Introduction
Lung cancer is the most common cause of
cancer-related mortality for males and females worldwide (1). Non-small cell lung cancer (NSCLC)
accounts for ~85% of all cases of lung cancer with an overall
5-year survival rate of <20.0%, as the majority of patients are
diagnosed at a late stage and are unsuitable for curative surgery
(2). Squamous cell carcinoma (SCC)
and adenocarcinoma (AC) represent the majority of NSCLCs.
According to a number of previous randomized
clinical trials, adjuvant chemotherapy is now considered to be the
unequivocal standard treatment for NSCLC patients. However, only a
proportion of patients benefit from adjuvant chemotherapy, while
others may succumb to metastasis derived from the malignancy
(3). Thus, it is imperative to
identify novel prognostic biomarkers that precisely predict
metastasis in patients with NSCLC. Such advances are likely to be
useful to stratify patients with NSCLC and select high-risk
patients who should receive aggressive adjuvant chemotherapy.
Girdin is overexpressed in various solid tumors,
including breast cancer, cervical carcinoma, lung, thyroid
(4) and colorectal (5) cancer, and glioblastoma (6). The present study, consistent with
previous studies, found that the expression of Girdin correlates
with tumor metastasis and may be a potential new distant metastasis
biomarker of breast and colorectal cancer (4,5,7–10).
Girdin locates at the crossroad of G protein and tyrosine kinase
receptor signaling (11), and
promotes cell migration via recruiting and controlling the actin
filaments (4,12). In addition, Girdin facilitates cell
proliferation by activating the mitogenic signals (13). Increasing evidence has confirmed
that Girdin is necessary for cell migration and proliferation, as
well as tumor metastasis and angiogenesis (4,13–15).
However, the correlation between Girdin expression and clinical
features in NSCLC remain unclear.
The aim of the present study was to assess the
expression of Girdin in a cohort of 36 consecutive patients with
NSCLC and correlate its expression with survival and other
clinicopathological parameters.
Materials and methods
Ethics statements
All experiments were performed in strict accordance
with the recommendations in the Guide for the Care and Use of
Laboratory Animals of the National Institutes of Health. The
protocol was approved by the Animal Care and Use Committee of The
309th Hospital of Chinese People’s Liberation Army (Beijing,
China).
Biopsy specimens
Paraffin-embedded sections of 36 NSCLCs were
obtained from the Department of Pathology of The 309th Hospital of
Chinese People’s Liberation Army, together with regional lymph node
dissection, between January 2010 and December 2012. Patients who
had received preoperative chemotherapy or radiotherapy were
excluded. The study was approved by the ethical committee of The
309th Hospital of Chinese People’s Liberation Army prior to
initiation.
Immunohistochemistry
All samples were fixed in 10% buffered formalin and
embedded in paraffin, and tissue sections (4 μm thick) were
obtained. All sections were deparaffinized and dehydrated with
graded alcohol. The sections were then washed for 10 min in
phosphate-buffered saline (PBS; pH 7.2). The endogenous peroxidase
activity was quenched by incubation in methanol containing 3%
H2O2 for 10 min at room temperature, then
heated for 30 min at 95°C to repair antigens and finally rinsed in
PBS. Following several washes in PBS, the sections were blocked
with goat serum for 15 min at room temperature and then incubated
with rabbit polyclonal Girdin primary antibody (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) overnight at 4°C in a
humidified chamber. In negative control sections, the primary
antibody was replaced by PBS. All slides were treated with polymer
enhancer (Reagent A; Zhongshan Jinqiao Biotechnology Co., Ltd.,
Beijing, China) for 20 min at room temperature. Following a
complete wash in PBS, the slides were treated with goat anti-rabbit
antibody (Reagent B; Zhongshan Jinqiao Biotechnology Co., Ltd.) for
30 min at room temperature. Following an additional complete wash
in PBS, the slides were developed in freshly prepared
diaminobenzidine solution for 8 min and then counterstained with
hematoxylin, dehydrated, air-dried and mounted.
Evaluation of score
Slides were reviewed independently by two
pathologists to evaluate the staining pattern of the protein
separately under the light microscope (Eclipse 80i, Nikon, Tokyo,
Japan). In scoring the expression of Girdin protein, the extent and
intensity of immunopositivity were considered. The intensity of
positivity was scored as follows: 0, negative; 1, weak; 2,
moderate; and 3, strong. The extent of positivity was scored
according to the percentage of cells showing positive staining as
follows: 1, <10%; 2, 11–50%; 3, 51–75%; and 4, >75%. The
final score was determined by multiplying the intensity and extent
of positivity scores, yielding a range between 0 and 12. The
expression for Girdin was considered positive when the scores were
>1. For the evaluation of immunoreactivity of Ki-67, 200 cells
from five representative fields of each section were randomly
selected and counted in a blinded manner by two independent
observers. Inconsistent data were discussed by the observers until
final agreements were reached. The expression positivity was graded
and counted as follows: Low, <25%; moderate, 25–50%; and high,
>50%.
Statistical analysis
All data were analyzed using SPSS 13.0 software
(SPSS, Inc., Chicago, IL, USA). The correlation between Girdin and
other parameters was investigated using the χ2 test,
Spearman’s rank correlation or an independent t-test when
appropriate. Linear regression was used to evaluate the correlation
among Girdin, Ki-67 and other parameters. P<0.05 was considered
to indicate a statistically significant difference.
Results
Clinical data
Specimens were obtained from archived
paraffin-embedded tissue sections of 36 patients with NSCLC. In the
cohort of NSCLC patients, 26 were male and 10 were female, with a
median age of 58 years (range, 31–77 years). According to the World
Health Organization classification of lung tumors published in 2001
(16), NSCLC patients were
classified as follows: AC, 21 cases; SCC, 12 cases; other types,
three cases; well- and moderately differentiated carcinoma, 24
cases; and poorly differentiated carcinoma, 12 cases. In the 36
NSCLC cases, 23 exhibited lymph node metastasis and 11 exhibited
blood vessel infiltration.
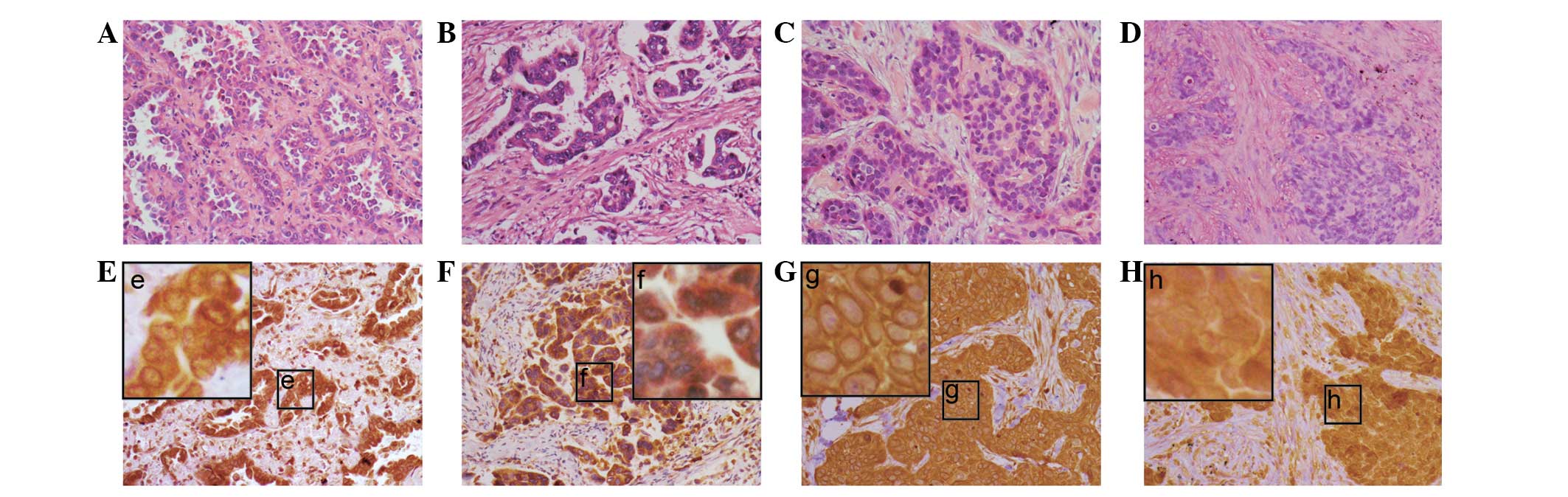
Expression of Girdin and Ki-67 in
NSCLC
In total, 26 (72.2%) of the 36 NSCLC cases showed
positive expression of Girdin. The expression of Girdin in NSCLC
tissues was predominantly observed in the cytoplasm or around the
nuclei membranes in SCC and AC (Fig.
1A–D). The perinucleus was characterized by thick, rounded,
densely stained material around the nucleus (Fig. 1; the lower panel). In total, 11
(30.6%) of the 36 cases exhibited the perinuclear pattern. A
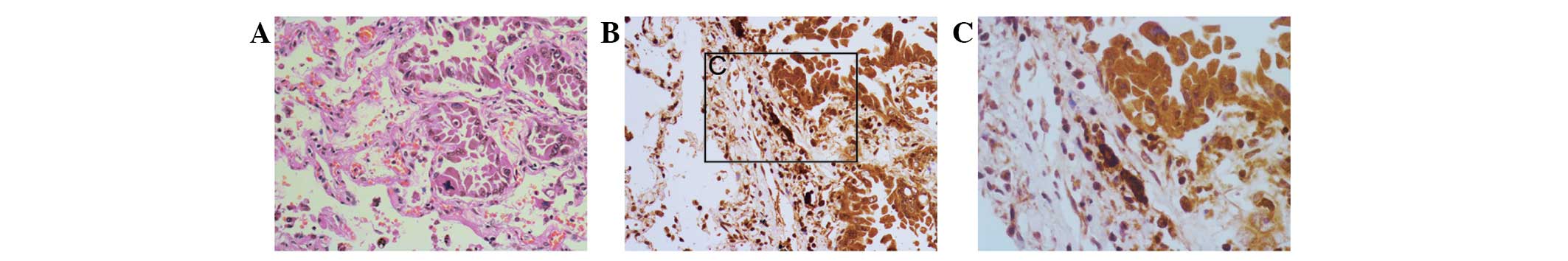
significant difference was identified between the NSCLC and distal
normal lung tissue (Fig. 2), and
eight (22.2%) of the 36 cases showed high expression of Ki-67.
Correlation between the protein
expression of Girdin and clinicopathological parameters in
NSCLC
Furthermore, correlation analysis demonstrated that
the expression of Girdin was found to significantly correlate with
blood vessel infiltration in NSCLC patients. High levels of Girdin
were found to correlate with significant blood vessel infiltration
(P=0.013). However, no correlations were observed between Girdin
expression patterns and the other clinicopathological parameters
studied (P>0.1; Table I).
 | Table ICharacteristics of 36 patients with
NSCLC and the correlation between Girdin protein expression and
clinicopathological variables. |
Table I
Characteristics of 36 patients with
NSCLC and the correlation between Girdin protein expression and
clinicopathological variables.
| | Girdin protein
expression | |
|---|
| |
| |
|---|
| Variables | Total (n=36), n | Low (n=10), n
(%) | High (n=26), n
(%) | P-value |
|---|
| Age, years | | | | 0.644 |
| <60 | 18 | 5 (27.8) | 13 (72.2) | |
| ≥60 | 18 | 5 (27.8) | 13 (72.2) | |
| Gender | | | | 0.580 |
| Male | 26 | 7 (26.9) | 19 (73.1) | |
| Female | 10 | 3 (30.0) | 7 (70.0) | |
| Tumor size, cm | | | | 0.519 |
| <3.5 | 16 | 4 (25.0) | 12 (75.0) | |
| ≥3.5 | 20 | 6 (30.0) | 14 (70.0) | |
| Histological
type | | | | 0.575 |
| SCC | 12 | 2 (16.7) | 10 (83.3) | |
| AC | 21 | 7 (33.3) | 14 (66.7) | |
| Others | 3 | 1 (33.3) | 2 (66.7) | |
| Lymphatic
infiltration | | | | 0.527 |
| Yes | 23 | 6 (26.1) | 17 (73.9) | |
| No | 13 | 4 (30.8) | 9 (69.2) | |
| Blood vessel
infiltration | | | | 0.013 |
| Yes | 11 | 0 (0.0) | 11 (100.0) | |
| No | 25 | 10 (40.0) | 15 (60.0) | |
| Histological
grade | | | | 0.330 |
|
Well-differentiated | 6 | 3 (50.0) | 3 (50.0) | |
| Moderately
differentiated | 18 | 5 (27.8) | 13 (72.2) | |
| Poorly
differentiated | 12 | 2 (16.7) | 10 (83.3) | |
In addition, the correlation between the perinuclear
expression pattern of Girdin and the clinicopathological parameters
was analyzed, but no significant difference was identified (all
P>0.1; Table II).
 | Table IICharacteristics of 36 patients with
NSCLC and the correlation between Girdin protein expression and
clinicopathological variables. |
Table II
Characteristics of 36 patients with
NSCLC and the correlation between Girdin protein expression and
clinicopathological variables.
| | Girdin expression
around nuclei | |
|---|
| |
| |
|---|
| Variables | Total (n=36), n | Sig. (n=11), n
(%) | Not sig. (n=25), n
(%) | P-value |
|---|
| Age, years | | | | 0.073 |
| <60 | 18 | 3 (16.7) | 15 (83.3) | |
| ≥60 | 18 | 8 (44.4) | 10 (55.6) | |
| Gender | | | | 0.335 |
| Male | 26 | 9 (34.5) | 17 (65.5) | |
| Female | 10 | 2 (20.0) | 8 (80.0) | |
| Tumor size, cm | | | | 0.391 |
| <3.5 | 16 | 4 (25.0) | 12 (75.0) | |
| ≥3.5 | 20 | 7 (35.0) | 13 (65.0) | |
| Histological
type | | | | 0.347 |
| SCC | 12 | 5 (41.7) | 7 (58.3) | |
| AC | 21 | 6 (28.6) | 15 (71.4) | |
| Others | 3 | 0 (0.0) | 0 (0.0) | |
| Lymphatic
infiltration | | | | 0.367 |
| Yes | 23 | 8 (34.5) | 15 (65.5) | |
| No | 13 | 3 (23.1) | 10 (76.9) | |
| Blood vessel
infiltration | | | | 0.551 |
| Yes | 11 | 3 (27.3) | 8 (72.7) | |
| No | 25 | 8 (32.0) | 17 (68.0) | |
| Histological
grade | | | | 0.182 |
|
Well-differentiated | 6 | 3 (50.0) | 3 (50.0) | |
| Moderately
differentiated | 18 | 3 (16.7) | 15 (83.3) | |
| Poorly
differentiated | 12 | 5 (41.7) | 7 (58.3) | |
Combined Ki-67 and Girdin analysis
The potential correlation between the protein
expression of Girdin and Ki-67 in NSCLC was evaluated. Spearman’s
rank correlation analysis showed that the expression of Girdin did
not correlate with Ki-67 expression in the NSCLC cohort (r=0.125;
P=0.468; Table III).
 | Table IIICorrelation between Girdin and Ki-67
protein expression. |
Table III
Correlation between Girdin and Ki-67
protein expression.
| Girdin protein
expression | Ki-67 (<25%), n
(%) | Ki-67 (25–50%), n
(%) | Ki-67 (>50%), n
(%) |
|---|
| High | 5 (71.4) | 14 (66.7) | 7 (87.5) |
| Low | 2 (28.6) | 7 (33.3) | 1 (12.5) |
Discussion
Previously, increasing evidence has shown that
Girdin expression is associated with tumor invasion/metastasis,
angiogenesis and growth in patients with colorectal and breast
cancer (4–13). In the present study, high expression
of Girdin protein was detected in 72.2% of NSCLCs, while the
expression level of Girdin in the normal lung tissues was extremely
low. Furthermore, statistical analysis showed that the expression
of Girdin was found to closely correlate with NSCLC differentiation
degree and blood vessel infiltration. The positive expression of
Girdin was more frequently observed in poorly differentiated cancer
and tumors with blood vessel infiltration.
Recently, Natsume et al reported that Girdin
is required for glioblastoma-initiating stem cells to sustain the
stemness and invasive properties. Stable Girdin knockdown in
isolated glioblastoma stem cells induced multilineage neural
differentiation (6). According to
this study, Girdin is pivotal for tumor cell differentiation in
NSCLC. Previous studies have suggested that Girdin produces a
marked effect by regulating the cytoskeleton of tumor cells
(4). Following binding to the actin
filaments, Girdin directly controls the migration and invasion of
breast cancer cells. In addition, Ohara et al found that
Girdin interacts with Par-3, a scaffolding protein that is a
component of the Par protein complex that has an established role
in determining cell polarity (17).
Thus, Girdin facilitates tumor metastasis by regulating the
cytoskeleton. These results suggested that the increased expression
of Girdin may facilitate the development and/or progression of
NSCLC.
In the present study, the Spearman’s rank
correlation analysis showed no correlation between the protein
expression of Girdin and Ki-67. However, previous studies have
revealed that Girdin is involved in tumor growth by upregulating a
variety of kinases, such as ERK 1/2, Src and STAT5 (13). Therefore, when the expression level
of Girdin is higher, it promotes tumor growth through these
molecules. The results of the present study indicated no
correlation between Girdin expression and tumor cell proliferation
in the tissues. It appears that more cases are required to
investigate the effect of Girdin expression on cancer cell
proliferation.
The current study confirmed that Girdin is highly
expressed in NSCLC. This result is consistent with previous
studies, which have reported that Girdin is highly expressed in
breast and colorectal cancer (4,5,7–10).
It has been documented that Girdin promotes cell proliferation and
migration (13). In addition,
increasing evidence has confirmed that high expression of Girdin is
associated with tumor metastasis and poorer postoperative,
disease-specific survival (5,9,10).
Based collectively on the aforementioned results, we proposed that
Girdin may also be involved in the tumorigenesis/progression of
NSCLC.
In conclusion, the present study is the first to
demonstrate that overexpression of Girdin closely correlates with
the malignant progression in patients with NSCLC. Girdin expression
may have clinical value as a new target for the treatment of lung
cancer. Future studies with larger cohorts of patients are required
to confirm the results of the current study and to establish a
prognostic role for this protein.
Acknowledgements
The current study was supported by grants from the
National Natural Science Foundation of China (no. 81072172), China
Postdoctoral Science Foundation special funded project (no.
201104043) and the Scientific Research Foundation for the Returned
Overseas Chinese Scholars, State Education Ministry (no.
jws1433).
References
|
1
|
Shin HR, Carlos MC and Varghese C: Cancer
control in the Asia Pacific region: current status and concerns.
Jpn J Clin Oncol. 42:867–881. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
3
|
Bonomi M, Pilotto S, Milella M, Massari F,
Cingarlini S, Brunelli M, Chilosi M, Tortora G and Bria E: Adjuvant
chemotherapy for resected non-small-cell lung cancer: future
perspectives for clinical research. J Exp Clin Cancer Res.
30:1152011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jiang P, Enomoto A, Jijiwa M, Kato T,
Hasegawa T, Ishida M, Sato T, Asai N, Murakumo Y and Takahashi M:
An actin-binding protein Girdin regulates the motility of breast
cancer cells. Cancer Res. 68:1310–1318. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Garcia-Marcos M, Jung BH, Ear J, Cabrera
B, Carethers JM and Ghosh P: Expression of GIV/Girdin, a
metastasis-related protein, predicts patient survival in colon
cancer. FASEB J. 25:590–599. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Natsume A, Kato T, Kinjo S, Enomoto A,
Toda H, Shimato S, Ohka F, Motomura K, Kondo Y, et al: Girdin
maintains the stemness of glioblastoma stem cells. Oncogene.
31:2715–2724. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu C, Zhang Y, Xu H, Zhang R, Li H, Lu P
and Jin F: Girdin protein: a new potential distant metastasis
predictor of breast cancer. Med Oncol. 29:1554–1560. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ling Y, Jiang P, Cui SP, Ren YL, Zhu SN,
Yang JP, Du J, Zhang Y, Liu JY and Zhang B: Clinical implications
for girdin protein expression in breast cancer. Cancer Invest.
29:405–410. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu C, Xue H, Lu Y and Chi B: Stem cell
gene Girdin: a potential early liver metastasis predictor of
colorectal cancer. Mol Biol Rep. 39:8717–8722. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jun BY, Kim SW, Jung CK, Cho YK, Lee IS,
Choi MG, Choi KY and Oh ST: Expression of girdin in human
colorectal cancer and its association with tumor progression. Dis
Colon Rectum. 56:51–57. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ghosh P, Garcia-Marcos M and Farquhar MG:
GIV/Girdin is a rheostat that fine-tunes growth factor signals
during tumor progression. Cell Adh Migr. 5:237–248. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Weng L, Enomoto A, Ishida-Takagishi M,
Asai N and Takahashi M: Girding for migratory cues: roles of the
Akt substrate Girdin in cancer progression and angiogenesis. Cancer
Sci. 101:836–842. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ghosh P, Beas AO, Bornheimer SJ,
Garcia-Marcos M, Forry EP, Johannson C, Ear J, Jung BH, Cabrera B,
Carethers JM and Farquhar MG: A Gαi-GIV molecular complex binds
epidermal growth factor receptor and determines whether cells
migrate or proliferate. Mol Biol Cell. 21:2338–2354. 2010.
|
|
14
|
Enomoto A, Murakami H, Asai N, Morone N,
Watanabe T, Kawai K, Murakumo Y, Usukura J, Kaibuchi K and
Takahashi M: Akt/PKB regulates actin organization and cell motility
via Girdin/APE. Dev Cell. 9:389–402. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kitamura T, Asai N, Enomoto A, Maeda K,
Kato T, Ishida M, Jiang P, Watanabe T, Usukura J, et al: Regulation
of VEGF-mediated angiogenesis by the Akt/PKB substrate Girdin. Nat
Cell Biol. 10:329–337. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brambilla E, Travis WD, Colby TV, Corrin B
and Shimosato Y: The new World Health Organization classification
of lung tumours. Eur Respir J. 18:1059–1068. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ohara K, Enomoto A, Kato T, Hashimoto T,
Isotani-Sakakibara M, Asai N, Ishida-Takagishi M, Weng L, Nakayama
M, et al: Involvement of Girdin in the determination of cell
polarity during cell migration. PLoS One. 7:e366812012. View Article : Google Scholar
|