, et al:
Genome-wide analysis of epigenetic silencing identifies BEX1 and
BEX2 as candidate tumor suppressor genes in malignant glioma.
Cancer Res. 66:6665–6674. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cadieux B, Ching TT, VandenBerg SR and
Costello JF: Genome-wide hypomethylation in human glioblastomas
associated with specific copy number alteration,
methylenetetrahydrofolate reductase allele status, and increased
proliferation. Cancer Res. 66:8469–8476. 2006. View Article : Google Scholar
|
|
22
|
Yu J, Zhang H, Gu J, et al: Methylation
profiles of thirty four promoter-CpG islands and concordant
methylation behaviours of sixteen genes that may contribute to
carcinogenesis of astrocytoma. BMC Cancer. 14:652004. View Article : Google Scholar
|
|
23
|
Uhlmann K, Rohde K, Zeller C, et al:
Distinct methylation profiles of glioma subtypes. Int J Cancer.
106:52–59. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Costello JF, Frühwald MC, Smiraglia DJ, et
al: Aberrant CpG-island methylation has non-random and
tumour-type-specific patterns. Nat Genet. 24:132–138. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ammerpohl O, Pratschke J, Schafmayer C, et
al: Distinct DNA methylation patterns in cirrhotic liver and
hepatocellular carcinoma. Int J Cancer. 130:1319–1328. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mayol G, Martín-Subero JI, Ríos J, et al:
DNA hypomethylation affects cancer-related biological functions and
genes relevant in neuroblastoma pathogenesis. PLoS One.
7:e484012012. View Article : Google Scholar
|
|
27
|
Fritz V and Fajas L: Metabolism and
proliferation share common regulatory pathways in cancer cells.
Oncogene. 29:4369–4377. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Davis CD, Emenaker NJ and Milner JA:
Cellular proliferation, apoptosis and angiogenesis: molecular
targets for nutritional preemption of cancer. Semin Oncol.
37:243–257. 2010. View Article : Google Scholar
|
|
29
|
Weinstein IB and Joe A: Oncogene
addiction. Cancer Res. 68:3077–3080. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
McCubrey JA, Steelman LS, Abrams SL, et
al: Roles of the RAF/MEK/ERK and PI3K/PTEN/AKT pathways in
malignant transformation and drug resistance. Adv Enzyme Regul.
46:249–279. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Testa JR and Bellacosa A: AKT plays a
central role in tumorigenesis. Proc Natl Acad Sci USA.
98:10983–10985. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Protzel C, Knoedel J, Zimmermann U,
Woenckhaus C, Poetsch M and Giebel J: Expression of proliferation
marker Ki67 correlates to occurrence of metastasis and prognosis,
histological subtypes and HPV DNA detection in penile carcinomas.
Histol Histopathol. 22:1197–1204. 2007.
|
|
33
|
Yamashita Y, Kasugai I, Sato M, et al:
CDC25A mRNA levels significantly correlate with Ki-67 expression in
human glioma samples. J Neurooncol. 100:43–49. 2010. View Article : Google Scholar
|