Introduction
Inorganic chemistry has its place in medicine, and
metals, particularly transition metals, have various clinical
applications (1,2). Cisplatin, as an inorganic
antineoplastic agent, has been extensively used to treat tumors,
but its clear side effects and tolerance limit its clinical
applications. In addition, platinum complex with new ligands did
not exhibit marked advantages in previous clinical trials. To date,
only carboplatin and oxaliplatin have been used clinically
(3,4).
β-diketone-cobalt complexes are polyoxometalates
containing cobalt and traditional methods have been modified for
the synthesis (5).
β-diketone-cobalt complexes have been shown to suppress SMMC-7721
and SK-OV-3 tumor cell viability and interact with λ-DN (6); however, the molecular mechanisms of
β-diketone-cobalt complexes against tumors remain unclear.
Brain glioma is a common central nervous system
tumor, with at least five new cases per 100,000 individuals
diagnosed worldwide each year (7,8).
Malignant brain glioma extensively infiltrates normal brain tissues
and is difficult to completely excise surgically. The relapse rate
is high and conventional therapies used are radiotherapy and
chemotherapy (9). Although present
therapeutic methods are markedly advanced, the majority of patients
cannot be cured (10). As present
chemotherapeutics do not obtain ideal outcomes, the development of
highly effective, low toxicity drugs for the treatment of brain
glioma is required.
The current study focused on the effects of
β-diketone-cobalt complexes against C6 rat glioma cell cytotoxicity
and their potential molecular mechanisms of action against tumor
cells.
Materials and methods
Antibodies and reagents
Anti-cyclin A, -cyclin E and -p21 polyclonal
antibodies were purchased from Santa Cruz Biotechnology, Inc.
(Santa Cruz, CA, USA), while GAPDH monoclonal antibody was
purchased from Kangchen Bio-tech, Inc. (Shanghai, China).
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
was obtained from Sigma-Aldrich (St. Louis, MO, USA).
Cell lines and culture
Rat C6 glioma cells were incubated in Dulbecco’s
modified Eagle’s medium (Gibco Life Sciences, Grand Island, NY,
USA) supplemented with 10% fetal bovine serum (Gibco Life
Sciences), 2 mM L-glutamate, 100 U/ml penicillin and 100 μg/ml
streptomycin at 37°C in a 5% CO2 incubator.
MTT assay
Rat C6 glioma cells at 104 cells/well
were seeded onto 96-well plates for 24 h at 37°C and then treated
with β-diketone-cobalt complexes. Next, 20 μl MTT solution (5
μg/ml) was added to each well and incubated for 4 h at 37°C prior
to the removal of the culture medium. Dimethyl sulfoxide (150 μl)
was then added and agitated for 10 min at room temperature.
Absorbance values were measured at 570 nm using a Micro ELISA
reader (Bio-Rad, Hercules, CA, USA). The inhibitory rate of
β-diketone-cobalt complexes was calculated as the ratio of
absorbance values of the experimental group to the control group.
IC10 and IC50 values were calculated by SPSS
version 19.0 (IBM, Armonk, NY, USA).
Cell cycle analysis
Rat C6 glioma cells were seeded at a density of
~106 cells/well in six-well plates at 37°C for 24 h.
Cells were washed twice with ice-cold phosphate-buffered saline
(PBS; pH 7.4), treated with β-diketone-cobalt complexes, fixed with
50% alcohol at 4°C overnight and then stained with propidium iodide
(1 mg/ml) containing 1% RNAase A for 30 min. The cell cycle was
analyzed using a flow cytometer (Epics XL ADC, Beckman Coulter,
Miami, FL, USA).
Western blot analysis
Rat C6 glioma cells were treated with
β-diketone-cobalt complexes for 12 h prior to the preparation of
cell lysates. Subsequently the cell lysates were separated through
a 12% SDS-PAGE gel. Following electrophoresis, proteins were
transferred to PVDF membranes, and blocked with 5% non-fat dry milk
in TBST buffer (20 mM Tris-HCl pH 7.6, 150 mM NaCl and 0.05%
Tween-20) for 1 h at room temperature. The membranes were
subsequently probed with diluted primary antibodies in 1% milk/TBST
at 4°C overnight, washed three times, incubated with HRP-conjugated
secondary antibodies for 30 min at room temperature, and washed
extensively prior to detection by chemiluminescence with the
ECL-Plus kit (Beyotime, Haimen, China).
[3H]-thymidine assay
Rat C6 glioma cell proliferation was quantified by
[3H]-thymidine (GE Healthcare, Milan, Italy)
incorporation, as described previously (12). Rat C6 glioma cells were seeded at a
density of 106 cells/well in six-well plates at 37°C for
24 h. Following treatment with β-diketone-cobalt complexes for 48
h, cells were incorporated with 20 μCi/ml [3H]-thymidine
at 37°C for 2 h. Cells were then washed three times with PBS, lysed
with 200 μl 4% trichloroacetic acid for 30 min and washed three
times with 200 μl NaOH (0.1 M). The liquid was poured into a
scintillating disc with the addition of 3 ml scintillation fluid.
The counts per minute value was detected using a liquid
scintillation counter (1450 MicroBeta TriLux, PerkinElmer Life
Sciences, Boston, MA, USA).
Cell morphology assay
Rat C6 glioma cells at 105 cells/well
were seeded onto 6-well plates for 24 h at 37°C and subsequently
treated with β-diketone-cobalt complexes and 5-Fu. The cells were
analyzed using a fluorescence microscope 48 h later. The images
were acquired using an Olympus IX71 fluorescence microscope
(Olympus, Tokyo, Japan)
Statistical analysis
Experiments were repeated at least three times with
four replicates per sample. Student’s t-test was used to calculate
the statistical significance of the experimental results. P<0.05
and P<0.01 were considered to indicate statistically significant
differences. Data are presented as the mean ± SD, unless stated
otherwise.
Results
β-diketone-cobalt complexes suppress rat
C6 glioma cell viability
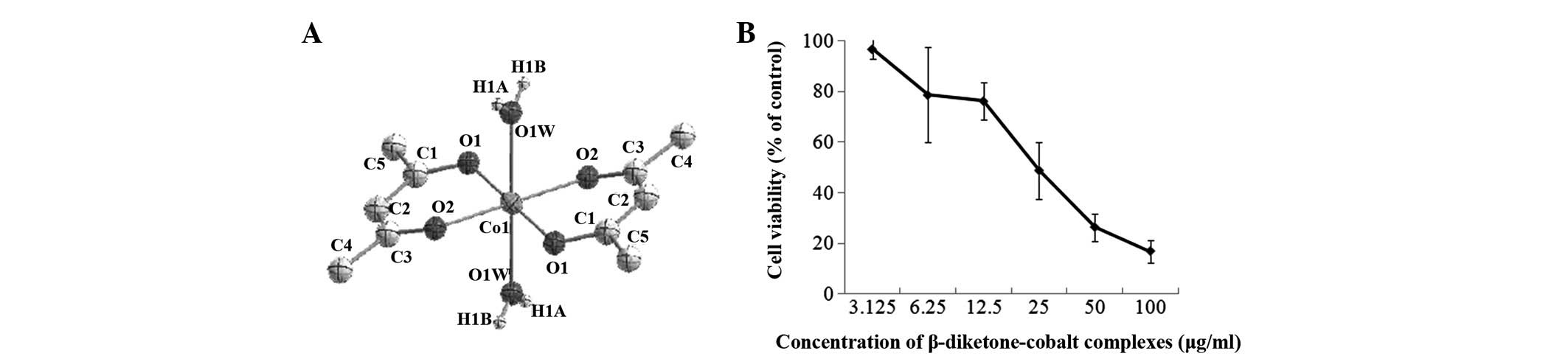
The chemical formula of the β-diketone-cobalt
complexes is Co(acac)2(H2O)2
[Co(acac)] and the structure is shown in Fig. 1A. The MTT results for rat C6 glioma
cells following treatment with β-diketone-cobalt complexes (3.125,
6.25, 12.5, 25, 50 or 100 μg/ml) for 48 h demonstrated that
β-diketone-cobalt complexes significantly suppress rat C6 glioma
cell viability in a dose-dependent manner. In rat C6 glioma cells,
the IC50 value of β-diketone-cobalt complexes was
24.7±3.395 μg/ml and IC10 value was 4.37±1.53 μg/ml
(Fig. 1B). The abovementioned
results revealed that β-diketone-cobalt complexes exhibit a marked
inhibitory effect on rat C6 glioma cells.
β-diketone-cobalt complexes inhibit rat
C6 glioma cell proliferation
To understand the mechanisms by which
β-diketone-cobalt complexes affect rat C6 glioma cell viability and
its antitumor capacity, β-diketone-cobalt complexes were compared
with an antineoplastic agent, 5-fluorouracil (5-Fu), in
vitro. Firstly, rat C6 glioma cells were separately treated
with β-diketone-cobalt complexes (10, 50 and 100 μg/ml) and 5-Fu
(10 and 50 μg/ml) for 48 h. β-diketone-cobalt complexes and 5-Fu
inhibited rat C6 glioma cell proliferation in a dose-dependent
manner (Fig. 2A). Rat C6 glioma
cells were analyzed using MTT assays following treatment with
β-diketone-cobalt complexes and 5-Fu (10, 25, 50 and 100 μg/ml) for
48 h. With increased concentration, the inhibitory effects of
β-diketone-cobalt complexes on rat C6 glioma cell proliferation
were significantly stronger than those of 5-Fu (Fig. 2B). The abovementioned results
revealed that β-diketone-cobalt complexes exert antitumor effects
by inhibiting rat C6 glioma cell proliferation.
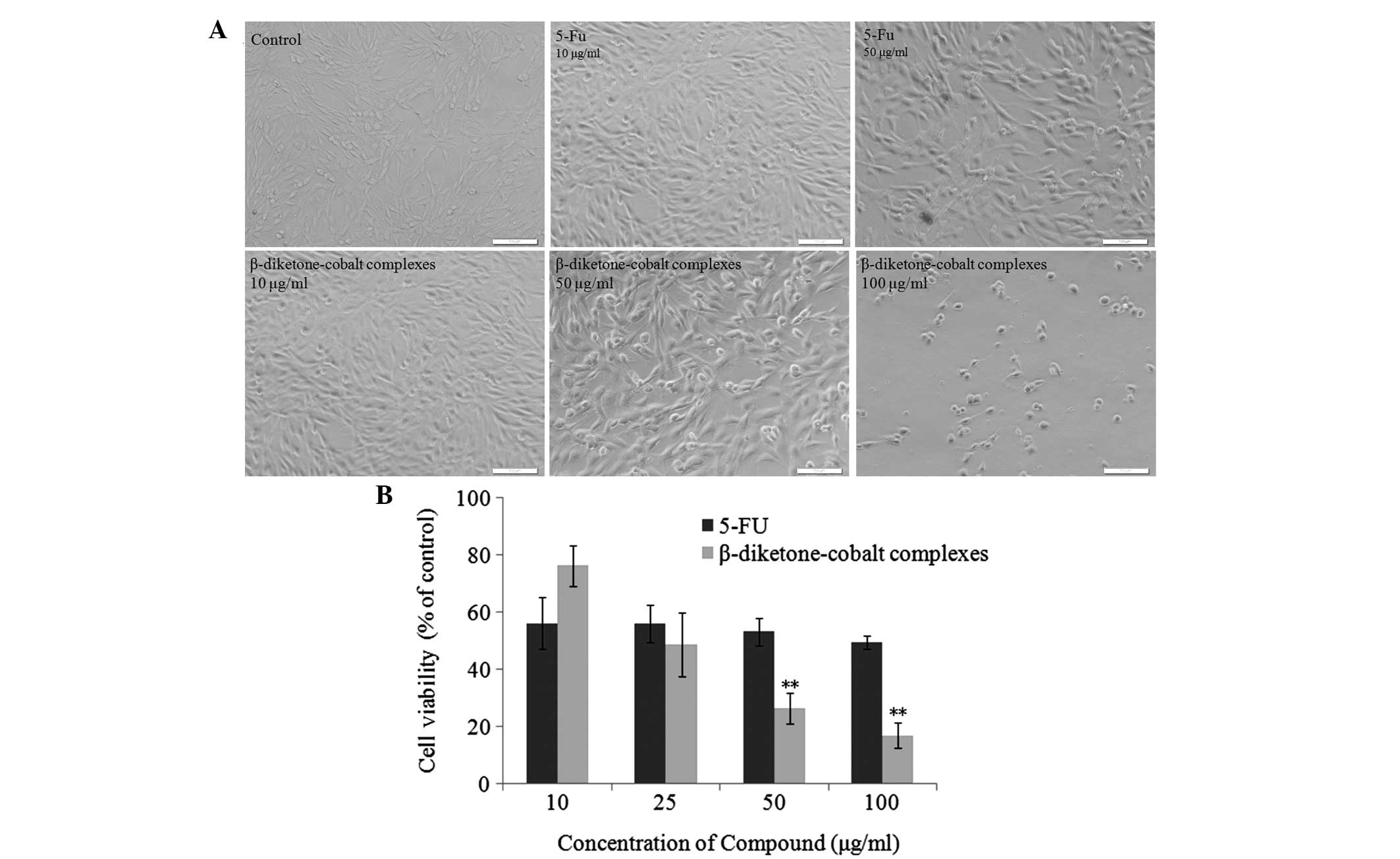 | Figure 2β-diketone-cobalt complexes inhibit
rat C6 glioma cell proliferation. (A) Rat C6 glioma cells were
treated with 10, 50 and 100 μg/ml β-diketone-cobalt complexes, and
10 and 50 μg/ml 5-Fu for 48 h. Images were captured under the light
microscope (scale bar, 100 μm; magnification, ×200).(B) Rat C6
glioma cells were treated with 10, 25, 50 and 100 μg/ml
β-diketone-cobalt complexes and 5-Fu for 48 hours. Dimethyl
sulfoxide [0.1% (v/v)] served as a negative control. Data are
presented as the mean ± SD of three independent experiments,
following 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide. **P<0.01, vs. the same concentration of
β-diketone-cobalt complexes and 5-Fu treatment groups. 5-Fu,
5-fluorouracil. |
β-diketone-cobalt complexes inhibit DNA
synthesis and induce S-phase arrest in rat C6 glioma cells
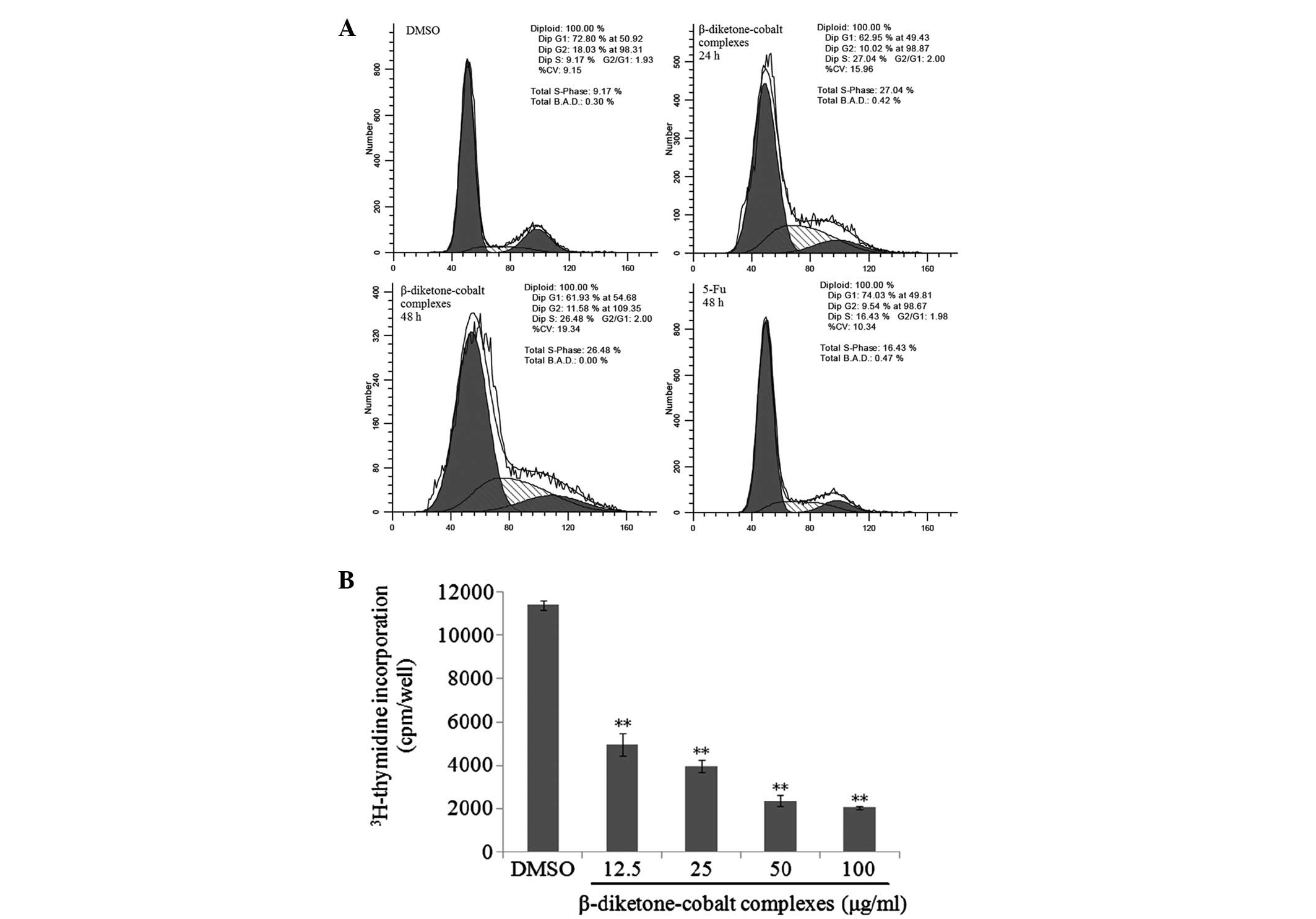
To further investigate the mechanisms by which
β-diketone-cobalt complexes suppress rat C6 glioma cell
proliferation, flow cytometry was utilized to identify the effects
of β-diketone-cobalt complexes and 5-Fu on the rat C6 glioma cell
cycle. Compared with the control group, the percentage of S-phase
cells significantly increased from 9.17 to 27.04 and 26.48%
following rat C6 glioma cell exposure to β-diketone-cobalt
complexes for 24 and 48 h, respectively. The percentage of cells in
S phase increased from 9.17 to 16.43% following exposure to 5-Fu
for 48 h (Fig. 3A). Subsequently,
[3H]-thymidine assay was employed to measure the effects
of various concentrations of β-diketone-cobalt complexes on the DNA
synthesis of rat C6 glioma cells for 24 h. Compared with the
control group, with increased concentration of β-diketone-cobalt
complexes, DNA synthesis in rat C6 glioma cells was evidently
inhibited in a dose-dependent manner (Fig. 3B). It was concluded that
β-diketone-cobalt complexes suppress rat C6 glioma cell
proliferation by inhibiting DNA synthesis and inducing S-phase cell
cycle arrest.
Effects of β-diketone-cobalt complexes on
cyclin A, cyclin E and p21 expression in rat C6 glioma cells
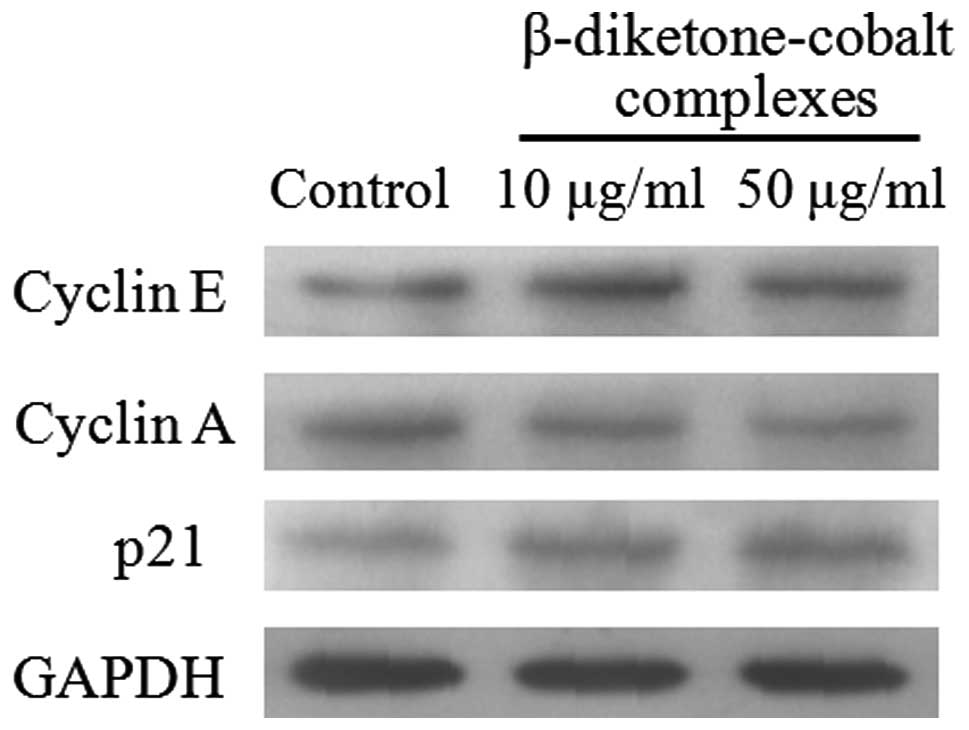
To identify proteins involved in S-phase arrest
induced by β-diketone-cobalt complexes in rat C6 glioma cells,
protein expression was detected in rat C6 glioma cells at 48 h
following exposure to β-diketone-cobalt complexes. Results showed
that β-diketone-cobalt complexes reduced cyclin A expression in rat
C6 glioma cells (13), but
increased cyclin E and p21 expression (Fig. 4).
Discussion
β-diketone-cobalt complexes, newly synthesized
non-platinum metal compounds, have been shown to inhibit SMMC-7721
and SK-OV-3 cell viability, but their antitumor mechanisms remain
unclear (14). Brain glioma is a
common tumor in the central nervous system and is difficult to
completely excise surgically. Its relapse rate is high and
conventional therapy is based on radiotherapy and chemotherapy;
however, the outcomes of current chemotherapy drugs are not ideal.
The present study first explored the mechanisms by which
β-diketone-cobalt complexes inhibit rat C6 glioma cell
proliferation and confirmed that β-diketone-cobalt complexes
suppress rat C6 glioma cell viability in a dose-dependent manner
(3.125–100 μg/ml). Of note, in rat C6 glioma cells, the
IC50 value of β-diketone-cobalt complexes was 24.7±3.395
μg/ml and IC10 value was 4.37±1.53 μg/ml, showing a good
inhibitory effect against tumors (Fig.
1B).
5-Fu, a common anticancer drug, is used for the
treatment of head and neck cancer (15). 5-Fu interacts with nucleic acid
metabolism, leading to cytotoxicity and cell death, thus, exerting
its antitumor activity (16,17).
Following comparison, the current study confirmed that
β-diketone-cobalt complexes exhibit marked antitumor activity in
vitro compared with 5-Fu (Fig.
2A). β-diketone-cobalt complexes at low concentrations
significantly inhibited DNA synthesis in rat C6 glioma cells
(Fig. 3B). Whether
β-diketone-cobalt complexes, similar to conventional chemotherapy
drugs, are simple cytotoxic drugs is poorly understood. The present
study revealed that the inhibitory effect of β-diketone-cobalt
complexes on rat C6 glioma cell proliferation correlates with
S-phase arrest (Figs. 2A and
3A). However, 5-Fu did not suppress
cell proliferation by cell cycle arrest (Fig. 3A), in contrast to the antitumor
mechanisms of β-diketone-cobalt complexes.
Cell cycle regulation depends on two protein
families, the cyclins and cyclin-dependent protein kinases (CDKs).
During the cell cycle, cyclin expression dynamically alters and
during the transition from G1 to S phase, cyclin E activates CDKs
and cyclin E expression increases. Cyclin E expression is
downregulated after entering S phase (18,19).
In the current study, cyclin E expression increased at 24 h
following treatment with β-diketone-cobalt complexes and diminished
at 48 h (Fig. 4). Cyclin A plays a
key role in S phase, but p21 causes cell cycle arrest by inhibiting
CDK activity (20–22). However, β-diketone-cobalt complexes
decreased the expression levels of cyclin A and p21 (Fig. 4). In conclusion, β-diketone-cobalt
complexes significantly suppress rat C6 glioma cell proliferation,
showing a potential ability for the development of novel antitumor
drugs.
Acknowledgements
The present study was supported by a grant from the
Youth Foundation Project of Department of Science and Technology of
Jilin Province of China (no. 20130522040JH).
References
|
1
|
Hambley TW: Chemistry. Metal-based
therapeutics. Science. 318:1392–1393. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hambley TW: Developing new metal-based
therapeutics: challenges and opportunities. Dalton Trans.
4929–4937. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kelland L: The resurgence of
platinum-based cancer chemotherapy. Nat Rev Cancer. 7:573–584.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Misset JL, Bleiberg H, Sutherland W,
Bekradda M and Cvitkovic E: Oxaliplatin clinical activity: a
review. Crit Rev Oncol Hematol. 35:75–93. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cotton FA and Elder RC: Crystal structure
of tetrameric cobalt(II) acetylacetonate. Inorg Chem. 4:1145–1151.
1965. View Article : Google Scholar
|
|
6
|
Zhang K, Cui S, Wang J, Wang X and Li R:
Study on antitumor activity of metal-based diketone complexes. Med
Chem Res. 21:1071–1076. 2012. View Article : Google Scholar
|
|
7
|
Lubin E: Brain tumors. N Engl J Med.
344:1478author reply 1479. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wen PY and Kesari S: Malignant gliomas in
adults. N Engl J Med. 359:492–507. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sant M, van der Sanden G and Capocaccia R:
Survival rates for primary malignant brain tumours in Europe.
EUROCARE Working Group. Eur J Cancer. 34:2241–2247. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Stupp R, Hegi ME, Mason WP, et al: Effects
of radiotherapy with concomitant and adjuvant temozolomide versus
radiotherapy alone on survival in glioblastoma in a randomised
phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet
Oncol. 10:459–466. 2009.
|
|
11
|
Zhou L, Bao YL, Zhang Y, et al: Knockdown
of TSP50 inhibits cell proliferation and induces apoptosis in P19
cells. IUBMB Life. 62:825–832. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Morrell NW, Upton PD, Kotecha S, et al:
Angiotensin II activates MAPK and stimulates growth of human
pulmonary artery smooth muscle via AT1 receptors. Am J Physiol.
277:L440–L448. 1999.PubMed/NCBI
|
|
13
|
Chen T and Wong YS: Selenocystine induces
S-phase arrest and apoptosis in human breast adenocarcinoma MCF-7
cells by modulating ERK and Akt phosphorylation. J Agric Food Chem.
56:10574–10581. 2008. View Article : Google Scholar
|
|
14
|
Xu W, Towers AD, Li P and Collet JP:
Traditional Chinese medicine in cancer care: perspectives and
experiences of patients and professionals in China. Eur J Cancer
Care (Engl). 15:397–403. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Grem JL: 5-Fluorouracil: forty-plus and
still ticking. A review of its preclinical and clinical
development. Invest New Drugs. 18:299–313. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang N, Yin Y, Xu SJ and Chen WS:
5-Fluorouracil: mechanisms of resistance and reversal strategies.
Molecules. 13:1551–1569. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Thomas DM and Zalcberg JR: 5-fluorouracil:
a pharmacological paradigm in the use of cytotoxics. Clin Exp
Pharmacol Physiol. 25:887–895. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schafer KA: The cell cycle: a review. Vet
Pathol. 35:461–478. 1998. View Article : Google Scholar
|
|
19
|
Vermeulen K, Van Bockstaele DR and
Berneman ZN: The cell cycle: a review of regulation, deregulation
and therapeutic targets in cancer. Cell Prolif. 36:131–149. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Michalides RJ, van de Brekel M and Balm F:
Defects in G1-S cell cycle control in head and neck cancer: a
review. Head Neck. 24:694–704. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ammit AJ and Panettieri RA Jr: Invited
review: the circle of life: cell cycle regulation in airway smooth
muscle. J Appl Physiol (1985). 91:1431–1437. 2001.PubMed/NCBI
|
|
22
|
Zhu H, Zhang L, Wu S, et al: Induction of
S-phase arrest and p21 overexpression by a small molecule
2[[3-(2,3-dichlorophenoxy)propyl] amino]ethanol in correlation with
activation of ERK. Oncogene. 23:4984–4992. 2004.PubMed/NCBI
|