Introduction
Collecting duct carcinoma (CDC) of the kidney, also
known as Bellini duct carcinoma, is an extremely rare variant of
renal cell carcinoma (RCC), accounting for 0.4–1.8% of all RCCs
(1). In contrast to the
considerably more common variants of RCC, arising from the
convoluted tubules of the renal cortex, CDC is derived from the
renal medulla, possibly from the distal collecting ducts of Bellini
(1–3). Approximately four decades ago,
Mancilla-Jimenez et al(4)
first observed the atypical hyperplasia of the adjacent collecting
ducts epithelium in three cases of papillary RCC. Therefore, the
authors speculated that a few papillary RCCs may derive from the
epithelium of the collecting ducts. Until 1979, the term Bellini
duct carcinoma was presented by Cromie et al(5) It is worth noting that CDC has other
synonyms besides Bellini duct carcinoma, including medullary renal
carcinoma, distal nephron carcinoma and distal renal tubular
carcinoma. In 1997, in accordance with the morphological aspect and
chromosome of the primary renal cancer, five histologic types was
defined in the Heidelberg classification (6), including the conventional,
chromophobe, papillary, collecting duct and unclassifiable
carcinoma. CDC is characterized by a tremendously aggressive
phenotype. Patients with CDC usually have metastatic diseases at
the time of presentation. Radical nephrectomy is the basis of
therapy. Several systemic treatment protocols, including
chemotherapy, radiotherapy and immunotherapy have been considered.
However, these treatments do not produce a favorable response in
the majority of CDC patients, and ~70% of patients succumb due to
CDC progression within 2 years of diagnosis.
In general, CDC is considered to have a poor
prognosis and early diagnosis is likely the only factor leading to
a prolonged survival for patients (7). However, due to the rarity of this
tumor and the lack of clinical awareness, no reliable diagnostic
protocol has been established. To achieve an improved understanding
of CDC and diagnosis, the present study analyzed the imaging
features of six CDC patients treated in Jinling Hospital, Clinical
school of Medical College, Nanjing University (Nanjing, China),
between June 2007 and October 2012.
Patients and methods
Patient characteristics
The current retrospective study was approved by the
institutional review board of Jinling Hospital, Clinical school of
Medical College, Nanjing University and written informed consent
forms were obtained from all patients.
In total, six patients (three males and three
females; age range, 22–70 years; mean age, 46 years) with
pathologically confirmed CDC of the kidney during the past five
years were included.
The clinical information included the age, gender
and clinical presentation of these patients. The radiological
results available for analysis included non-contrast computed
tomography (CT) in all six patients, contrast-enhanced CT in five
patients, magnetic resonance (MR) urography in one patient, renal
dynamic imaging and glomerular filtration rate (GFR) measuring in
one patient and conventional whole-body
18F-fluorodeoxyglucose (FDG) positron emission
tomography (PET)/CT in two patients.
CT analysis
Abdominal CT was performed using a Siemens Somatom
Emotion 6 or Somatom Definition (Siemens Healthcare, Erlangen,
Germany), with the following scan parameters for imaging
acquisition: 120–130 kVp, 110–340 mA, and a reconstruction
thickness of 1–8 mm. Following the non-contrast CT scan, 100–120 ml
IV contrast agent was injected into an antecubital vein at a rate
of 3.0 ml/sec in five patients. Triphasic contrast-enhanced CT was
performed, including arterial, nephrographic and excretory phases,
with 25, 60 and 180 sec, respectively. A series of characteristic
parameters describing the tumors consisted of the number of the
tumors, tumor size, solid, cystic or complex mass, CT attenuation
of the solid component, tumor location, inside features of the
tumor (calcification, pseudocapsules and cystic components), degree
and pattern of enhancement, metastatic lesions of the tumors
(direct invasion to the renal pelvis and ureter, perinephric
invading, region lymphadenopathies and distant metastases) and
pattern of tumor growth.
The CT attenuation of the solid component was
classified as high, equal or low compared with contralateral normal
kidney. The location of tumor was classified as medullary, cortical
or exophytic depending on the predominance. Medullary location was
supported by intrusion of the renal pelvis, replacement of the
renal sinus fat or distortion of the intrarenal collecting system.
Cortical location was supported by a peripheral location of the
tumor and contact with the outer renal capsule. An exophytic
location was considered to be present when the major section of the
tumor extended beyond the predicted renal confines. The presence of
calcification was described on the non-contrast CT scan. A cystic
component was considered to be present if a well-defined,
liquid-like attenuation area was noted in the tumor.
In five cases where the contrast-enhanced CT was
available, the degree and pattern of enhancement were determined on
the nephrographic phase. The presence of vascular invasion was
described on the contrast-enhanced CT scan and the presence of an
infiltrative or expansile pattern of growth was defined by which
pattern predominated in each case. On CT, infiltrative growth was
characterized by poorly marginated borders between the tumor and
normal renal parenchyma. On the contrary, expansible growth was
characterized by well-defined bulging tumor margins that displaced
the normal parenchyma.
Lymphadenopathy was defined when a lymph node was
enlarged by >1 cm in diameter. Perinephric invading was defined
when there was evidence of nodules with soft-tissue attenuation in
the perinephric area and thickening of Gerota’s fascia. In
addition, chest CT and cranial MR were performed in each patient to
detect extra-abdominal metastatic lesions.
MR urography analysis
MR urography was performed by a 3-Tesla scanner
(Siemens Healthcare) using a torso phased array coil. Breath-hold,
coronal thin slice and thick-slab T2-weighted single-shot fast
spin-echo were obtained. Technical parameters for thin section
T2-weighted single-shot fast spin-echo sequences were as follows:
Repetition time (2,400 msec)/echo time (710 msec); 384×384 matrix;
1.5-mm section thickness; and 48-cm field of view. Technical
parameters for the thick-slab T2-weighted sequences were as
follows: 256×256 matrix; 5-mm thickness; and 40-cm field of view.
The tumors that presented in the renal collecting system and ureter
were evaluated.
Single photon emission computed
tomography (SPECT) analysis
SPECT (Siemens E.Cam; Siemens Healthcare) was used
to perform renal dynamic imaging and the measurement of GFR. In
total, 185 MBq 99Tcm-DTPA was used for the
patient. The radioactivity (counts) of the pre-injection syringe
containing 99Tcm-DTPA was determined at a
distance of 20 cm from the detector for 60 sec. The patient
consumed 300 ml of water prior to imaging and was then kept supine
with the back facing the detector. The renal images were captured
dynamically following a ‘bolus’ injection of 1 ml
99Tcm-DTPA (185 MBq). The acquisition
conditions were as follows: Low-energy collimator; energy peak, 140
KeV; window width, 20%; and matrix, 128×128. In total, 20 frames of
slow dynamic acquisition at a rate of one frame per 60 sec were
collected following 30 frames of rapid dynamic acquisition at a
rate of one frame per 2 sec. Once the images were captured, the
radioactivity (counts) of the post-injection empty syringe was
determined at a distance of 20 cm from the detector for 60 sec. GFR
normalized to body surface area was calculated automatically from
the renal dynamic images. The observations of renal dynamic imaging
and GFR measuring were analyzed.
PET/CT analysis
Conventional whole-body PET/CT was performed using a
Siemens Biograph Sensation 16 (Siemens Healthcare). The patients
were fasted for ≥6 h to maintain the blood glucose level at 3.9–6.1
mmol/l. A mean dose of 5.55 MBq/kg (0.15 mci/kg) of
18F-FDG was administrated intravenously to each patient.
Imaging was initiated following an 18F-FDG uptake period
of 60 min. Each patient underwent a total body scan that contained
two steps of body and brain scanning. The non-contrast CT scan was
performed immediately prior to the PET scan with a 16-slice
multidetector spiral CT scanner. The CT results on the combined
scanner were used for PET attenuation correction. CT, PET and
PET/CT fused images were reconstructed in coronal, sagittal and
transaxial projections on a computer screen with ordered subset
expectation maximization iterative algorithm. All PET/CT images
were interpreted using visualization and semi-quantitative analysis
[maximum standardized uptake value (SUVmax)]. The SUVmax of each
lesion, which was found by CT scanning or showed a high
18F-FDG uptake (SUVmax, >2.5), was measured and
analyzed carefully.
Surgical analysis
In total, five patients underwent nephrectomy and
one patient underwent nephroureterectomy. The gross and microscopic
features of the tumors were described by two pathologists. In
addition, one patient underwent pleural biopsy and was diagnosed
with multiple pleural metastases of CDC.
The time intervals between each examination and the
surgery were <14 days. All images were retrospectively reviewed
by three experienced radiologists, to reach a consensus in each
patient.
Results
Clinical observations
The predominate manifestations that brought the
patients to clinical attention included flank pain (n=4), fever
(n=3), weight loss (n=3), gross hematuria (n=2), palpable mass
(n=2) and chest pain (n=1).
CT observations
In total, seven tumors were found in the six cases,
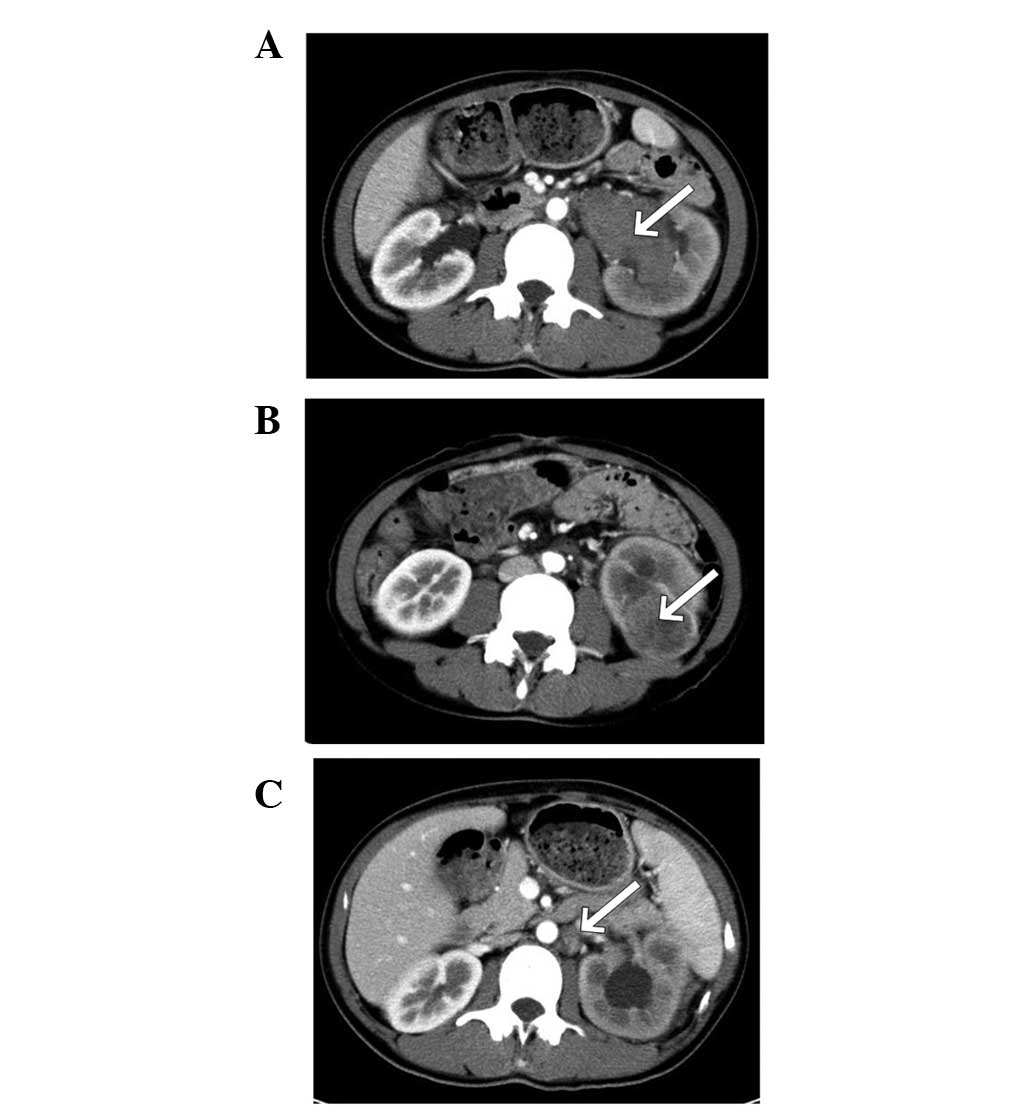
with two tumors detected in the left kidney of patient 2 (Fig. 1A and B). The longest diameter of the
tumors ranged between 4.0 and 7.5 cm, and the mean size was 5.3 cm.
In one case, the boundary of the tumor was not defined by CT
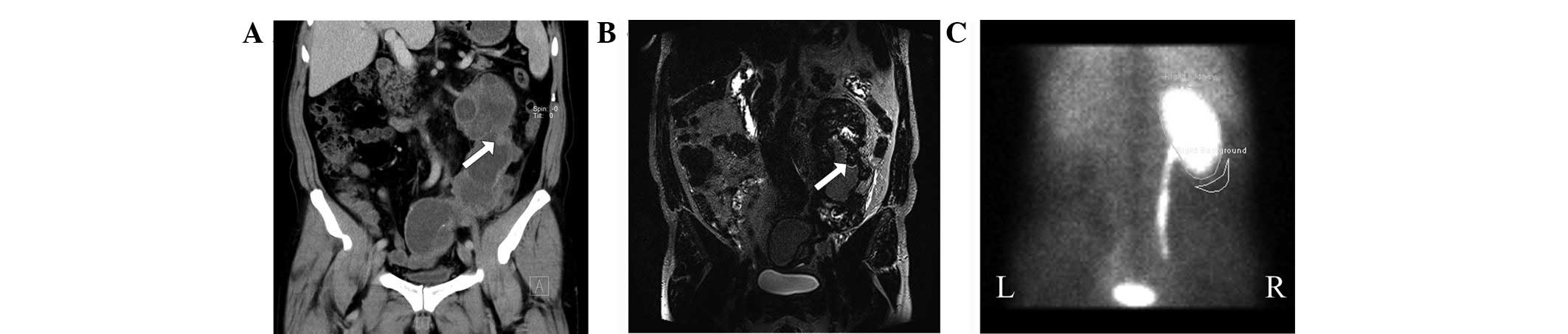
scanning (patient 4; Fig. 2A);
therefore, size was determined on the gross specimen. The tumors
appeared solid (2/7) or complex (5/7) on CT. On non-contrast CT
scanning, high, equal and low attenuation was observed in two, four
and one tumors, respectively. In total, six tumors were located in
medullary areas and only 1 tumor was found in the cortical
location. A tiny calcification was present in only one tumor and
cystic components were observed in five tumors, but no
pseudocapsule was observed. Weak enhancements were observed in all
six tumors examined with contrast-enhanced CT, and heterogeneous
enhancements were also observed in the majority of these tumors
with the exception of one tumor. An infiltrative pattern of tumor
growth was present in five tumors, with an expansible appearance in
the remaining two tumors.
Metastatic lesions were found in all six patients.
Regional lymphadenopathies were observed in five cases, located in
renal hilum and retroperitoneal areas. No evidence of lymph node
metastases was shown in one of these five cases by pathology
(patient 2; Fig. 1C), although
multiple lymph nodes were found in the renal hilum area.
Perinephric invading was observed in one case and direct invasion
of the renal pelvis and ureter were observed in two cases.
Distention of the renal pelvis and almost total ureter, multiple
nodular thickening on the wall of the ureter, extensive destruction
of the calyceal structure and hydronephrosis and hydroureterosis
existed in one patient (patient 4; Fig.
2A), in which pyonephrosis and inflammatory infiltrates were
found. Renal or inferior vein tumor thrombus were not observed.
Multiple pleural metastases were detected by chest CT in one
patient (patient 6; Table I).
 | Table ICT observations of six CDCs. |
Table I
CT observations of six CDCs.
| Patient no. | Age,
years/gender | Longest diameter,
cm | CT attenuation | Location | Pattern of
enhancement | Inside features | Pattern of tumor
growth | Metastatic
lesions |
|---|
| 1 | 70/F | 6.5 | Equal | Medullary | Weak and
homogeneous | None | Infiltrative | Multiple lymph node
metastases in renal hilum area |
| 2a | 22/F | 4.0/4.0 | High/high |
Medullary/cortical | Weak and
heterogeneous/weak and heterogeneous | Cystic
component/cystic component |
Infiltrative/expansible | Direct invasion to
the renal pelvis and multiple enlarged lymph nodes in renal
hilumb |
| 3c | 53/M | 6.0 | Low | Medullary | - | None | Infiltrative | Direct invasion to
the perirenal fat and multiple lymph node metastases in renal hilum
and retroperitoneal areas |
| 4d | 50/M | 7.5 | Equal | Medullary | Weak and
heterogeneous | Cystic component | Infiltrative | Direct invasion to
the renal pelvis and ureter |
| 5 | 30/F | 5.0 | Equal | Medullary | Weak and
heterogeneous | Cystic component | Infiltrative | Lymph node metastasis
in renal hilum area |
| 6 | 46/M | 4.0 | Equal | Medullary | Weak and
heterogeneous | Cystic component and
calcification | Expansible | Lymph node
metastasis in renal hilum area and multiple pleural metastases |
MR urography observations
The MR urography was performed on only one patient
(patient 4). Similar to the CT observations, the boundary of the
tumor was not clearly defined (Fig.
2B). However, the destruction of the renal pelvis and wall of
the ureter and the extent of the hydronephrosis and hydroureterosis
were shown more distinctly.
Renal dynamic imaging and measurement of
GFR
The renal dynamic imaging was performed on only one
patient (patient 4). In these images, the left kidney was not
detected. This denoted that the renal function of the left kidney
had been lost (Fig. 2C). However,
the renal function of the right kidney increased complementally and
the normalized GFR was 121.5 ml/min.
PET/CT observations
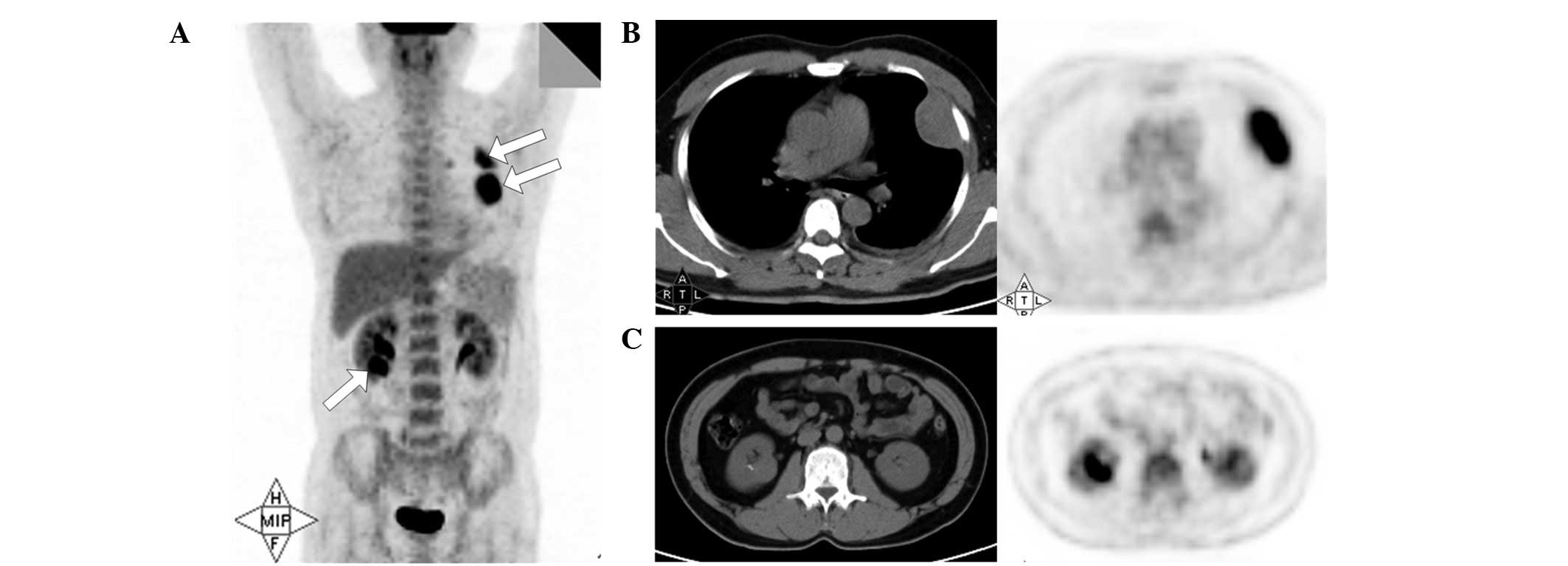
The 18F-FDG PET/CT was performed on two
patients (patients 1 and 6). The malignant lesions, including
primary tumors, regional lymphadenopathies and distant metastases,
found by PET/CT were consistent with those detected by pre- and
post-contrast CT scanning. In addition, the SUVmax was >2.5 in
each lesion (Fig. 3).
Discussion
CDC is a rare epithelial neoplasm in the kidney and
is recognized as a distinct entity in the 2004 World Health
Organization classification (8–10).
Tokuda et al(11) reported
the largest series of exclusive CDC cases in 2006, which were
collected throughout Japan across 66 institutions. Of these, the
median age was 58.2 years and males comprised of 71.6% of the
patient population. However, this demographic profile also applies
to the more common RCCs and is not an effective differential
point.
Clinical manifestations of CDC in the present study
were consistent with those of more common RCCs, including flank
pain, hematuria and palpable mass. Constitutional symptoms,
including fever and weight loss, are also common, but no particular
paraneoplastic syndrome was observed (3,12). In
addition, one of the patients showed evident chest pain, which may
have been caused by the pleural metastasis.
To date, the imaging features of CDC are not well
characterized, since only case reports or studies involving small
numbers of patients have been published (1,3,12–18).
Previously, Pickhardt et al (§1) described the radiological
observations of 17 patients with histopathologically confirmed CDC.
In the authors’ series, medullary involvement (94%) and an
infiltrative appearance (65%) were common observations of CDC, and
a cystic component (35%) and calcification were frequently
identified within the tumors. An additional study by Yoon et
al(14) has reported the
largest radiological series in the literature. In the total 18
cases, the authors found that medullary location (94%), weak (69%)
and heterogeneous (85%) enhancement, involvement of the renal sinus
(94%), infiltrative growth (67%), preserved renal contour (61%) and
a cystic component (50%) were CT observations frequently identified
in patients with CDC. At the same time, regional lymphadenopathy,
perinephric stranding, vascular invasion and distant metastases
were observed in 56, 56, 28 and 33% of the patients.
In the present study, a total of six patients,
including monofocal and multifocal cases, exhibited seven tumors.
In general, the tumors presented as solid or complex solid and
cystic on CT. Renal medullary involvement was the most common
observation of CDC identified in six tumors. In contrast to the
more common RCCs, weak and heterogeneous enhancement were the
general appearance in contrast-enhanced CT scans of the CDCs.
Calcification, cystic components and pseudocapsule were observed in
1, 5 and 0 tumors, respectively. An infiltrative pattern of tumor
growth was present in the majority of the tumors. In addition,
local, lymphatic or hematogenous spreading was noted in all CDCs,
which predicted an aggressive biological behavior and a poor
long-term prognosis. Regional lymphadenopathies were observed in
five cases, but no lymph node metastases were detected in one of
these cases. This demonstrated that lymphadenopathies are not
necessarily caused by lymph nodes metastases. Pyonephrosis and
inflammatory infiltrates were detected in one case, which may have
been caused by the secondary upper urinary tract obstruction.
MR urography is an evolving member of the urologic
imaging armamentarium. It evaluates the renal parenchyma and
surrounding structures besides the renal collecting systems,
ureters and bladder (19–23). The two most common sequences used in
MRU are a heavily T2-weighted hydrographic sequence without
contrast material and a T1-spoiled GRE sequence during the
excretory phase following gadolinium based contrast administration.
Previous studies have shown that MRU detects tumors of the upper
urinary tract with high accuracy using T2-weighted MRU only
(22,23). In the current study, the extent and
the surrounding structures of the tumor were shown more clearly by
MR urography. From these images, the doctors of urinary surgery
determined that the patient undergo nephroureterectomy rather than
nephrectomy.
The GFR, the plasma volume filtering through the
glomerulus per minute, is a significant index for the assessment of
the renal function. Currently, renal dynamic imaging is widely used
in clinical practice to calculate the GFR (24–26).
In the present study, the purpose of this examination was to
evaluate the renal function of the healthy kidney. The renal
function of the involved kidney was virtually lost, at the same
time, the renal function of the healthy kidney increased
complimentally and the normalized GFR was 121.5 ml/min. Therefore,
the renal insufficiency was not likely to occur following
nephroureterectomy.
The most commonly used radionuclide in PET is
18F-FDG, which is the analog of D-glucose. Malignant
tumors are more metabolically active than their normal surrounding
tissues and are likely to uptake more 18F-FDG. This high
concentration of the radiotracer produces a detectable signal
greater than the background, allowing the isolation of tumor
location. However, in previous studies, the detection of common
RCCs with PET scanning has been hampered by the fact that
18F-FDG is excreted via the kidneys (27–29).
Due to the rarity of the CDC, few previous studies have analyzed
the appearances of PET imaging (1,30). In
a previous study by Ye et al(30), a CDC, with the longest diameter of
4.6 cm and SUVmax of 7, located in the right kidney was reported.
Yang et al(1) also described
PET/CT images of a CDC with distal ureteral seeding metastasis.
However, in this study, only faint nodular 18F-FDG
uptake was observed in the primary tumor. In the current series,
PET/CT scanning was performed on two patients and an evidently high
uptake of 18F-FDG was observed in each tumor. In
addition, the PET/CT images showed a marked 18F-FDG
uptake in the regional lymphadenopathies and pleural metastases,
which is consistent with the study by Yang et al(1).
The differential diagnoses for CDC include renal
clear cell carcinoma, invasive transitional cell or squamous cell
carcinoma, renal lymphoma and metastases, mesoblastic nephroma,
renal medullary carcinoma and bacterial pyelonephritis (12,14).
As the most common renal malignant tumor, renal clear cell
carcinoma usually locates in the renal cortex with a pseudocapsule
and is hypervascular, in contradistinction to CDC. The invasive
transitional cell or squamous cell carcinoma locates in the pelvis
and ureter, but usually invades to the renal medulla and is
hypovascular. It is difficult to distinguish these two types of
cancer from CDC. Renal lymphoma locates in the renal medulla, but
rarely shows cystic components or calcification prior to treatment.
Renal metastatic lesion, usually from a primary lung cancer, is
typically multiple and bilateral. Mesoblastic nephroma often occurs
in infancy and rarely in adults. Renal medullary carcinoma is an
aggressive malignancy that is closely associated with sickle cell
trait. Bacterial pyelonephritis is distinguished on a clinical
basis. However, all of these entities demonstrate significant
overlap on imaging observations.
To date, few studies have analyzed the imaging
characteristics of CDC. In addition to confirming observations
reported by previous studies, the current study identified several
additional features regarding the imaging appearance of CDC.
Firstly, to the best of our knowledge, the present study is the
first to report multifocal CDC in the same kidney. It demonstrated
that multifocus may occasionally be observed in the patients of
CDC, although the majority of patients were monofocal. Secondly,
the widespread infiltration of renal pelvis and ureter was
observable. Although a few cases of ureteral metastasis have been
reported in the previous literature, the extent of the malignant
lesions has been shorter than in the present study (1,18).
Thirdly, the current study suggested that PET/CT scanning may
provide additional information for detecting and grading CDC, due
to the high uptake of the 18F-FDG.
There were several limitations of the present study.
Firstly, the imaging results obtained of CDC were too small,
particularly for MRU, renal dynamic imaging and PET/CT. Therefore,
the study was limited in terms of the statistical analysis of
imaging observations. Secondly, not all enlarged lymph nodes
obtained reliable pathological results, due to the difficulties of
the surgery and, finally, specific imaging features of CDC were not
obtained. Certain common imaging observations may have appeared for
the other subtypes of RCC; therefore, future studies with large
numbers of patients is necessary.
The informative imaging observations of the CDC
obtained in the present study include monofocal or multifocal
lesions, solid or complex solid and cystic mass, medullary
location, weak and heterogeneous enhancement, infiltrative growth,
a cystic component, damage of renal function in the involved kidney
and a marked uptake of 18F-FDG. Furthermore, direct
invasion of the perirenal fascia, renal pelvis and ureter, regional
lymph nodes and distant metastases were observed. However, these
imaging features may be observed in other more common renal
diseases as aforementioned. Therefore, these imaging appearances
are non-specific and may not allow CDC to be reliably distinguished
from these diseases. However, when a renal tumor exhibits these
imaging observations, CDC may be suggested as a valuable
differential diagnosis.
References
|
1
|
Yang G, Seo J and Park J: Distal ureteral
seeding metastasis of collecting duct carcinoma manifesting as deep
vein thrombosis. Clin Radiol. 67:936–939. 2012. View Article : Google Scholar
|
|
2
|
Sironi M, Delpiano C, Claren R and
Spinelli M: New cytological findings on fine-needle aspiration of
renal collecting duct carcinoma. Diagn Cytopathol. 29:239–240.
2003. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Auguet T, Molina JC, Lorenzo A, Vila J,
Sirvent JJ and Richart C: Synchronus renal cell carcinoma and
Bellini duct carcinoma: a case report on a rare coincidence. World
J Urol. 18:449–451. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mancilla-Jimenez R, Stanley RJ and Blath
RA: Papillary renal cell carcinoma: a clinical, radiologic, and
pathologic study of 34 cases. Cancer. 38:2469–2480. 1976.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cromie WJ, Davis CJ and DeTure FA:
Atypical carcinoma of kidney: possibly originating from collecting
duct epithelium. Urology. 13:315–317. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Antonelli A, Portesi E, Cozzoli A, et al:
The collecting duct carcinoma of the kidney: a cytogenetical study.
Eur Urol. 43:680–685. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tsui K-H, Shvarts O, Smith RB, Figlin RA,
deKernion JB and Belldegrun A: Prognostic indicators for renal cell
carcinoma: a multivariate analysis of 643 patients using the
revised 1997 TNM staging criteria. J Urol. 163:1090–1095. 2000.
View Article : Google Scholar
|
|
8
|
Srigley JR and Delahunt B: Uncommon and
recently described renal carcinomas. Mod Pathol. 22(Suppl 2):
S2–S23. 2009. View Article : Google Scholar
|
|
9
|
Lopez-Beltran A, Carrasco JC, Cheng L,
Scarpelli M, Kirkali Z and Montironi R: 2009 update on the
classification of renal epithelial tumors in adults. Int J Urol.
16:432–443. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lopez-Beltran A, Scarpelli M, Montironi R
and Kirkali Z: 2004 WHO classification of the renal tumors of the
adults. Eur Urol. 49:798–805. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tokuda N, Naito S, Matsuzaki O, Nagashima
Y, Ozono S and Igarashi T: Collecting duct (Bellini duct) renal
cell carcinoma: a nationwide survey in Japan. J Urol. 176:40–43;
discussion 43. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pickhardt PJ, Siegel CL and McLarney JK:
Collecting duct carcinoma of the kidney: are imaging findings
suggestive of the diagnosis? AJR Am J Roentgenol. 176:627–633.
2001. View Article : Google Scholar
|
|
13
|
Fukuya T, Honda H, Goto K, et al: Computed
tomographic findings of Bellini duct carcinoma of the kidney. J
Comput Assist Tomogr. 20:399–403. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yoon SK, Nam KJ, Rha SH, et al: Collecting
duct carcinoma of the kidney: CT and pathologic correlation. Eur J
Radiol. 57:453–460. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hsiao HL, Yeh HC, Chang TH, et al: Renal
collecting duct carcinoma and concomitant bladder urothelial
carcinoma: a case report. Kaohsiung J Med Sci. 24:157–162. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Maestroni U, Ferretti S, Dinale F, et al:
A renal cancer with intermediate characteristics between collecting
(Bellini) duct carcinoma and urothelial carcinoma: case report and
review of the literature. Tumori. 92:545–548. 2006.
|
|
17
|
Ohnishi S, Dazai M, Iwasaki Y, Tsuzaka K,
Takahashi T and Miyagishima T: Undiagnosed collecting duct
carcinoma presenting as meningeal carcinomatosis and multiple bone
metastases. Intern Med. 49:1541–1544. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nakamura H, Kuirhara Y, Matsushita K,
Sakai A, Yamaguchi T and Nakajima Y: Extrarenal multiorgan
metastases of collecting duct carcinoma of the kidney: a case
series. J Med Case Rep. 2:3042008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Leyendecker JR and Gianini JW: Magnetic
resonance urography. Abdom Imaging. 34:527–540. 2009. View Article : Google Scholar
|
|
20
|
Leyendecker JR, Barnes CE and Zagoria RJ:
MR urography: techniques and clinical applications. Radiographics.
28:23–46; discussion 46-27. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Takahashi N, Glockner JF, Hartman RP, et
al: Gadolinium enhanced magnetic resonance urography for upper
urinary tract malignancy. J Urol. 183:1330–1365. 2010. View Article : Google Scholar
|
|
22
|
Chahal R, Taylor K, Eardley I, Lloyd S and
Spencer J: Patients at high risk for upper tract urothelial cancer:
evaluation of hydronephrosis using high resolution magnetic
resonance urography. J Urol. 174:478–482. 2005. View Article : Google Scholar
|
|
23
|
Shokeir AA, El-Diasty T, Eassa W, et al:
Diagnosis of noncalcareous hydronephrosis: role of magnetic
resonance urography and noncontrast computed tomography. Urology.
63:225–229. 2004. View Article : Google Scholar
|
|
24
|
Li Q, Zhang CL, Fu ZL, Wang RF, Ma YC and
Zuo L: Development of formulae for accurate measurement of the
glomerular filtration rate by renal dynamic imaging. Nucl Med
Commun. 28:407–413. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xun L, Cheng W, Hua T, et al: Assessing
glomerular filtration rate (GFR) in elderly Chinese patients with
chronic kidney disease (CKD): a comparison of various predictive
equations. Arch Gerontol Geriatr. 51:13–20. 2010. View Article : Google Scholar
|
|
26
|
Ozulker F, Özülker T, Uzun AK and Özpaçacı
T: Investigation of the efficacy of 99 mTc-DTPA scintigraphic GFR
measurement with Gates method in the detection of cisplatin-induced
nephrotoxicity in comparison with plasma urea and creatinine
measurement. Med Oncol. 28:1101–1106. 2011. View Article : Google Scholar
|
|
27
|
Aide N, Cappele O, Bottet P, et al:
Efficiency of [(18)F]FDG PET in characterising renal cancer and
detecting distant metastases: a comparison with CT. Eur J Nucl Med
Mol Imaging. 30:1236–1245. 2003.
|
|
28
|
Kang DE, White R Jr, Zuger JH, Sasser HC
and Teigland CM: Clinical use of fluorodeoxyglucose F 18 positron
emission tomography for detection of renal cell carcinoma. J Urol.
171:1806–1809. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lawrentschuk N, Davis ID, Bolton DM and
Scott AM: Positron emission tomography (PET), immuno-PET and
radioimmunotherapy in renal cell carcinoma: a developing diagnostic
and therapeutic relationship. BJU Int. 97:916–922. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ye XH, Chen LH, Wu HB, et al: 18F-FDG
PET/CT evaluation of lymphoma with renal involvement: comparison
with renal carcinoma. South Med J. 103:642–649. 2010. View Article : Google Scholar : PubMed/NCBI
|