Introduction
Extramedullary hematopoiesis (EMH) is the formation
of blood cells outside the bone marrow (BM). It may also be
referred to as myeloid metaplasia and is usually observed as
secondary to BM insufficiency (1).
The most common location is in the liver and spleen, but it may be
observed in other tissues as well as in tumors (2,3). Among
the tumors, EMH has been reported in renal oncocytoma and clear
cell type renal carcinoma (RCC) (4).
Despite being widely considered as an epiphenomenon,
recent molecular evidence for EMH points to stem cells and the
microenvironment, i.e. colonization within the liver, spleen and
RCC, as a niche is unlikely to be indiscriminate. Historically,
hematopoietic stem cells (HSCs) are found in the BM as well as in
circulatory system. When BM insufficiency occurs, circulatory HSCs
home toward the liver and spleen. However, there must be a possible
crosstalk between hematopoietic and osteogenic cells, as well as a
microenvironment created by certain tumors. It was found that liver
sinusoidal cells had a chemokine receptor that plays important
roles in the homing of HSCs (5). It
was also shown that erythropoietin (EPO), which is secreted from
the kidney has receptors that are present outside of hematopoietic
tissues and acute bleeding may trigger bone formation as well as
hematopoiesis (6) Written informed
consent was obtained from the patient.
Case report
The current report presents a case of a 69-year-old
female who was incidentally found to have a renal cyst in the right
kidney. The patient’s past medical history was unremarkable with
the exception of a complaint of right back pain in April 2008.
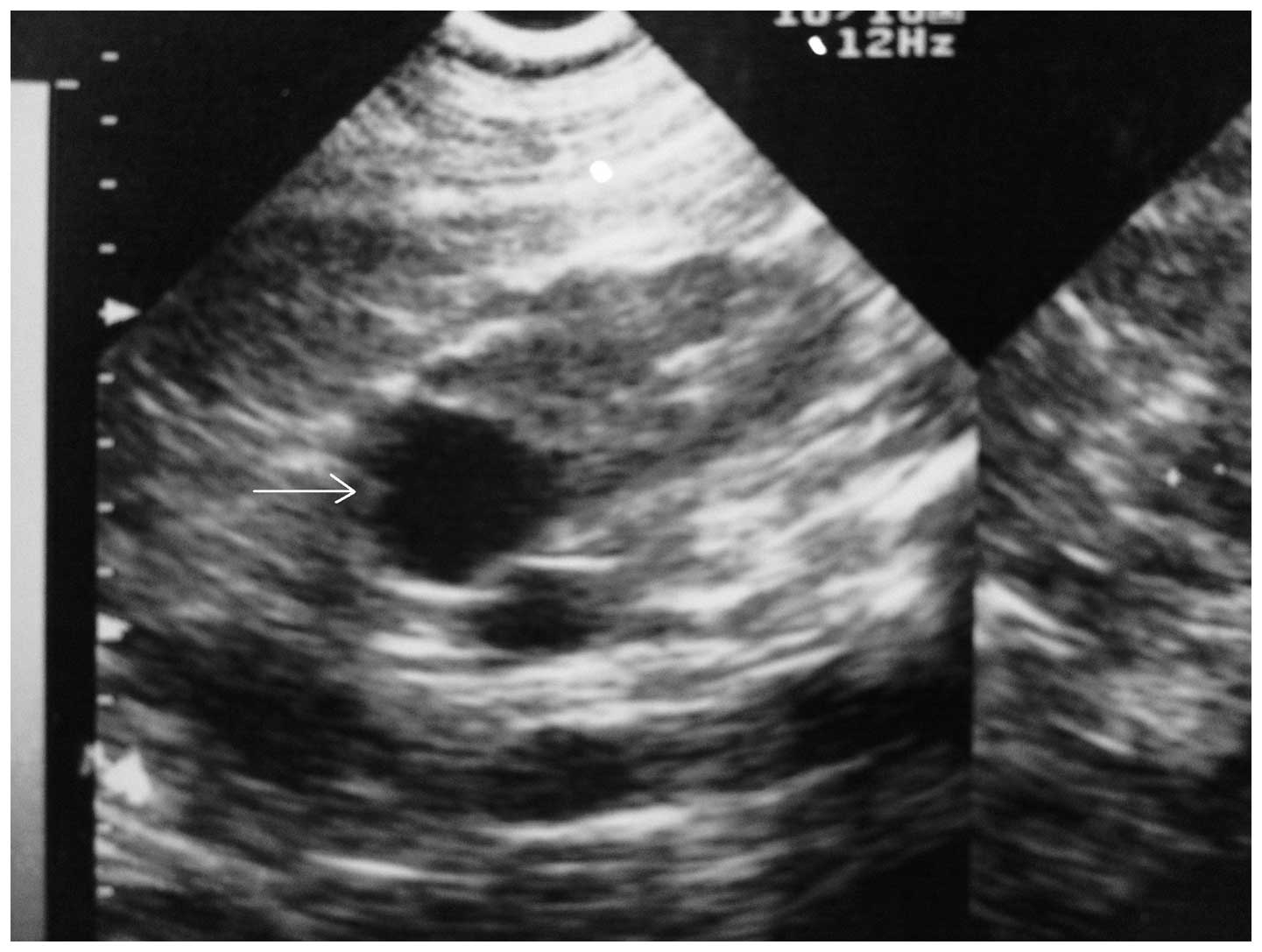
Further investigation revealed a right renal cortical cyst, 29 mm
in diameter, by ultrasonography (USG) (Fig. 1). Later admission to the Antalya
Ataturk Hospital (Antalya, Turkey) in April 2012 for the same
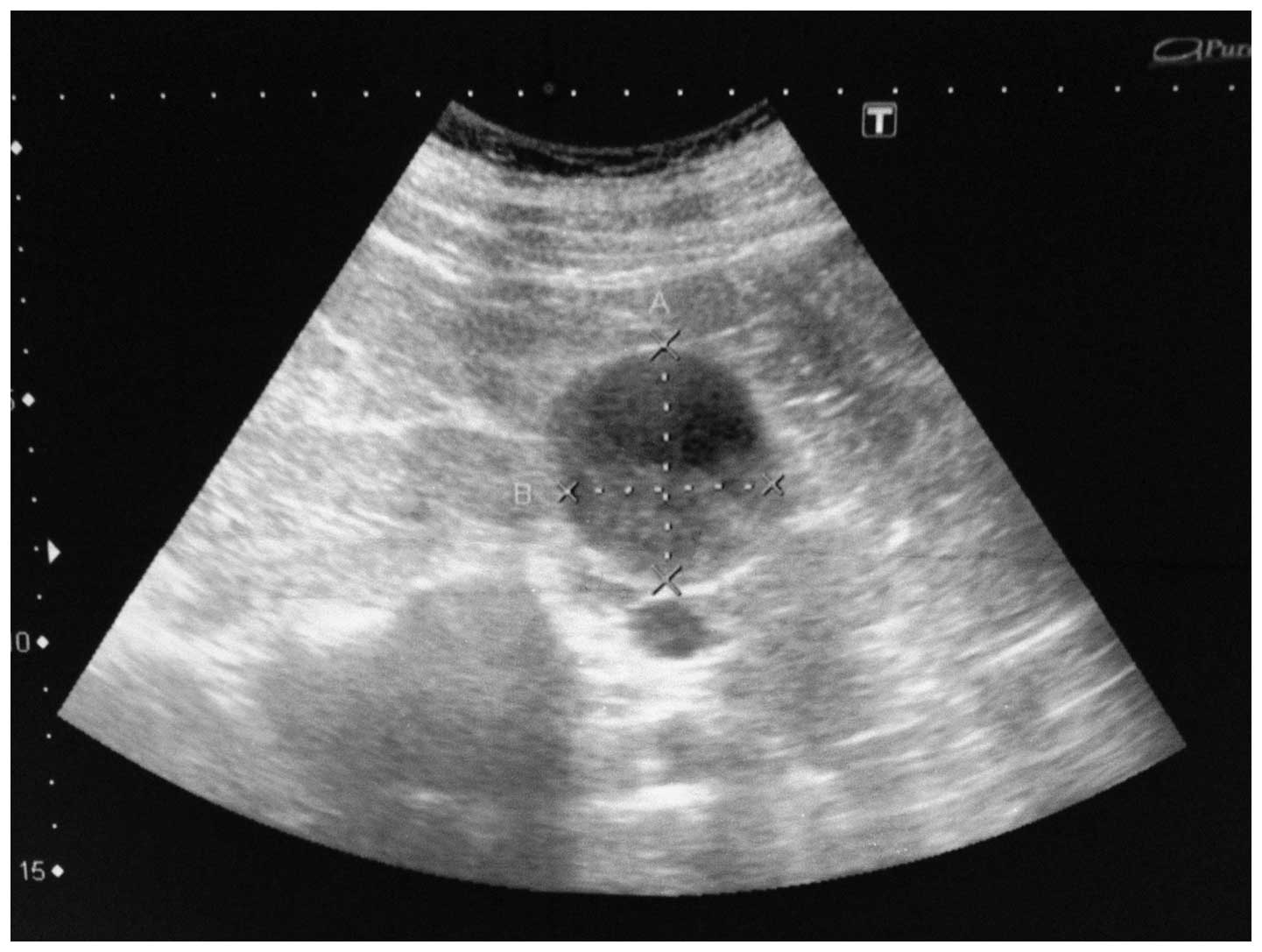
complaint revealed an increase in the size of the right renal
cortical cyst to 46×40 mm by USG (Fig.
2). The cyst was more complex and, in the base of the mass, a
semilunar hyper echoic region was identified that may belong to the
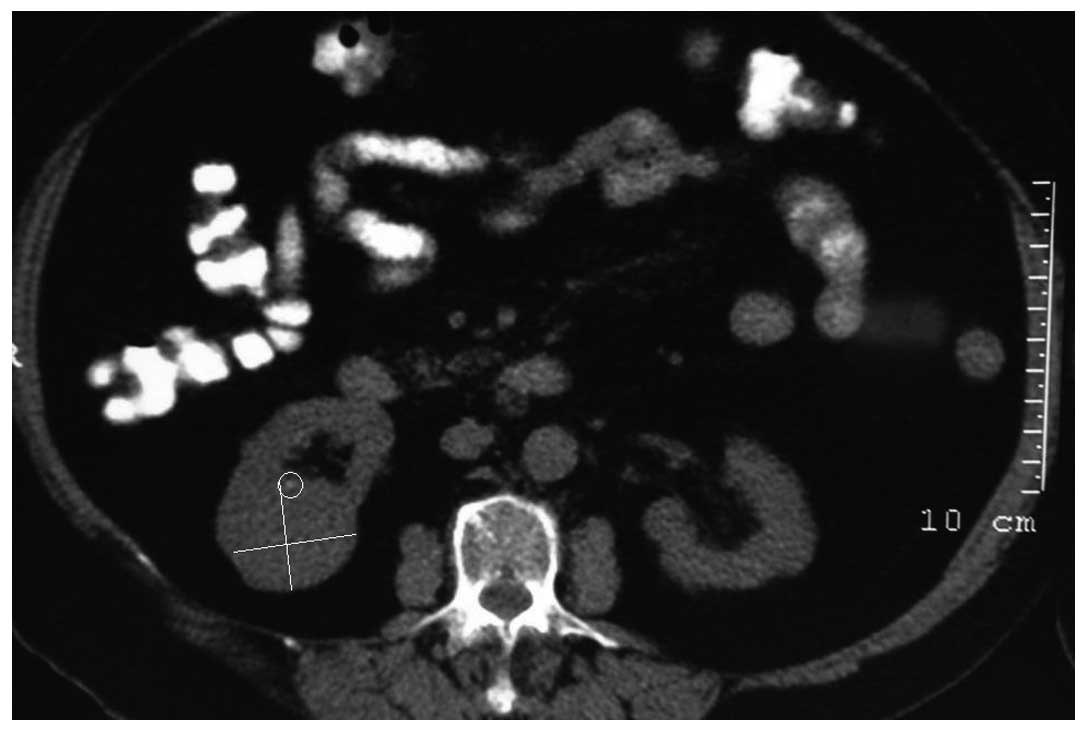
solid component of the tumor lesion. Abdominal tomography showed a
semi-solid, exophytic situated cyst, averaging 40×35 mm in size,
located at the posterior aspect of the inferior pole of the right
kidney (Fig. 3). Upon admission to
the hospital, the patient’s hemoglobin (Hgb) level was 13 g/dl,
white blood cell (WBC) count was
6.16×103/mm3, red blood cell (RBC) count was
4.36×103/mm3, hematocrit (HCT) result was
39.7% and platelet (PLT) count was
244×103/mm3 (Table I). Biochemical investigations were
within normal limits. During surgery, the surgeon also identified a
5-mm mass on the cyst wall and cystectomy was performed.
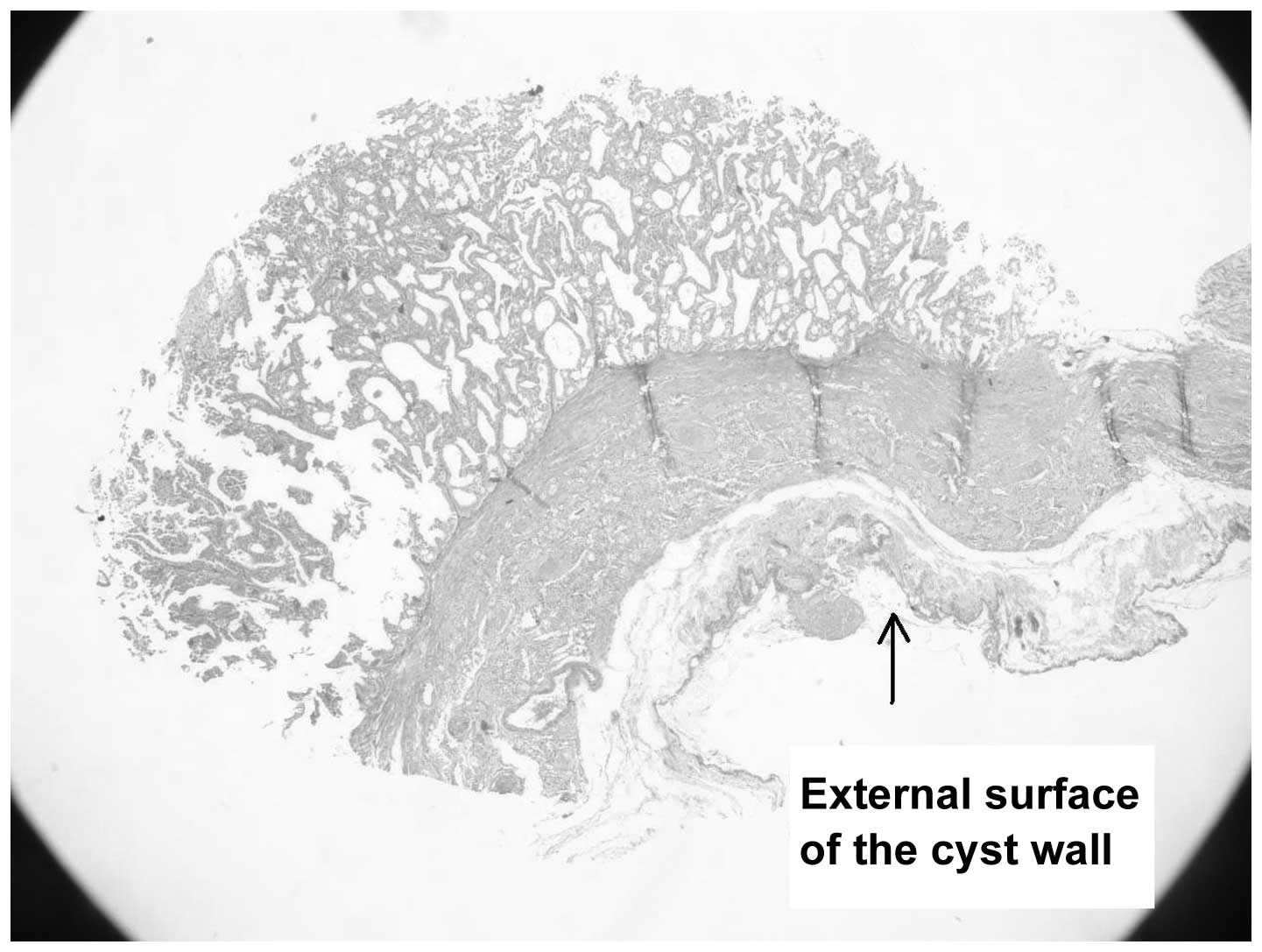
Histopathologically, the outer aspect of the cyst revealed atrophic
tubules and sclerotic glomeruli consistent with the
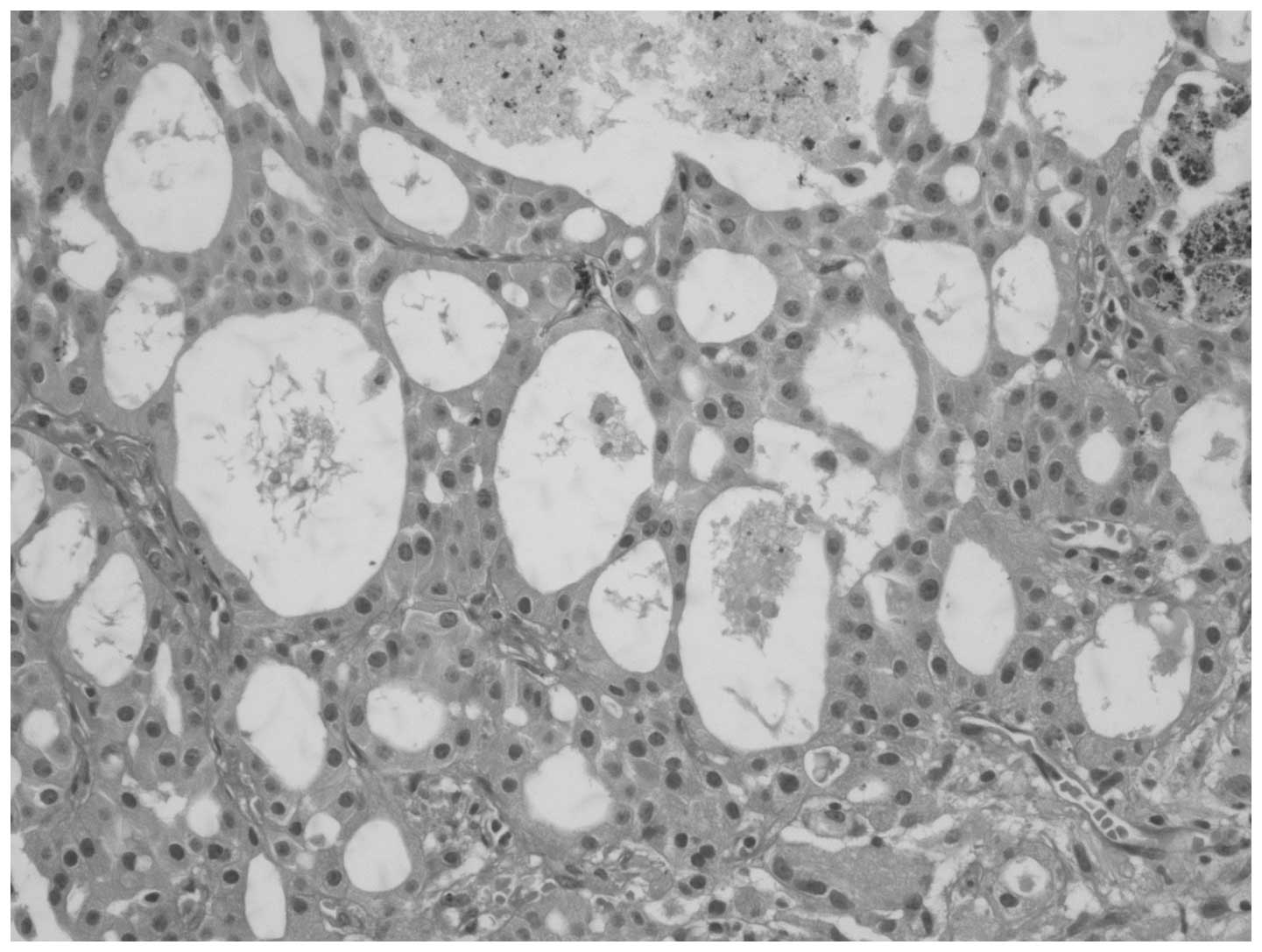
intraparenchymal location of the cyst (Fig. 4). The epithelial lining of the cyst
consisted of cells with monotonous nuclei and oncocytic cytoplasm
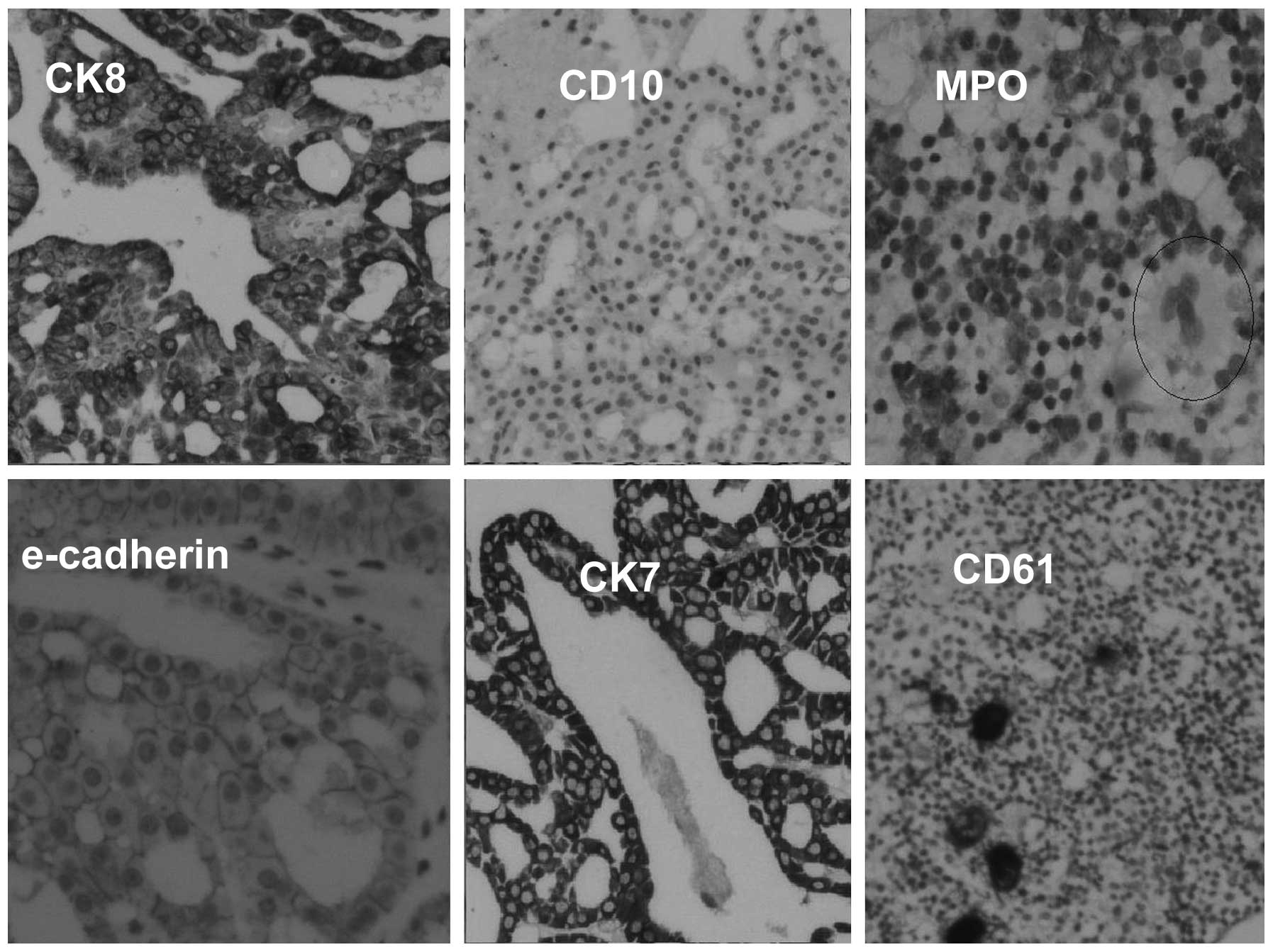
(Fig. 5). Tumor cells were negative
for clear cell type RCC markers, CD10 and vimentin, and positive
for chromophobe cell type RCC markers, E-cadherin and CK7, as well
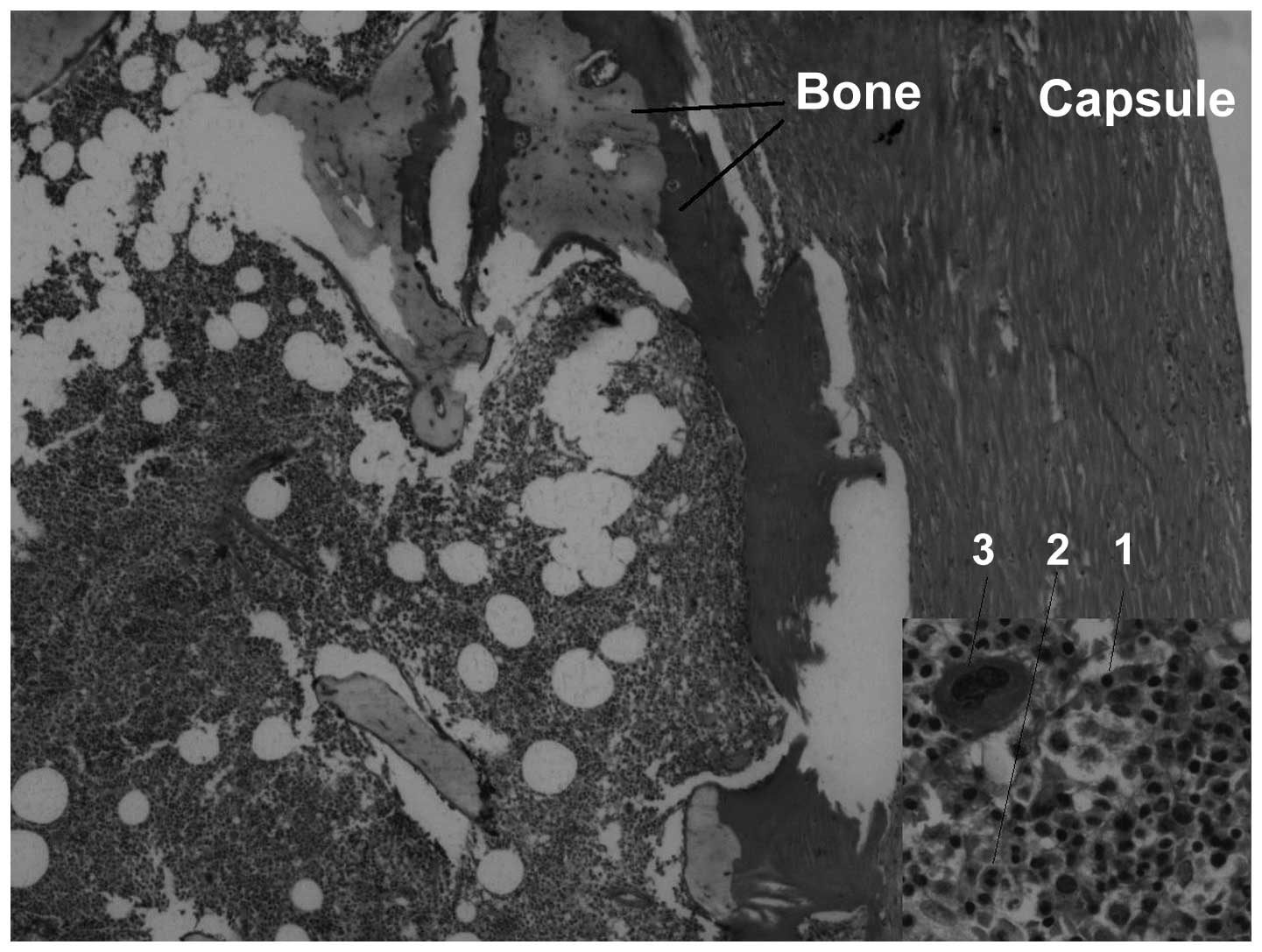
as oncocytic cell markers, CK8 and CK18 (Fig. 6). An examination of the mural nodule
revealed bone trabeculae with evident BM elements (Fig. 7), which were positive for the
erythroblast (glycophorin), myeloid (myeloperoxidase) and
megakaryocytic (CD61) markers (Fig.
6). The histopathological appearance of the mass along with the
immunohistological observations were similar to EMH.
 | Table IBiochemical values at different time
points. |
Table I
Biochemical values at different time
points.
| Values | March 2001 | May 2012 | July 2012 | September 2012 | January 2013 |
|---|
| WBC,
mm3 | 6.82 | 6.16 | 6.3 | 6.3 | 6.6 |
| RBC,
mm3 | 4.37 | 4.36 | 4.2 | 4.1 | 4.4 |
| Hgb, g/dl | 12.9 | 13 | 12.1 | 12.1 | 12.7 |
| HCT, % | 40.6 | 39.7 | 38.6 | 37.5 | 39.9 |
| PLT,
mm3 | 228 | 244 | 193 | 199 | 202 |
| Blood calcium,
mg/dl | 9.9 | ND | 9.5 | 9.6 | 9.9 |
| EPO, mU/ml | | | | 17,900 | |
| JAK-2 | | | | Negative | |
| t(9;22) | | | | Negative | |
BM biopsy was planned postoperatively but the
patient did not agree to this diagnostic procedure. At the fourth
postoperative month, the patient’s Hgb level was 12.1 g/dl, WBC
count was 6.3×103/mm3, RBC count was
4.1×103/mm3, HCT result was 37.5% and PLT
count was 199×103/mm3. The Janus kinase 2
mutations and reciprocal translocation between chromosome 9 and 22,
t(9;22), were not detected in the peripheral blood sample. However,
they are commonly found in polycythemia vera and chronic
myelogenous leukemia, respectively (7). EPO was 17,900 mU/ml in the third
postoperative month. Computed tomography did not detect any
residual tumor postoperatively.
Discussion
EMH is a condition defined as the appearance of
hematopoietic elements outside of the BM. It is associated with
various hematological diseases, mostly chronic myeloproliferative
diseases (1). The most common sites
for EMH are the liver and spleen (2). However it has been previously reported
in the majority of organs. EMH is usually found microscopically,
but may present as a mass-forming lesion (8). The most common locations for
mass-forming lesions are the paravertebral and intrathoracic spaces
(9). Occasionally, these masses
reach ≤8 cm in diameter, but even with this size, bone trabeculae
has not previously been reported within the mass.
With the exception of the liver and spleen, EMH may
affect the thoracic spinal region, which is rarely observed, and
<10 cases of EMH have previously been reported in association
with RCC or as occurring in the kidney (10). A number of EMHs are incidentally
found and are not associated with hematological diseases. Although
polycythemia is a common peripheral blood observation in RCC
patients, the exact pathogenesis of EMH within RCC is not known; it
has been previously reported that 74% of RCCs show EPO
immunohistochemically. EPO is a hormone secreted by the kidney and
fetal liver that stimulates RBC production from the BM. Expression
of EPO within the tumor tissue of RCC is more frequent in clear
cell type RCC (11) and has rarely
been reported in oncocytomas (4).
Expression of EPO has never been reported in cystic RCC or
chromophobe cell type RCC, as in the present case.
Hematopoiesis begins in the yolk sac and then takes
place transitorily in the liver (12,13).
In adults, HSCs are primarily seen in the BM, but also in the
circulatory system. Under certain circumstances, including
myelofibrosis, circulating HSCs from the peripheral blood filter
into the tissues. Colonization of filtered HSCs are considered to
reside in specific niches, which are specialized microenvironments,
such as osteoblastic cells, vascular endothelial cells, liver
sinusoidal cells and reticular cells (14–16).
With the collaboration of several other molecular findings
(17) fetal hematopoiesis as well
as EMH occurs mainly in the liver (5).
Based on aforementioned findings, it is not possible
to consider EMH, which is observed in RCC or other tissues as an
incidental finding. By contrast, it is possible to reflect on the
association between stem cell niches and the tissues where EMH
colonizes (microenvironments), i.e. certain tissues may signal
HSCs. Focus of EMH is frequently observed in hepatoblastomas and,
similar to RCC, EPO has been previously detected in the tumor
tissues of 11 out of 15 hepatoblastomas, i.e. tissues rich in EPO,
as in hepatoblastoma. In addition, RCC may signal HSCs to colonize
in these tissues. EMH is also observed within the breast following
therapy with granulocyte colony-stimulating factor (G-CSF) for
breast cancer (18). G-CSF is a
growth factor that stimulates BM to produce granulocytes and,
subsequently, stem cells stimulate the BM to release the
granulocytes into the blood. Breast cancer patients are treated
with G-CSF for chemotherapy-related BM suppression. Since adipose
tissue contains various types of adult stem cells as well as HSCs
(19), the elevated levels of HSCs
within the blood of the present patient may have preferentially
migrated to the adipose breast tissue.
In adults, HSCs are primarily observed in the BM,
but also in the blood. Notably, HSCs are the only immature cells
that pass through the BM. Under certain circumstances, i.e.
myelofibrosis, circulating HSCs from the peripheral blood
infiltrate into the tissues. Colonization of infiltrated HSCs are
considered to reside in specific niches, which are specialized
microenvironments, including osteoblastic, vascular endothelial and
reticular cells (16). A previous
study found that the colonization of HSCs to the BM was mainly
regulated by a factor named stromal-derived factor-1 (SDF1). In an
additional study, SDF1 and its receptor were found to be expressed
in liver sinusoidal endothelial cells (5). These two studies explain why fetal
hematopoiesis occurs mainly in the liver. Based on these molecular
observations and the present case report, we hypothesized that EPO
produced by RCC cells raises the levels of circulating HSCs and the
vascular endothelium-rich RCC acts as a niche that allows
circulating HSCs to colonize within the tumor and initiate BM
formation.
Bone trabecula has never been reported in RCC or
cystic RCC. The precise pathway of bone trabecula in the EMH focus
of the current study remains unknown. Bone trabecula has never been
reported in hepatoblastomas; however, similar bone trabecula
associated with EMH has been observed in the endometrium and
thyroid gland (3,20). Hematological disorders have not been
observed in these cases. Dystrophic calcification is a common
finding in nodular goiter specimens and the presence of bone in the
endometrial samples has been attributed to metaplasia following
abortion and local osteogenic factors (3,20).
Once bone trabecula is formed, it may serve as a niche for EMH,
which has been observed in these patients. Since hypercalcemia is
the most common paraneoplastic complication of RCC, bone trabecula
observed in RCC may be attributed to hypercalcemia, but the current
patient was normocalcemic and normocytic, pre- and
postoperatively.
Although EMH is observed secondary to BM
insufficiency, hematopoietic focus is observed without underlying
BM insufficiency as a consequence of the presence of factors
excreted by tumor cells. Hypercalcemia as well as polycythemia are
frequently observed in RCC patients and EPO produced by tumor cells
raises the level of blood stem cells that preferentially colonize
within highly vascularized RCC. Clear cell type RCC, particularly,
but also chromophobe and oncocytic cell type RCC, exhibit
biological properties that result in EMH focus.
Acknowledgements
In November 2012, part of the current case report
was presented in the poster section of the 22nd National Congress
of Pathology (Manavgat, Antalya, Turkey). The authors would like to
thank Dr Iclal Erdem Toslak for assistance in the radiological
evaluation of the present case.
References
|
1
|
Koch CA, Li CY, Mesa RA and Tefferi A:
Nonhepatosplenic extramedullary hematopoiesis: associated diseases,
pathology, clinical course, and treatment. Mayo Clin Proc.
78:1223–1233. 2003. View Article : Google Scholar
|
|
2
|
Palatnik A, Narayan R and Walters M:
Extramedullary hematopoiesis involving uterus, fallopian tubes, and
ovaries, mimicking bilateral tuboovarian abscesses. Int J Gynecol
Pathol. 31:584–587. 2012. View Article : Google Scholar
|
|
3
|
Akbulut S, Yavuz R, Akansu B, Sogutcu N,
Arikanoglu Z and Basbug M: Ectopic bone formation and
extramedullary hematopoiesis in the thyroid gland: report of a case
and literature review. Int Surg. 96:260–265. 2011. View Article : Google Scholar
|
|
4
|
Radopoulos D, Tzakas K and Tahmatzopoulos
A: A rare case of renal oncocytoma associated with erythrocytosis:
case report. BMC Urol. 6:262006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mendt M and Cardier JE: Stromal-derived
factor-1 and its receptor, CXCR4, are constitutively expressed by
mouse liver sinusoidal endothelial cells: implications for the
regulation of hematopoietic cell migration to the liver during
extramedullary hematopoiesis. Stem Cells Dev. 21:2142–2151. 2012.
View Article : Google Scholar
|
|
6
|
McGee SJ, Havens AM, Shiozawa Y, Jung Y
and Taichman RS: Effects of erythropoietin on the bone
microenvironment. Growth Factors. 30:22–28. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kumar R, Abbas A, Fausto N and Aster JC:
Diseases of white blood cells, lymph nodes, spleen, and thymus.
Robbins and Cotran Pathologic Basis of Disease. 8th edition.
Saunders Elsevier; Philadelphia, PA: pp. 589–638. 2010
|
|
8
|
Policarpio-Nicolas ML, Bregman SG, Ihsan M
and Atkins KA: Mass-forming extramedullary hematopoiesis diagnosed
by fine-needle aspiration cytology. Diagn Cytopathol. 34:807–811.
2006. View
Article : Google Scholar
|
|
9
|
Lemos LB, Baliga M, Benghuzzi HA and Cason
Z: Nodular hematopoiesis of the liver diagnosed by fine-needle
aspiration cytology. Diagn Cytopathol. 16:51–54. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Broucqsault A, Ouzzane A, Launay D, et al:
Renal extramedullary hematopoiesis. Prog Urol. 21:575–579. 2011.(In
French).
|
|
11
|
Wiesener MS, Münchenhagen P, Gläser M, et
al: Erythropoietin gene expression in renal carcinoma is
considerably more frequent than paraneoplastic polycythemia. Int J
Cancer. 21:2434–2442. 2007. View Article : Google Scholar
|
|
12
|
Tavian M, Biasch K, Sinka L, Vallet J and
Péault B: Embryonic origin of human hematopoiesis. Int J Dev Biol.
54:1061–1065. 2010. View Article : Google Scholar
|
|
13
|
Johns JL and Christopher MM:
Extramedullary hematopoiesis: a new look at the underlying stem
cell niche, theories of development, and occurrence in animals. Vet
Pathol. 49:508–523. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Robin C, Bollerot K, Mendes S, et al:
Human placenta is a potent hematopoietic niche containing
hematopoietic stem and progenitor cells throughout development.
Cell Stem Cell. 5:385–395. 2009. View Article : Google Scholar
|
|
15
|
Miyamoto K, Yoshida S, Kawasumi M, et al:
Osteoclasts are dispensable for hematopoietic stem cell maintenance
and mobilization. J Exp Med. 208:2175–2181. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schepers K, Hsiao EC, Garg T, Scott MJ and
Passegué E: Activated Gs signaling in osteoblastic cells alters the
hematopoietic stem cell niche in mice. Blood. 120:3425–3435. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li J, Zhao Z, Carter C, Ehrlich LI,
Bedford MT and Richie ER: Coactivator-associated arginine
methyltransferase 1 regulates fetal hematopoiesis and thymocyte
development. J Immunol. 190:597–604. 2013. View Article : Google Scholar
|
|
18
|
Wang J and Darvishian F: Extramedullary
hematopoiesis in breast after neoadjuvant chemotherapy for breast
carcinoma. Ann Clin Lab Sci. 36:475–478. 2006.PubMed/NCBI
|
|
19
|
Han J, Koh YJ, Moon HR, et al: Adipose
tissue is an extramedullary reservoir for functional hematopoietic
stem and progenitor cells. Blood. 115:957–964. 2010. View Article : Google Scholar
|
|
20
|
Singh P, Kapur K, Singla S and Naz N:
Endometrial osseous metaplasia and mature bone formation with
extramedullary hematopoiesis. J Hum Reprod Sci. 4:56–57. 2011.
View Article : Google Scholar : PubMed/NCBI
|