Introduction
Pancreatic cancer is one of the most lethal
malignant diseases with the poorest prognosis and is the fourth
leading cause of tumor-associated mortality in the industrialized
world (1). Due to the absence of
specific symptoms and the lack of early detection, pancreatic
cancer is usually diagnosed at an advanced and incurable stage.
Therefore, the median overall survival is only 5–6 months after
conventional therapies for locally advanced and metastatic disease,
and the 5-year survival rate is only 5% (2–4). Such
a short survival rate is primarily due to late diagnosis, intrinsic
and extrinsic drug resistance, which contributes to tumor
recurrence and metastasis. Epithelial to mesenchymal transition
(EMT) represents a fundamental step in tumor invasion and
metastasis (5). Tumor cells
involved in EMT lose epithelial cell adhesion molecules and start
to express mesenchymal-specific cytoskeletal components (6). This process is followed by
morphological changes from a flattened epithelial to an elongated
fibroblastic phenotype.
MicroRNAs (miRNAs or miRs) have important regulatory
roles in controlling proliferation, development, differentiation
and apoptosis through a fine modulation of key players in these
cellular pathways (7). Several miR
families have been implicated in EMT transition and EMT regulators
differentially modulate their expression in healthy tissues
compared to invasive tumors (8).
miR-203 is a keratinocyte-derived miR that promotes epithelial
differentiation from proliferative basal progenitors in the dermis
by suppressing p63, a member of the p53 family (9,10).
miR-203 is overexpressed in pancreatic adenocarcinoma compared with
levels in normal pancreatic tissues and chronic pancreatitis,
suggesting that miR-203 may be linked to specific characteristics
of tumors and their progression patterns (11). miR-203 was also upregulated in the
pancreatic cancer cells, as shown by miR array analysis, compared
with normal human pancreatic duct epithelial cells, suggesting that
miR-203 expression is a new prognostic marker in pancreatic
adenocarcinoma patients (12).
miR-203 transcription is specifically repressed by the EMT
activator Zeb-1, contributing to the invasive and metastatic
behavior of pancreatic and colorectal cancer cells (13).
In this study we show that miR-203 inhibits cancer
cell migration, invasion and EMT transition, in pancreatic cancer.
We also showed that miR-203 inhibits cell migration and invasion
via caveolin-1. The present study suggests that miR-203 expression
may be a useful indicator of the metastatic potential and provide a
new therapeutic target in this common malignancy.
Materials and methods
Cell culture
The Panc-1 cell line, which was derived from an
invasive intraductal extension of a primary tumor, had an
intermediate expression level of caveolin-1 and was obtained from
the Typical Culture Preservation Commission Cell Bank (Chinese
Acadamy of Sciences, Shanghai. China). The stable human pancreatic
cancer Panc-1 cell lines were cultured in DMEM medium (Invitrogen
Life Technologies, Carlsbad, CA, USA) supplemented with 5% fetal
bovine serum (FBS), 2 mmol/l glutamine, 50 units/ml penicillin and
50 μg/ml streptomycin (Invitrogen Life Technologies). The cells
were maintained in a 5% CO2-humidified atmosphere at
37ºC.
Transfection of miRNA mimics or small
interfering RNA (siRNA)
Panc-1 cells were seeded at a density of
2×105 cells per well in six-well plates and transfected
with miR-203 mimics or negative miRNA control (GenePharma,
Shanghai, China), or human caveolin-1 siRNA or a control siRNA
(BIONEEC, Shanghai, China) using the using Lipofectamine 2000
(Invitrogen), following the manufacturer’s instructions. The media
were removed after a 24-h transfection and the cells were incubated
in media containing 5% FBS for an additional 24 h.
Wound healing assay
A wound-healing assay was performed to examine the
capacity for cell migration. Briefly, after the cells grew to 90%
confluence in six-well plates, a single scratch wound was generated
with a 200-μl disposable pipette tip. The scratch wounds were
photographed over 7 h with a Nikon inverted microscope (Nikon,
Tokyo, Japan) with an attached digital camera (DXM 1200, Nikon),
and the scratch widths were quantitated with the ImageJ software
(rsbweb.nih.gov/ij). The data were plotted as the
percentage of wound closure, setting the initial scratch width as
100%.
Cell invasion assay
The Transwell chambers (Millipore, Billerica, MA,
USA) (8-μm pore size) were coated with Matrigel (BD Biosciences,
Franklin Lakes, NJ, USA) (15 μg/filter). Cells (2.0×104)
in serum-free medium were plated into the upper chamber and the
bottom wells were filled with complete medium. The cells were
allowed to invade across the Matrigel-coated membrane for 72 h.
Following incubation, the cells were removed from the upper surface
of the filter by scraping with a cotton swab. The invaded cells
that adhered to the bottom of the membrane were fixed with methanol
and stained with DAPI. The number of cells that penetrated the
membrane was determined by counting the mean cell number of five
randomly selected high-power fields.
Quantitative polymerase chain reaction
(qPCR)
RNA was extracted using TRIzol (Invitrogen) or
miRVANA (Ambion, Austin, TX, USA) kits according to the
manufacturer’s instructions. Complementary DNA (cDNA) was generated
with the High-Capacity cDNA Reverse Transcription kit (Roche
Diagnostics GmbH, Mannheim, Germany). The quantitative analysis of
the change in expression levels was calculated by the qPCR machine
(iQ5, Bio-Rad, Hercules, CA, USA). qPCR was used to quantify the
mRNA expression. The reactions were performed in duplicate and the
Δ-Δ-cycle threshold values were calculated on the basis of the
average of the normalization genes and the results were normalized
to the average of the results obtained for glyceraldehyde
3-phosphate dehydrogenase (GAPDH) or RNU6B.
Western blot analysis
The protein expression levels were assessed using
western blot analysis. In brief, total cell lysates from different
experiments were obtained by lysing the cells in RIPA buffer. The
total cell lysates were separated on SDS-PAGE gels, transferred to
PVDF membranes (Millipore), immunoblotted with antibodies
[anti-snail rabbit polyclonal antibody, anti-ZO-1 rabbit polyclonal
antibody, anti-β catenin rabbit polyclonal antibody, anti-vimentin
rabbit polyclonal antibody, anti-fibronectin rabbit polyclonal
antibody, anti-caveolin-1 rabbit polyclonal antibody, (all
antibodies supplied by Proteintech, Wuhan, China), and anti-GAPDH
monoclonal antibody (Bioworld, Nanjing, China)] and visualized
using an enhanced chemiluminescence detection system (Amersham
Biosciences, Chalfont St Giles, UK). The protein bands were
quantitated by densitometry using gel analysis software ImageJ. The
values were normalized to GAPDH expression.
Statistical analysis
The results presented are the average of at least
three experiments, each performed in triplicate with standard
deviations. Statistical analyses were performed by analysis of
variance followed by Tukey’s multiple comparison test or Student’s
t-test using SPSS 15.0. P<0.05 was considered to indicate a
statistically significant result and is indicated with
asterisks.
Results
miR-203 inhibited cell migration and
invasion in Panc-1 cells
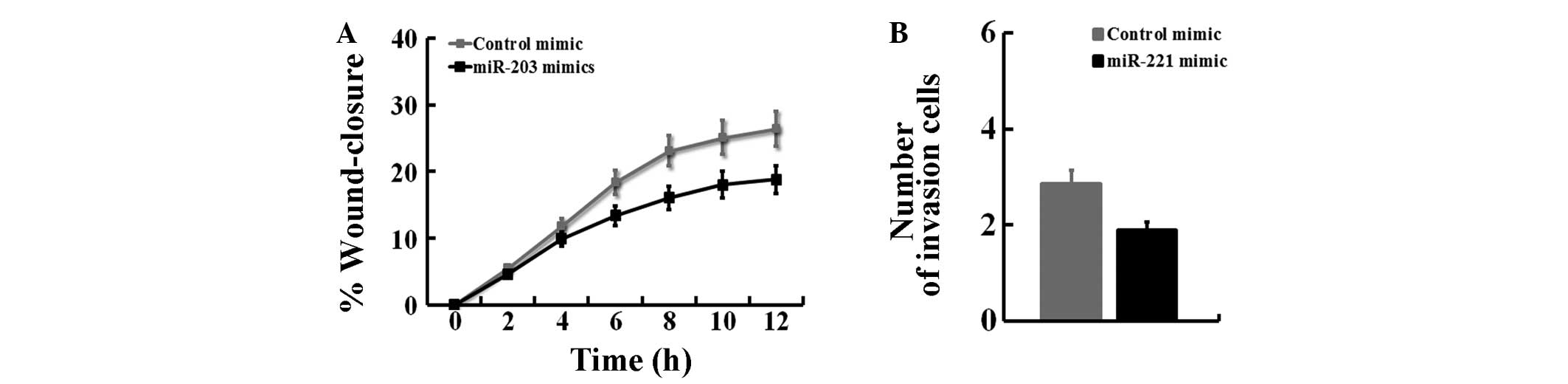
We first investigated whether miR-203 repressed cell
migration using an in vitro scratch-wound assay. One day
after the Panc-1 cells were treated with control or miR-203 mimics,
a single scratch wound was created in the well, and the time-course
of wound closure was monitored (Fig.
1A). In Panc-1 cells, treatment with miR-203 mimics strongly
inhibited cell migration. To determine whether miR-203 affected the
invasive ability of Panc-1 cells, we performed cell invasion assays
using a Transwell system. The results of the cell invasion assay
indicated that miR-203 inhibited the invasiveness of Panc-1 cells
compared with blank cells, as indicated by a marked decrease in the
number of cells that invaded the bottom well (P<0.05, Fig. 1B). These results suggest that
miR-203 suppresses cell invasion in Panc-1 cells.
miR-203 altered the expression of
associated proteins in Panc-1 cells
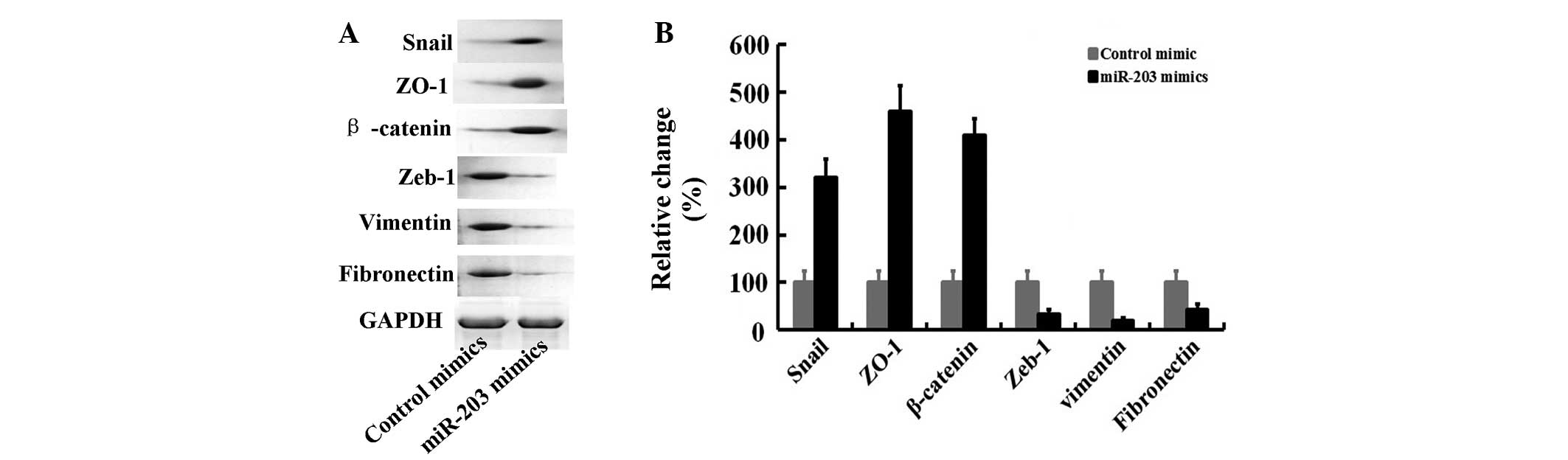
In this study, using western blot analysis, we also
observed that miR-203 mimic treatment for 24 h significantly
upregulated the expression of epithelial markers (Snail, ZO-1 and
β-catenin) followed by a decrease of mesenchymal marker expression
(Zeb-1, vimentin and fibronectin) (Fig.
2). These results suggest that miR-203 altered the expression
of associated proteins of the EMT phenotype in Panc-1 cells.
Regulation of caveolin-1 expression by
miR-203 in Panc-1 cells
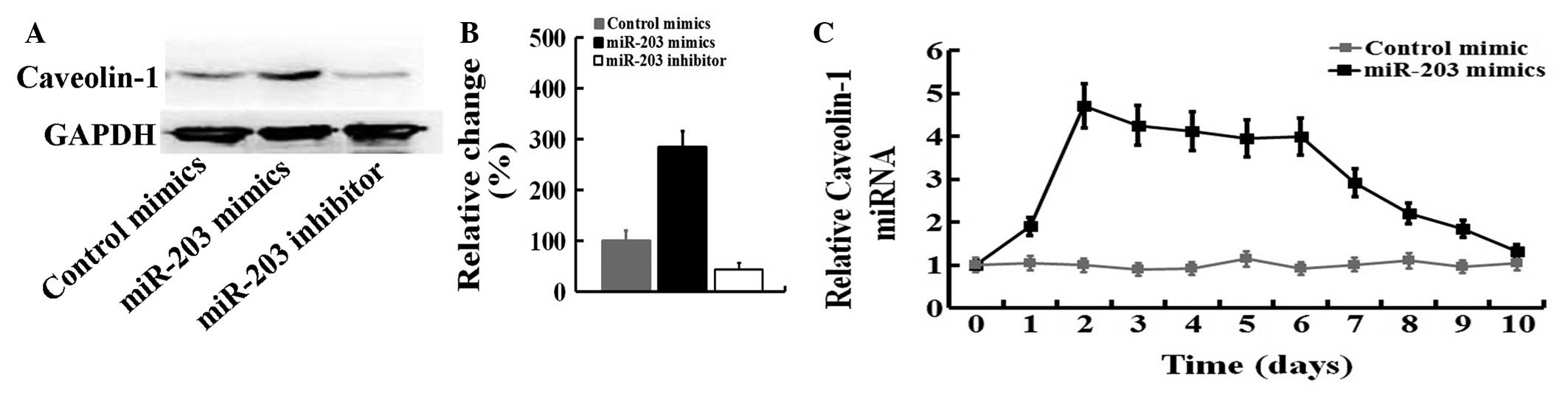
We investigated whether the caveolin-1 mRNA or
protein levels are modulated by miR-203 in Panc-1 cells. We
observed that the caveolin-1 protein level was increased by
approximately 330% (Fig. 3A and B)
upon treatment of Panc-1 cells with miR-203 for 24 h under
conditions that significantly inhibited the expression of
mesenchymal markers (Fig. 2). By
contrast, miR-203 inhibitor was able to inhibit caveolin-1 protein
expression. A time-course of the expression of caveolin-1 was
examined after miR-203 mimics-treatment in Panc-1 cells. Caveolin-1
mRNA was induced 4.7-fold after 2 days of treatment with miR-203
mimics and gradually decreased after 7 days (Fig. 3C), suggesting that caveolin-1 is
likely to be regulated by miR-203 signaling.
Downregulation of caveolin-1 expression
promoted cell migration and invasion in Panc-1 cells
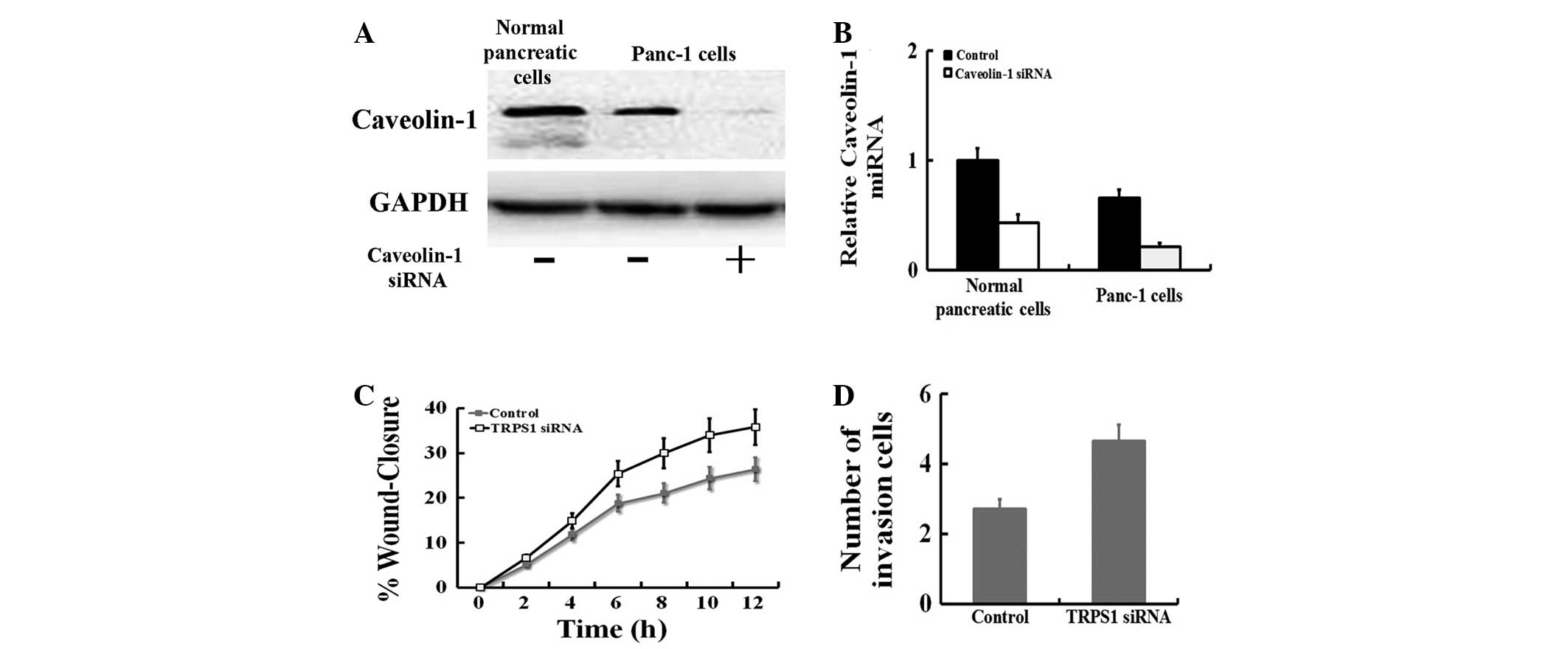
The baseline mRNA and protein levels of caveolin-1
in Panc-1 cells and normal pancreatic cells were determined by qPCR
and western blotting. The results showed that the mRNA and protein
of caveolin-1 was highly expressed in Panc-1 cells compared with
normal pancreatic cells (P<0.05; Fig. 4A). Thus, Panc-1 cells were
transiently transfected with caveolin-1 siRNA. Compared with the
blank (no siRNA) Panc-1 cells, the expression of FoxM1 was markedly
suppressed in cells transfected with caveolin-1 siRNA at the mRNA
(P<0.05) and protein levels. In this study, we also observed
that caveolin-1 siRNA treatment significantly promoted cell
migration and invasion in Panc-1 cells (Fig. 4C and D).
miR-203 inhibits the EMT by targeting
caveolin-1
To assess whether caveolin-1 is responsible for the
miR-203-dependent inhibition of cell migration and invasion, the
miR-203 mimic was transfected into Panc-1 cells treated with
caveolin-1 siRNA, and then to examine whether induction of
caveolin-1 by miR-203 plays a role in cell migration (Fig. 5A) and invasion (Fig. 5B). When caveolin-1 was silenced by
caveolin-1 siRNA, miR-203 did not suppress cell migration and
invasion. By contrast, transfection of the miR-203 mimic alone
significantly elevated cell migration (Fig. 1A) and invasion (Fig. 5B). The protein of Zeb-1, fibronectin
and vimentin were examined by western blot analysis (Fig. 5C). We observed that exogenous
miR-203 altered the expression of associated proteins following
caveolin-1 knockdown by siRNA. These results indicate that
caveolin-1 is essential for miR-203-dependent cell migration and
invasion.
Discussion
miR-203 is an antiproliferative microRNA involved in
skin differentiation that targets the 3′-UTR of the
‘stemness-maintaining’ transcription factor ΔNp63α (14). The downregulation of miR-203
expression has been described in several types of cancer, including
hepatocellular carcinomas (15),
breast cancer (16) and pancreatic
cancer (12). miR-203 acts as a
tumor suppressor in chronic myelogenous leukemias, acute
lymphoblastic leukemias and in hepatocellular carcinomas where its
expression is silenced by chromosomal deletion or promoter CpG
island hypermethylation (17).
miR-203 transcription is specifically repressed by the EMT
activator Zeb-1, contributing to the invasive and metastatic
behavior of pancreatic and colorectal cancer cells (16).
A previous study showed that miR-203 is
downregulated in metastatic pancreatic cancer cell lines compared
to normal epithelial pancreatic cells, a result that suggests that
miR-203 deficiency contributes to prostate cancer progression and
metastasis (12). Previous results
have correlated miR-203 downregulation with increased
proliferative, invasive and metastatic potential of transformed
cells in other malignancies (14).
In the present study, we showed that this is also true in
pancreatic cancers and, using the metastatic cell line (Panc-1), we
investigated the molecular mechanisms downstream of miR-203. In
vitro wound healing assays and Transwell/Matrigel invasion
assays following miR-203 transfection in Panc-1 cells suggest that
its expression is sufficient to reduce migratory ability and
invasiveness. Overexpression of miR-203 in Panc-1 cells was
sufficient to induce upregulation of epithelial markers (Snail,
ZO-1 and β-catenin) and was followed by a decrease of mesenchymal
marker expression (Zeb-1, vimentin and fibronectin).
In our study, we have identified caveolin-1 as
miR-203 direct target mRNAs involved in these events. We observed
that the caveolin-1 mRNA or protein levels are modulated by miR-203
in Panc-1 cells. Previous studies indicated that caveolin-1 is a
crucial modulator of EMT and cell differentiation in pancreatic
cancer cells (18). Using
transfected with caveolin-1 siRNA suppress the expression in the
Panc-1 cells that showed the least caveolin-1 expression. The
absence of caveolin-1 expression was sufficient to promote
pancreatic cancer cell migration and invasion. When caveolin-1 was
silenced by caveolin-1 siRNA, miR-203 did not alter the level of
cell migration and invasion. We observed that exogenous miR-203
altered the expression of associated proteins after caveolin-1
knockdown by siRNA. Our data suggest that miR-203 inhibits cell
migration and invasion via caveolin-1 in pancreatic cancer
cells.
miR-203 pleiotropically regulates the expression of
a cohort of effectors involved in cancer stem cell self-renewal,
cytoskeletal remodeling and tissue-specific metastatic spreading
pathways. Our studies suggest that miR-203 may also be considered a
suppressor of cancer cell migration, invasion and EMT transition in
pancreatic cancer, and that miR-203 expression may be a useful
indicator of the metastatic potential and provide a new therapeutic
target in this common malignancy.
References
|
1
|
Michaud DS: Epidemiology of pancreatic
cancer. Minerva Chir. 59:99–111. 2004.
|
|
2
|
Ducreux M, Boige V and Malka D: Treatment
of advanced pancreatic cancer. Semin Oncol. 34(2 Suppl 1): S25–S30.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Goulart BH, Clark JW, Lauwers GY, Ryan DP,
Grenon N, Muzikansky A and Zhu AX: Long term survivors with
metastatic pancreatic adenocarcinoma treated with gemcitabine: a
retrospective analysis. J Hematol Oncol. 2:132009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Edwards BK, Brown ML, Wingo PA, Howe HL,
Ward E, Ries LA, Schrag D, Jamison PM, Jemal A, Wu XC, et al:
Annual report to the nation on the status of cancer, 1975–2002,
featuring population-based trends in cancer treatment. J Natl
Cancer Inst. 97:1407–1427. 2005.
|
|
5
|
Royer C and Lu X: Epithelial cell
polarity: a major gatekeeper against cancer? Cell Death Differ.
18:1470–1477. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Savagner P: The epithelial-mesenchymal
transition (EMT) phenomenon. Ann Oncol. 21(Suppl 7): vii89–vii92.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sassen S, Miska EA and Caldas C: MicroRNA:
implications for cancer. Virchows Arch. 452:1–10. 2008. View Article : Google Scholar
|
|
8
|
Joyce CE, Zhou X, Xia J, Ryan C, Thrash B,
Menter A, Zhang W and Bowcock AM: Deep sequencing of small RNAs
from human skin reveals major alterations in the psoriasis
miRNAome. Hum Mol Genet. 20:4025–4040. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yuan Y, Zeng ZY, Liu XH, Gong DJ, Tao J,
Cheng HZ and Huang SD: MicroRNA-203 inhibits cell proliferation by
repressing ΔNp63 expression in human esophageal squamous cell
carcinoma. BMC Cancer. 11:572011.
|
|
10
|
Furuta M, Kozaki KI, Tanaka S, Arii S,
Imoto I and Inazawa J: miR-124 and miR-203 are epigenetically
silenced tumor-suppressive microRNAs in hepatocellular carcinoma.
Carcinogenesis. 31:766–776. 2010. View Article : Google Scholar
|
|
11
|
Greither T, Grochola LF, Udelnow A,
Lautenschläger C, Würl P and Taubert H: Elevated expression of
microRNAs 155, 203, 210 and 222 in pancreatic tumors is associated
with poorer survival. Int J Cancer. 126:73–80. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ikenaga N, Ohuchida K, Mizumoto K, Yu J,
Kayashima T, Sakai H, Fujita H, Nakata K and Tanaka M: MicroRNA-203
expression as a new prognostic marker of pancreatic adenocarcinoma.
Ann Surg Oncol. 17:3120–3128. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wellner U, Schubert J, Burk UC,
Schmalhofer O, Zhu F, Sonntag A, Waldvogel B, Vannier C, Darling D,
zur Hausen A, et al: The EMT-activator ZEB1 promotes tumorigenicity
by repressing stemness-inhibiting microRNAs. Nat Cell Biol.
11:1487–1495. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Viticchiè G, Lena AM, Latina A, Formosa A,
Gregersen LH, Lund AH, Bernardini S, Mauriello A, Miano R, Spagnoli
LG, et al: MiR-203 controls proliferation, migration and invasive
potential of prostate cancer cell lines. Cell Cycle. 10:1121–1131.
2011.PubMed/NCBI
|
|
15
|
Wei W, Wanjun L, Hui S, Dongyue C, Xinjun
Y and Jisheng Z: miR-203 inhibits proliferation of HCC cells by
targeting survivin. Cell Biochem Funct. 31:82–85. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang Z, Zhang B, Li W, Fu L, Fu L, Zhu Z
and Dong JT: Epigenetic silencing of miR-203 upregulates SNAI2 and
contributes to the invasiveness of malignant breast cancer cells.
Genes Cancer. 2:782–791. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bueno MJ, Pérez de Castro I, Gómez de
Cedrón M, Santos J, Calin GA, Cigudosa JC, Croce CM,
Fernández-Piqueras J and Malumbres M: Genetic and epigenetic
silencing of microRNA-203 enhances ABL1 and BCR-ABL1 oncogene
expression. Cancer Cell. 13:496–506. 2008. View Article : Google Scholar
|
|
18
|
Salem AF, Bonuccelli G, Bevilacqua G,
Arafat H, Pestell RG, Sotgia F and Lisanti MP: Caveolin-1 promotes
pancreatic cancer cell differentiation and restores membranous
E-cadherin via suppression of the epithelial-mesenchymal
transition. Cell Cycle. 10:3692–3700. 2011. View Article : Google Scholar
|