Introduction
Laryngeal carcinoma is a common type of head and
neck cancer with poor prognosis. The disease occurs mainly in adult
males who abuse tobacco and alcohol and is characterized by
squamous differentiation (1).
Laryngeal carcinoma is usually identified in patients at late stage
leading to reduced treatment efficacy and a high rate of
recurrence. Despite the advances in the use of molecular markers
for monitoring human cancer over the past decades, no reliable
markers exist to screen laryngeal carcinoma and follow-up patients
after treatment. Based on the structure, chromosomal location and
biological/biochemical properties of the melanoma
differentiation-associated gene-7 (MDA-7), it has now been
classified as a novel member of the interleukin (IL)-10 gene family
(2–4).
This tumor suppressor gene associated with
differentiation, growth and apoptosis was initially identified from
human melanoma cells (5,6). Mapped within the IL-10 family cytokine
cluster to chromosome 1q32.2-q41, the gene encodes a protein
consisting of 206 amino acids, secreted in mature form as a 35–40
kDa-phosphorylated glycoprotein (7,8).
MDA-7 is expressed by diverse cell types, including
B cells, natural killer cells, dendritic cells, monocytes and
melanocytes. Although its physiological role is poorly understood,
the forced expression of MDA-7 in cancer cells results in
irreversible growth inhibition, reversal of the malignant phenotype
and terminal differentiation (9).
Thus, the biological impact of MDA-7 on the behavior of laryngeal
carcinoma cells was evaluated in the present study.
Materials and methods
Cells and main reagents
Hep-2 (ATCC, Manassas, VA, USA), the human laryngeal
cancer cell line and 293A, a subclone of the 293 cell line, were
preserved at the Key Laboratory for Modern Medicine and Technology
of Shandong Province (address?) and maintained in RPMI 1640
supplemented with 10% heat-inactivated fetal calf serum. Human
umbilical vein endothelial cells (HUVECs) were obtained from the
umbilical vein of healthy adults. The Ethics Committee of Shandong
University School of Medicine approved the study and all patients
provided written informed consent. Recombinant Ad-hIL-24 was
constructed and the total RNA extract kit was produced by our
laboratory. M-MLV reverse transcriptase and Taq DNA polymerase were
purchased from Promega Corporation (Madison, WI, USA). Methyl
thiazolyl tetrazolium (MTT) was purchased from Sigma-Aldrich (St.
Louis, MO, USA) and RPMI-1640 was purchased from Gibco-BRL
(Carlsbad, CA, USA). Serum from newborn calf was obtained from
Hangzhou Sijiqing Biological Engineering Materials Co., Ltd.
(Hangzhou, China). Human IL-24 monoclonal antibody was purchased
from Abcam (Cambridge, UK), human Bcl-2 monoclonal antibody was
purchased from Trevigen, Inc. (Gaithersburg, MD, USA), human Bax
polyclonal antibody was purchased from Beijing Biosynthesis
Biotechnology Co., Ltd. (Beijing, China), human caspase-3
monoclonal antibody was purchased from Bioworld Technology, Inc.
(St. Louis Park, MN, USA) and actin polyclonal antibody was
purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). Horseradish peroxidase-labeled goat anti-rabbit and
anti-mouse IgG were purchased from Beijing Zhongshan Golden Bridge
Biotechnology Co., Ltd. (Beijing, China).
Recombinant adenovirus amplification and
titer determination
The 70% adherent 293A cells were infected with
Ad-hIL-24 or empty adenovirus (Ad-GFP) and collected following 48
h. The cell suspension was frozen and thawed three times at −80 and
37°C, respectively. The supernatant was then removed, infections
were repeated and the cells were amplified. The virus solution was
stored at −80°C.
For virus titer determination, 1×105 293A
cells/ml were seeded in 96-well plates (100 μl/well) and cultured
under 5% CO2 at 37°C for 24 h. The virus stock solution
was then diluted from 1:10 to 1:1010 with 2% fetal
bovine serum cell culture fluid. Then, 100 μl of 1:103
to 1:1010 dilutions of the virus were added in the
96-well plates. In total, three wells were infected for each
dilution of virus and the negative control was set. The 96-well
plates were cultured at 37°C in a 5% CO2 incubator and
the cytopathic effect was observed every day. After 96 h (4 days),
>50% and <50% lesion well virus dilution were recorded in
order to calculate the 50% tissue culture infective dose
(TCID50) and subsequently calculate the PFU using the
formula: Virus titer (pfu/ml) = 0.7 × TCID50.
Identification of exogenous hIL-24 mRNA
and protein in Hep-2 cells and HUVECs
Hep-2 cells and HUVECs were seeded in 6-well plates
(2×105/well) and then treated with phosphate-buffered
saline (PBS) without calcium and magnesium ions or 100 multiplicity
of infection (MOI) of Ad-GFP or 100 MOI of Ad-hIL-24 following 24
h. The cells were collected following culture at 37°C in a 5%
CO2 incubator for 48 h.
The sequences of the IL-24 and β-actin primers are
listed in Table I. β-actin controls
were designed to be 18–24 nucleotides in length and to have 100%
homology with particular regions of the gene. The gene sequences
were obtained using the Oligo Primer analysis software, version 5.0
(NBA; Software and Research Services for Tomorrow’s Discoveries;
National Biosciences, Inc., Plymouth, MN, USA) and polymerase chain
reaction (PCR) oligomers were synthesized by a DNA/RNA synthesizer
(Applied Biosystems, Inc., Foster City, CA, USA) at the BioSune
Biotechnology (Shanghai) Co., Ltd. (Shanghai, China). The reverse
transcription (RT)-PCR method was used as previously described
(10). Briefly, RNA was extracted
from tissues using the acid guanidinium phenol-chloroform method.
The quality of the RNA yield was assessed by electrophoresis
(EC250–90, E-C Apparatus Corporation, Milford, MA, USA) on a 1.5%
agarose gel in 0.5 M Tris/borate/EDTA buffer, demonstrating the
typical 28S and 18S bands of the total RNA in all RNA yielded from
the cells. The amount of each RNA sample was measured by optical
density reading and only RNA samples showing a A260-A280 ratio
between 1.8 and 2.0 were used to obtain complementary DNA (cDNA).
RT-PCR was performed using RNA PCR kit (Promega Corporation). Cell
RNA (1 μg) was reverse transcribed into cDNA in a reaction mixture
containing 1X buffer, 1 mM dNTP, 2.5 μM oligo (dT) primer, 1 unit
RNAse inhibitor and 2.5 units reverse transcriptase. Following
incubation at 37°C for 60 min, the reaction was terminated by
heating at 95°C for 5 min. PCR was performed using the forward and
reverse primers described in Table
I. The PCR reaction buffer (25 μl), consisting of 2 mM
MgCl2, 0.5 μM of each primer and 2 units AmpliTaq DNA
polymerase (2 μl of each reverse-transcriptase solution) was added
to an amplification tube. PCR was run for 33 cycles and each cycle
consisted of 95°C for 1 min, 55°C for 1 min and 72°C for 1 min,
followed by a final extension for 7 min. In total, 12 μl aliquots
of the amplified product was fractionated on a 1.5% agarose gel and
visualized by ethidium bromide staining. The band intensity of
ethidium bromide fluorescence was measured using NIH/1D image
analysis software version 1.61 (National Institutes of Health,
Bethesda, MD, USA). The relative intensity of each band was
determined by the ratio to β-actin. To exclude the possibility of
carry-over contamination, reactions containing all the RT-PCR
reagents, including cytokine PCR primers without sample RNA, were
used as negative controls. No contamination was detected.
 | Table IOligonucleotide-specific primers used
to demonstrate associated gene messenger RNA expression in Hep-2
cells and HUVECs. |
Table I
Oligonucleotide-specific primers used
to demonstrate associated gene messenger RNA expression in Hep-2
cells and HUVECs.
| Target gene | Oligonucleotide
sequence | Length (bp) |
|---|
| β-actin |
| F |
5′-gtggggcgccccaggcacca-3′ | 539 |
| R |
5′-ctccttaatgtcacgcacgattt-3′ | |
| IL-24 |
| F |
5′-tactcgagagatgaattttcaacagaggct-3′ | 621 |
| R |
5′-gcgtctagatatcagagcttgtagaat-3′ | |
| Bcl-2 |
| F |
5′-cgacgacttctcccgccgctaccgc-3′ | 319 |
| R |
5′-ccgcatgctggggccgtacagttcc-3′ | |
| Bax |
| F |
5′-tccaccaagaagctgagcgag-3′ | 355 |
| R |
5′-gtccagcccatgatggttct-3′ | |
| Caspase-3 |
| F |
5′-cccatttctccatacgcact-3′ | 358 |
| R |
5′-tgacagccagtgagacttgg-3′ | |
| IL-20R1 |
| F |
5′-tcaaacagaacgtggtcccagtg-3′ | 386 |
| R |
5′-tccgagatattgagggtgataaag-3′ | |
| IL-22R |
| F |
5′-ccccactgggacactttcta-3′ | 243 |
| R |
5′-tggccctttaggtactgtgg-3′ | |
SDS-PAGE and immunoblotting was performed as
previously described in the legend to each figure using standard
techniques. In brief, the prepared cells were lysed at 4°C for 30
min in lysis buffer [20 mM tris(hydroxymethyl)aminomethane-HCl (pH
7.5), 140 mM NaCl, 1 mM ethylenediaminetetraacetic acid, 50 U/ml
aprotinin, 1 mM phenylmethylsulfonyl fluoride and 1 mM sodium
orthovanadate] containing 1% Nonidet P-40 detergent (11) and the protein samples were boiled
for 10 min. The boiled samples were loaded onto a 14% SDS-PAGE gel
and electrophoresis was run for 2 h. Proteins were
electrophoretically transferred onto 0.22 μm nitrocellulose
membrane and immunoblotted with IL-24 monoclonal and β-actin
antibodies against different proteins. The immunoblots were
visualized using a LAS4000 Chemiluminescence Imager (Fijifilm,
Tokyo, Japan) with associated software. For presentation,
immunoblots were opened in PhotoShop CS2 (Adobe Systems, Mountain
View, CA, USA); the color was removed and figures were generated in
PowerPoint (Microsoft Corporation, Redmond, WA, USA).
Cytotoxicity of Ad-hIL-24
Hep-2 cells and HUVECs were seeded in culture
plates, 24 h following the addition of PBS without calcium and
magnesium ions or infection with 100 MOI of Ad-GFP or 100 MOI of
Ad-hIL-24. The cells were cultured at 37°C in a 5% CO2
for 48 h. Morphological changes were observed under an inverted
fluorescence microscope (IX70, Olympus, Tokyo, Japan).
Ad-hIL-24 effect on cell growth by MTT
assay
Hep-2 cells and HUVECs were inoculated in 96-well
plates, separately, at 100 μl/well (5×104/ml). The cells
were divided into three groups following cell adherence and the
assay was repeated three times for each group. The cells were added
to PBS or infected with 100 MOI of Ad-GFP or 100 MOI of Ad-hIL-24
(100 μl/well) and observed for four days. A total of 10 μl MTT (5
mg/ml) was added to each well of the three groups every 24 h and
incubated at 37°C for 4 h. Then, 100 μl SDS-HCl (10%) stopping
solution was added to each well to fully dissolve the formazan
particles. The groups were measured with a microplate reader at 570
nm wavelength absorbance (A) and a growth curve of the time effect
was drawn with the A value as the vertical axis and incubation time
as the abscissa.
IL-24 effect on Bcl-2, Bax, caspase-3 and
IL-24 receptor mRNA expression in Hep-2 cells and HUVECs by
RT-PCR
IL-24 receptor includes IL-20R1, IL-20R2 and IL-22R.
IL-20R1 and IL-22R were selected as the IL-24 receptors to detect
expression in Hep-2 cells and HUVECs. The sequences of Bcl-2, Bax,
caspase-3, IL-20R1 and IL-22R primers are listed in Table I. Cell preparation, RNA extraction,
reverse transcription and PCR were performed as described
above.
IL-24 effect on Bcl-2, Bax and caspase-3
protein expression in Hep-2 cells and HUVECs by western blot
analysis
Hep-2 cells and HUVECs were seeded separately in
culture plates. Following 24 h, the cells were added to PBS or
infected with 100 MOI of Ad-GFP or 100 MOI of Ad-hIL-24. The cells
were then incubated at 37°C and 5% CO2 for 48 h,
digested with trypsin and collected. SDS-PAGE and immunoblotting
were performed as previously described. Proteins were
electrophoretically transferred onto 0.22 μm nitrocellulose
membranes and immunoblotted with various primary antibodies (Bcl-2,
Bax, caspase-3 and β-actin) against different proteins. Immunoblots
were visualized using a LAS4000 Chemiluminescence Imager (Fijifilm)
with associated software.
Statistical analysis
Comparison of the effects of various treatments was
performed using one-way analysis of variance (ANOVA) using the
statistical software SPSS 11.5 (SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Amplification and titer determination of
the recombinant adenovirus
Following infection of 293A cells with Ad-GFP or
Ad-hIL-24 for 24 h, green fluorescence was observed in the cells
under an inverted fluorescence microscope. Determination of the
amplified adenovirus by the TCID50 method demonstrated
that the titer of recombinant adenovirus was 7×108
pfu/ml following multiple rounds of amplification.
Identification of exogenous hIL-24 mRNA
and protein in Hep-2 cells and HUVECs
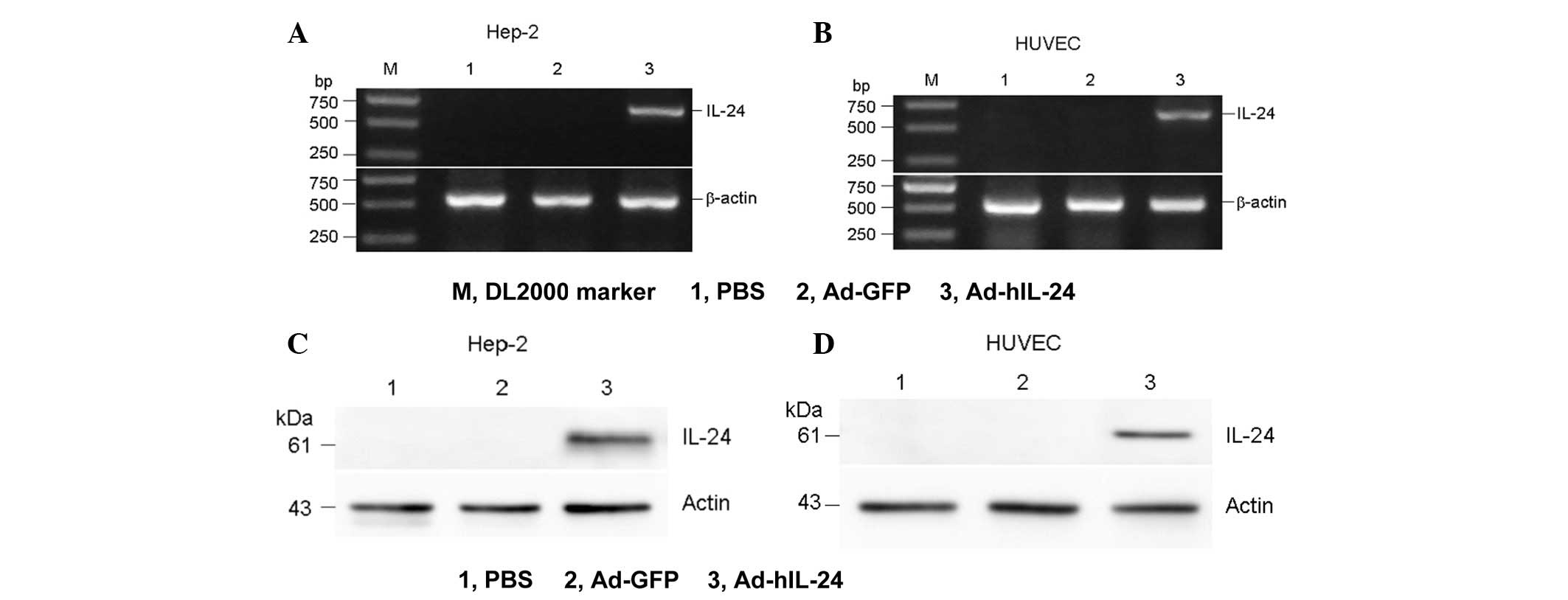
The Ad-hIL-24 group was found to exhibit a specific
DNA band at the 500–750-bp position and a protein band at the
51-kDa position, while the PBS and Ad-GFP groups did not show any
bands. This finding indicated that the adenovirus-mediated hIL-24
gene and protein was successfully transcripted and translated in
the Hep-2 and HUVECs, respectively (Fig. 1).
Cytotoxicity of Ad-hIL-24
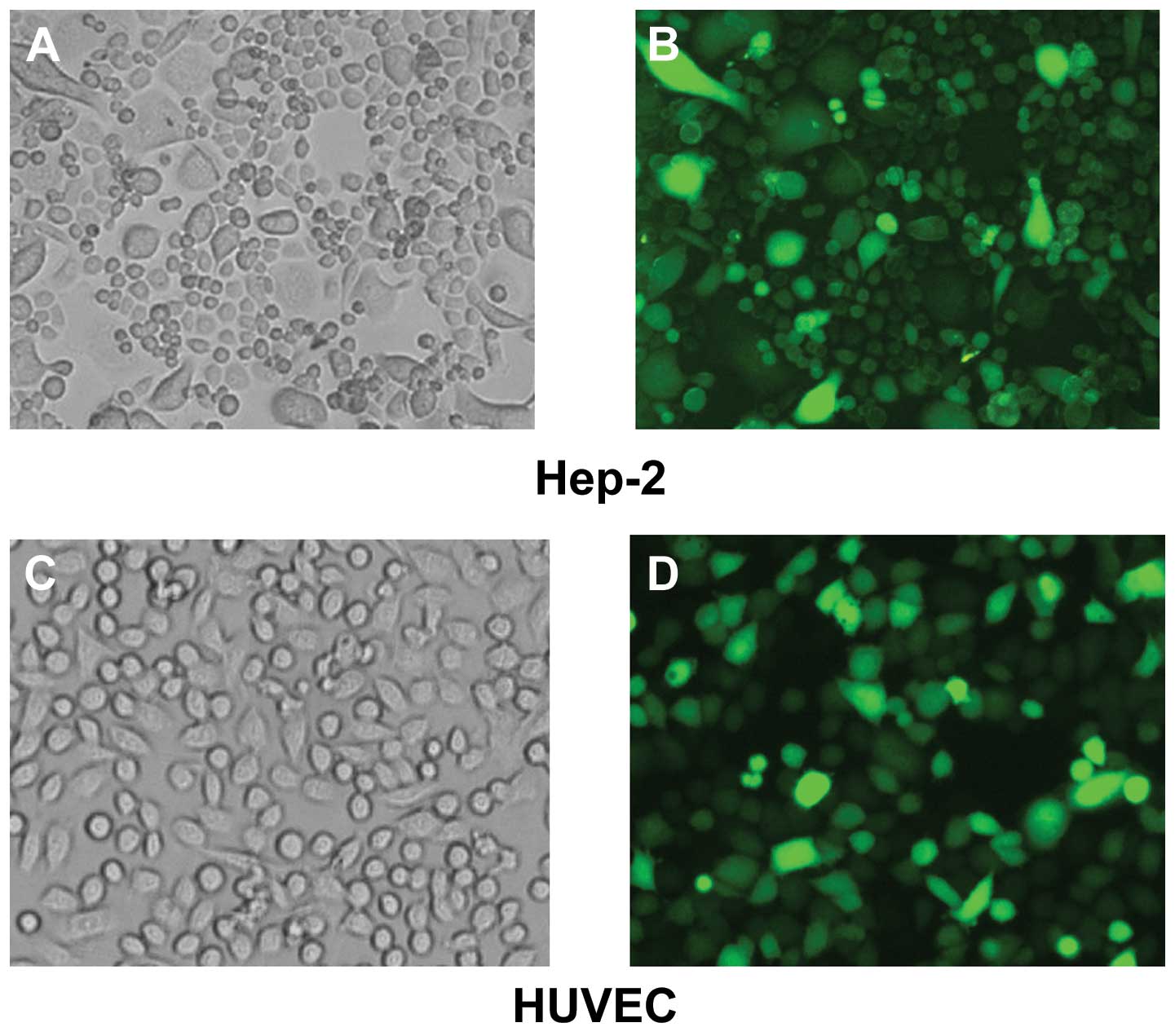
Under the microscope the living Hep-2 cells were
observed to adhere to the culture plate and were fusiform in shape.
Following 48 h the Ad-hIL-24-infected cells underwent apoptosis and
the cell shape became rounder and the cells detached from the
plate. Subsequently, the cell membranes shrank and the cells
ruptured. Hep-2 cells treated with PBS and Ad-GFP and HUVECs
treated with Ad-hIL-24, PBS and Ad-GFP did not show these changes
(Fig. 2).
Ad-hIL-24 effect on cell growth by MTT
assay
Hep-2 cell proliferation was significantly inhibited
following infection with Ad-hIL-24 and indicated a time-dependent
trend. Cell proliferation was significantly different between the
Ad-hIL-24-treated, PBS control or Adv-treated groups by ANOVA
(P<0.01). No statistically significant difference was identified
between the PBS control and Adv-treated groups (P>0.05; Fig. 3). These results showed that
Ad-MDA-7/IL-24 inhibited the proliferation of laryngeal cancer
cells. In addition, no change was identified between the
Ad-hIL-24-treated, PBS control or Adv-treated groups (P>0.05) in
HUVECs.
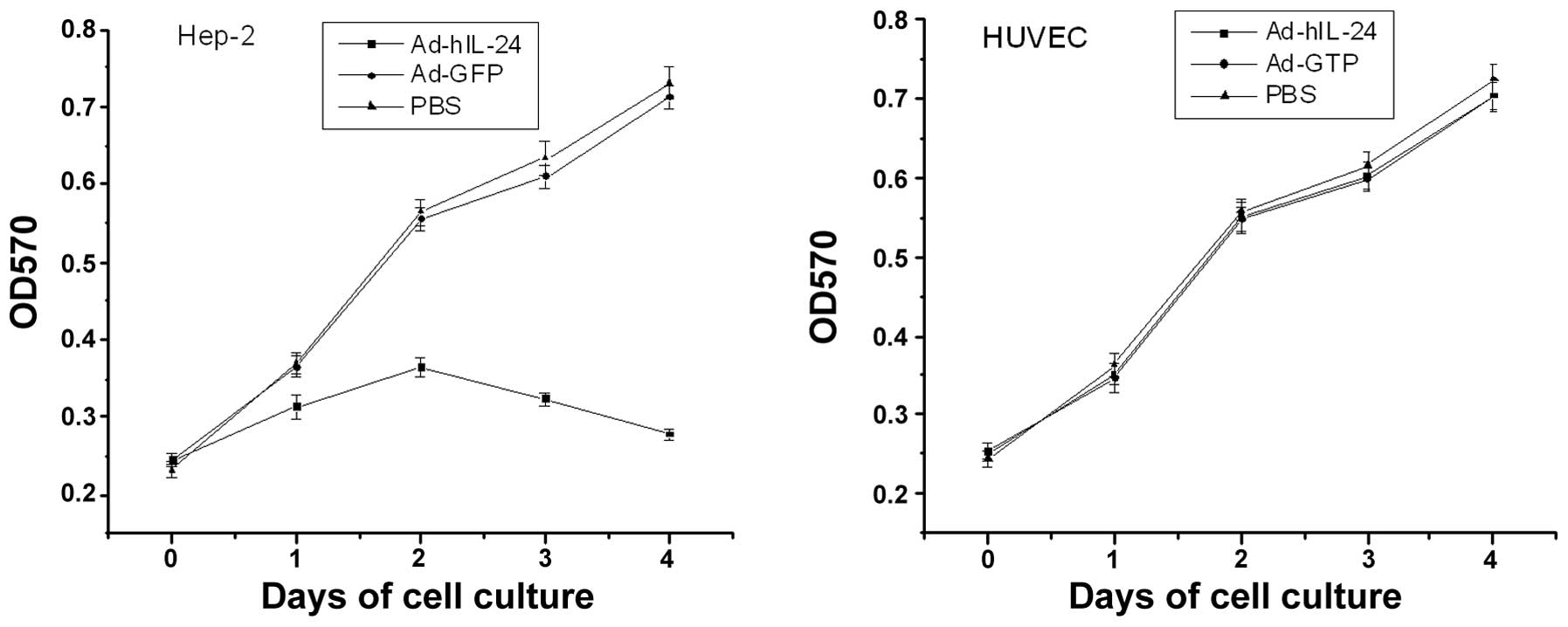 | Figure 3Time effect of Ad-hIL-24 on Hep-2
cells and HUVECs. Hep-2 cells and HUVECs were treated with
Ad-hIL-24 at a multiplicity of infection of 100 or with Ad-GFP or
PBS, serving as controls for four days. The survival of cells was
evaluated on days 0, 1, 2, 3 and 4 following infection by methyl
thiazolyl tetrazolium assay. The growth of Hep-2 tumor cells
treated with Ad-hIL-24 was significantly inhibited following
infection (P<0.05, vs. Ad-GFP and PBS groups at days 2, 3 and
4), but was not significantly inhibited in the Ad-GFP group
(P>0.05, vs. PBS group, via ANOVA). In addition, Ad-hIL-24 had
no effect on HUVECs (P>0.05, vs. Ad-GFP and PBS groups, via
ANOVA). Experiments were repeated three times per condition.
HUVECs, human umbilical vein endothelial cells; PBS,
phosphate-buffered saline; ANOVA, one-way analysis of variance; OD,
optical density. |
RT-PCR detection of the mRNA of related
apoptosis molecules
The mRNA expression of apoptosis-related molecules,
Bcl-2, Bax and caspase-3, was detected by RT-PCR assay. The results
showed that IL-24 induced proapoptotic gene Bax expression and
increased caspase-3 mRNA expression. Antiapoptotic gene Bcl-2
expression was significantly decreased while the IL-24 receptor was
markedly expressed in Hep-2 cells. In HUVECs, the Bax and caspase-3
expression was similar to that of Hep-2 cells, but Bcl-2 expression
did not change and no expression of the IL-24 receptor was
identified (Fig. 4). This result
showed that IL-24 inhibits antiapoptotic genes and increases the
expression of apoptotic genes to promote tumor cell apoptosis. In
addition, IL-24 also enhanced the expression of the IL-24 receptor,
thus, promoting apoptosis in Hep-2 cells.
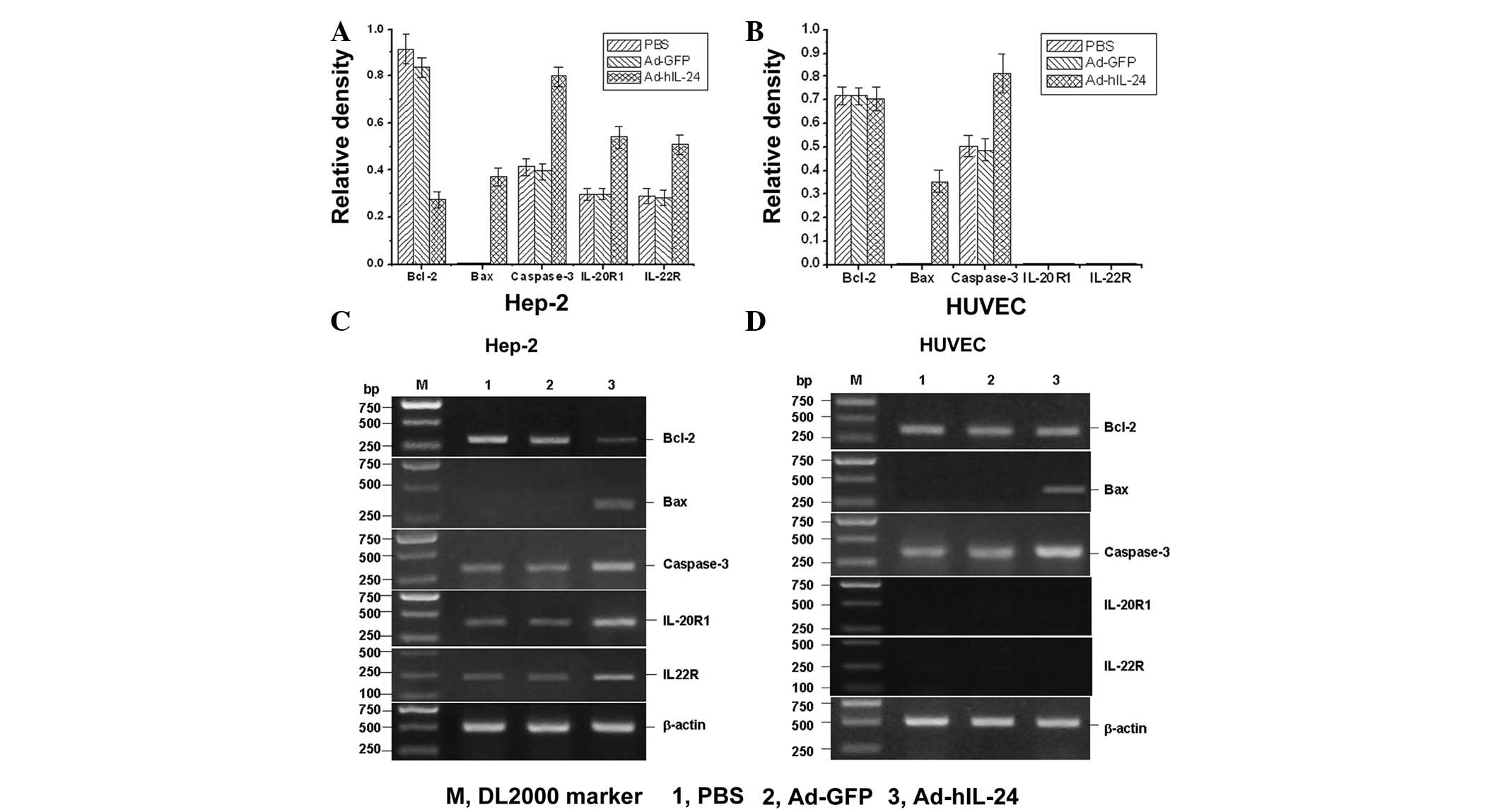 | Figure 4Reverse transcription polymerase chain
reaction analysis of the mRNA expression of apoptosis-related genes
and the IL-24 receptor. Average mRNA expression of Bcl-2, Bax,
caspase-3, Il-20R1 and IL-22R in (A) Hep-2 cells and (B) HUVECs.
All experiments were repeated twice and each experiment was
performed in triplicate for each sample. (C) Gel electrophoresis of
the mRNA expression of Bcl-2, Bax, caspase-3, Il-20R1 and IL-22R in
Hep-2 cells. IL-24 induced the proapoptotic gene Bax expression and
increased caspase-3, IL-20R1 and IL-22R mRNA expression and
antiapoptotic gene Bcl-2 expression was significantly reduced in
Hep-2 cells. (D) Gel electrophoresis of the mRNA expression of
Bcl-2, Bax, caspase-3, Il-20R1 and IL-22R in HUVECs. The Bax and
caspase-3 expression levels were similar to that of Hep-2 cells,
but Bcl-2 expression did not change and no expression of IL-20R1
and IL-22R was identified. mRNA, messenger RNA; IL, interleukin;
HUVECs, human umbilical vein endothelial cells; PBS,
phosphate-buffered saline. |
Western blot analysis detection of the
protein of related apoptosis molecules
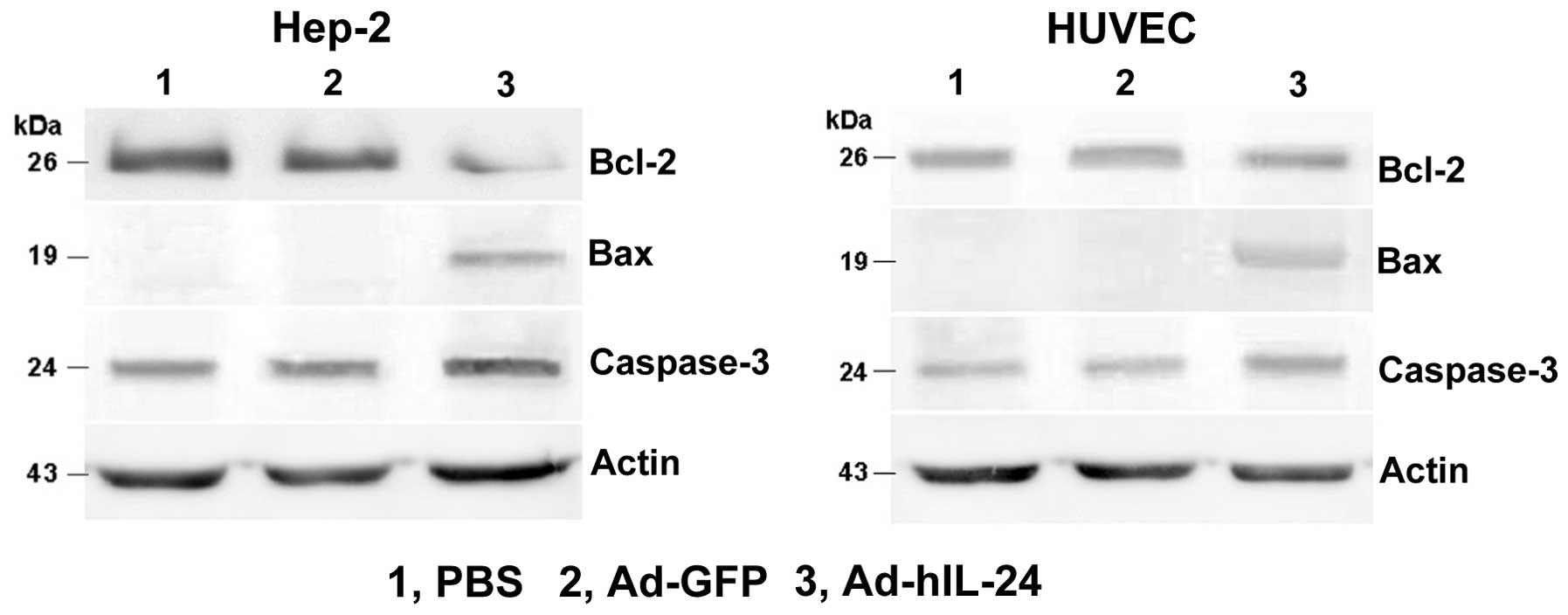
The protein expression of apoptosis-related
molecules, Bcl-2, Bax and caspase-3, was analyzed by western blot
analysis. The results revealed that IL-24 induced proapoptotic gene
Bax protein expression and increases caspase-3 protein expression.
Antiapoptotic gene Bcl-2 protein expression was significantly
reduced in Hep-2 cells. In HUVECs, the Bax and caspase-3 protein
expression was similar to that of Hep-2 cells, but Bcl-2 protein
expression did not change (Fig. 5).
This showed that IL-24 inhibited the expression of the
antiapoptotic protein and increased the expression of the apoptotic
protein to promote tumor cell apoptosis.
Discussion
MDA-7/IL-24 was identified by subtraction
hybridization strategy in the mid-1990s (5). The MDA-7 gene was isolated from human
melanoma cells induced to terminally differentiate by treatment
with interferon and mezerein. The protein expression of MDA-7/IL-24
is decreased during melanoma progression, with almost imperceptible
levels in metastatic disease (5,6,12,13).
MDA-7/IL-24 has been mapped within the IL-10 family cytokine
cluster to 1q32.2-q41 and the gene encodes a protein consisting of
206 amino acids, secreted in mature form as a 35–40
kDa-phosphorylated glycoprotein (7,8).
One of the essential requirements of utilizing a
therapeutic gene in gene therapy is that its expression must not
induce any deleterious effects in normal cells. Therefore,
MDA-7/IL-24 fits the requirements of a therapeutic gene. Previous
studies analyzing MDA-7/IL-24 have clearly shown the absence of
deleterious effects on normal human cells, including normal
melanocytes, endothelial cells, astrocytes, mammary and prostate
epithelial cells and skin fibroblasts (9,14–18).
MDA-7/IL-24 is a potent therapeutic cancer gene due
to its broad-spectrum cancer-specific apoptosis-inducing properties
as well as its multipronged indirect antitumor activities (19). Although its physiological role is
poorly understood, forced expression of MDA-7 in cancer cells
results in irreversible growth inhibition, reversal of the
malignant phenotype and terminal differentiation (9). Previous in vitro and in
vivo studies have demonstrated these attributes to be
tumor-selective and applicable to numerous solid malignancies. The
ectopic expression of MDA-7 (by transfection or adenovirus
transduction) exerts potent growth-suppressive and
apoptosis-inducing effects, not only in human melanoma cells, but
also in a wide spectrum of human cancer cells, including malignant
glioma, osteosarcoma, mesothelioma and carcinomas of the breast,
cervix, colon, lung, ovary and prostate (2–4,14,16,20).
Notably, similar effects are not apparent following transduction
into their non-malignant counterparts (18). Specific antitumor activity has also
been established in a range of human tumor xenograft models and in
several early phase clinical trials involving patients with
advanced solid cancers (2,20–22).
MDA-7 is emerging as a differentiation-, growth- and
apoptosis-associated gene with potential utility for the gene-based
therapy of several types of human cancer (7).
The apoptotic pathways by which MDA-7/IL-24 kills
tumor cells remain to be fully understood; however, current
evidence suggests an inherently high degree of complexity and an
involvement of proteins important for the onset of growth
inhibition and apoptosis, including Bcl-XL, Bcl-2 and Bax (3,4,14,17,23–25).
MDA-7 has also been shown to influence endothelial cells, exerting
a potentially antiangiogenic effect within the tumor vasculature
(26). Ad-MDA-7 has been found to
mediate p53-independent inhibition of tumor growth, cell cycle
arrest and apoptosis, associated with the downregulation of Bcl-2
and Akt. In previous in vivo studies, growth inhibition has
been demonstrated in multiple xenograft models. Furthermore,
Ad-MDA-7 has been demonstrated to have an additive or synergistic
effect in cellular and animal studies when combined with
chemotherapy, biological therapies and radiotherapy. These effects
have been associated with a decreased Bcl-2 expression and Bax
upregulation (27).
Laryngeal carcinoma, one of the most common tumors
of the head and neck, occurs mainly in adult males who abuse
tobacco and alcohol and is characterized by squamous
differentiation. Although early-stage glottic cancer has a
favorable prognosis, with five-year survival rates of >70%
(1), numerous types of supraglottic
and subglottic cancer are not diagnosed until severe signs develop,
by which time the five-year survival rate has decreased to <50%.
Locoregional recurrence, cervical lymph node metastases and distant
metastases are the factors significantly affecting prognosis in
laryngeal squamous carcinoma patients (28). The recognition and identification of
tumor markers associated with recurrence and/or metastasis are key
elements in predicting the biological behavior of the tumor and
deciding on the most appropriate therapeutic strategy.
MDA-7 induces cell cycle arrest at the G2/M phase,
induces apoptosis in cancer cells, inhibits new blood vessel
formation essential for tumor growth and stimulates the immune
system. In addition, MDA-7 is a secreted protein, which allows it
to exhibit bystander effects resulting in amplified tumor cell
killing.
In the present study, the human MDA-7/IL-24 gene was
transfected into the human laryngeal cancer Hep-2 cell line and
HUVECs with a replication-incompetent adenovirus vector. The
expression of Bcl-2 was significantly decreased while the IL-24
receptor was markedly expressed in Hep-2 cells following infection
with Ad-hIL-24, but not in HUVECs. In addition, the expression of
Bax and caspase-3 was increased in Hep-2 cells and HUVECs. This
finding showed that IL-24 inhibits antiapoptotic genes and
increases the expression of apoptotic genes to promote tumor cell
apoptosis. Furthermore, IL-24 also enhances the expression of the
IL-24 receptor, thus, stimulating apoptosis in Hep-2 cells. Bcl-2
expression did not change and no expression of the IL-24 receptor
was identified in the HUVECs. In addition to the IL-24 receptor,
other methods may exist that enhance the increased expression of
Bax and caspase-3. The MTT assay of the present study indicated
that Ad-hIL-24 induces growth suppression in Hep-2 cells but not in
HUVECs. Therefore, the results have shown that Ad-hIL-24
selectively inhibits proliferation and induces apoptosis of Hep-2
cells. No visible damage was identified in the normal cells under
the microscope. Therefore, the present study, evaluating
MDA-7vIL-24 in the context of laryngeal carcinoma, may prove to be
extremely valuable for developing an effective gene therapy
strategy for laryngeal carcinoma.
Acknowledgements
The present study was supported by grants from the
Shandong Province Outstanding Young Scientist Award Fund (no.
BS2009SW007) and Natural Science Foundation of Shandong Province
(no. ZR2010CM067) of China.
References
|
1
|
Karatzanis AD, Psychogios G, Zenk J,
Waldfahrer F, Hornung J, Velegrakis GA and Iro H: Comparison among
different available surgical approaches in T1 glottic cancer.
Laryngoscope. 119:1704–1708. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lebedeva IV, Sauane M, Gopalkrishnan RV,
et al: MDA-7/IL-24: exploiting cancer’s Achilles’ heel. Mol Ther.
11:4–18. 2005.
|
|
3
|
Gupta P, Su ZZ, Lebedeva IV, et al:
mda-7/IL-24: multifunctional cancer-specific apoptosis-inducing
cytokine. Pharmacol Ther. 111:596–628. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fisher PB: Is mda-7/IL-24 a ‘magic bullet’
for cancer? Cancer Res. 65:10128–10138. 2005.
|
|
5
|
Jiang H, Lin JJ, Su ZZ, Goldstein NI and
Fisher PB: Subtraction hybridization identifies a novel melanoma
differentiation associated gene, mda-7, modulated during human
melanoma differentiation, growth and progression. Oncogene.
11:2477–2486. 1995.
|
|
6
|
Wei N, Fan JK, Gu JF and Liu XY:
Double-regulated oncolytic adenovirus-mediated interleukin-24
overexpression exhibits potent antitumor activity on gastric
adenocarcinoma. Hum Gene Ther. 21:855–864. 2010. View Article : Google Scholar
|
|
7
|
Huang EY, Madireddi MT, Gopalkrishnan RV,
et al: Genomic structure, chromosomal localization and expression
profile of a novel melanoma differentiation associated (mda-7) gene
with cancer specific growth suppressing and apoptosis inducing
properties. Oncogene. 20:7051–7063. 2001. View Article : Google Scholar
|
|
8
|
Sauane M, Gupta P, Lebedeva IV, et al:
N-glycosylation of MDA-7/IL-24 is dispensable for tumor
cell-specific apoptosis and ‘bystander’ antitumor activity. Cancer
Res. 66:11869–11877. 2006.PubMed/NCBI
|
|
9
|
Jiang H, Su ZZ, Lin JJ, Goldstein NI,
Young CS and Fisher PB: The melanoma differentiation associated
gene mda-7 suppresses cancer cell growth. Proc Natl Acad Sci USA.
93:9160–9165. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tian Z, Shen X, Feng H and Gao B: IL-1
beta attenuates IFN-alpha beta-induced antiviral activity and STAT1
activation in the liver: involvement of proteasome- dependent
pathway. J Immunol. 165:3959–3965. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Maarof G, Bouchet-Delbos L, Gary-Gouy H,
Durand-Gasselin I, Krzysiek R and Dalloul A: Interleukin-24
inhibits the plasma cell differentiation program in human germinal
center B cells. Blood. 115:1718–1726. 2010. View Article : Google Scholar
|
|
12
|
Ekmekcioglu S, Ellerhorst J, Mhashilkar
AM, et al: Down-regulated melanoma differentiation associated gene
(mda-7) expression in human melanomas. Int J Cancer. 94:54–59.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ellerhorst JA, Prieto VG, Ekmekcioglu S,
Broemeling L, Yekell S, Chada S and Grimm EA: Loss of MDA-7
expression with progression of melanoma. J Clin Oncol.
20:1069–1074. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Su ZZ, Madireddi MT, Lin JJ, et al: The
cancer growth suppressor gene mda-7 selectively induces apoptosis
in human breast cancer cells and inhibits tumor growth in nude
mice. Proc Natl Acad Sci USA. 95:14400–14405. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Saeki T, Mhashilkar A, Chada S, Branch C,
Roth JA and Ramesh R: Tumor-suppressive effects by
adenovirus-mediated mda-7 gene transfer in non-small cell lung
cancer cell in vitro. Gene Ther. 7:2051–2057. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Madireddi MT, Su ZZ, Young CS, Goldstein
NI and Fisher PB: Mda-7, a novel melanoma differentiation
associated gene with promise for cancer gene therapy. Adv Exp Med
Biol. 465:239–261. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lebedeva IV, Su ZZ, Chang Y, Kitada S,
Reed JC and Fisher PB: The cancer growth suppressing gene mda-7
induces apoptosis selectively in human melanoma cells. Oncogene.
21:708–718. 2002. View Article : Google Scholar
|
|
18
|
Su ZZ, Lebedeva IV, Sarkar D, et al:
Melanoma differentiation associated gene-7, mda-7/IL-24,
selectively induces growth suppression, apoptosis and
radiosensitization in malignant gliomas in a p53-independent
manner. Oncogene. 22:1164–1180. 2003. View Article : Google Scholar
|
|
19
|
Lebedeva IV, Su ZZ, Vozhilla N, et al:
Mechanism of in vitro pancreatic cancer cell growth inhibition by
melanoma differentiation-associated gene-7/interleukin-24 and
perillyl alcohol. Cancer Res. 68:7439–7447. 2008. View Article : Google Scholar
|
|
20
|
Fisher PB, Gopalkrishnan RV, Chada S, et
al: mda-7/IL-24, a novel cancer selective apoptosis inducing
cytokine gene: from the laboratory into the clinic. Cancer Biol
Ther. 2(4 Suppl 1): S23–S37. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cunningham CC, Chada S, Merritt JA, et al:
Clinical and local biological effects of an intratumoral injection
of mda-7 (IL24; INGN 241) in patients with advanced carcinoma: a
phase I study. Mol Ther. 11:149–159. 2005. View Article : Google Scholar
|
|
22
|
Tong AW, Nemunaitis J, Su D, et al:
Intratumoral injection of INGN 241, a nonreplicating adenovector
expressing the melanoma-differentiation associated gene-7
(mda-7/IL24): biologic outcome in advanced cancer patients. Mol
Ther. 11:160–172. 2005. View Article : Google Scholar
|
|
23
|
Su ZZ, Lebedeva IV, Sarkar D, et al:
Ionizing radiation enhances therapeutic activity of mda-7/IL-24:
overcoming radiation and mda-7/IL-24-resistance in prostate cancer
cells overexpressing the antiapoptotic proteins bcl-xL or bcl-2.
Oncogene. 25:2339–2348. 2006. View Article : Google Scholar
|
|
24
|
Park MA, Walker T, Martin AP, et al:
MDA-7/IL-24-induced cell killing in malignant renal carcinoma cells
occurs by a ceramide/CD95/PERK-dependent mechanism. Mol Cancer
Ther. 8:1280–1291. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Eulitt PJ, Park MA, Hossein H, et al:
Enhancing mda-7/IL-24 therapy in renal carcinoma cells by
inhibiting multiple protective signaling pathways using sorafenib
and by Ad.5/3 gene delivery. Cancer Biol Ther. 10:1290–1305. 2010.
View Article : Google Scholar
|
|
26
|
Ramesh R, Mhashilkar AM, Tanaka F, et al:
Melanoma differentiation-associated gene 7/interleukin (IL)-24 is a
novel ligand that regulates angiogenesis via the IL-22 receptor.
Cancer Res. 63:5105–5113. 2003.
|
|
27
|
Chada S, Mhashilkar AM, Liu Y, et al:
mda-7 gene transfer sensitizes breast carcinoma cells to
chemotherapy, biologic therapies and radiotherapy: correlation with
expression of bcl-2 family members. Cancer Gene Ther. 13:490–502.
2006. View Article : Google Scholar
|
|
28
|
Cosetti M, Yu GP and Schantz SP: Five-year
survival rates and time trends of laryngeal cancer in the US
population. Arch Otolaryngol Head Neck Surg. 134:370–379. 2008.
|