Introduction
Ovarian cancer is the fifth most common cause of
cancer-related mortality in females and accounts for the highest
tumor-associated mortality of gynecological malignancies (1). Late diagnosis of ovarian cancer and
ineffective chemotherapy has resulted in the number of mortalities
from ovarian cancer exceeding that of any other cancer of the
female reproductive system (2).
More than 70% of patients with ovarian cancer are diagnosed in the
advanced stages (International Federation of Gynecology and
Obstetrics stages III and IV), where the cancer has spread beyond
the ovary and treatment becomes increasingly ineffective (3). Currently, the preferred treatment of
human ovarian cancer is cisplatin-centered chemotherapy, which can
markedly decrease the mortality rate and lengthen the survival time
for patients. However, a major obstacle in ovarian cancer treatment
is the development of drug resistance. The mechanism responsible
for cisplatin chemoresistance in ovarian cancer remains poorly
understood (4).
Recently, twist basic helix-loop-helix transcription
factor 2 (Twist2), a highly homologous protein of Twist1, has
attracted increasing attention. Overexpression of Twist2 has been
reported to correlate with poor prognosis of colorectal cancer, as
well as head and neck squamous cell carcinomas (5,6). Zhou
et al (7) and Li et
al (8) indicated that Twist2 is
associated with the invasion and metastasis of salivary adenoid
cystic carcinoma, cervical malignant conversion and tumor
metastasis separately. In addition, Koh et al (9) reported that Twist2 increased the
resistance to galectin-1-mediated apoptosis, which facilitated the
progression of T cells into tumors. Twist2 is also considered to be
an inducer of epithelial-mesenchymal transition (EMT) (10), a well-known process involved in
embryogenesis (11), tumor
invasion, metastasis (12) and drug
resistance (13). Evidently, Twist2
plays a critical role in human tumors. Thus, the present study
investigated Twist2 expression patterns in ovarian cancer, the role
in chemoresistance and also a possible underlying mechanism.
The aim of the present study was to investigate the
Twist2 expression pattern in a cisplatin-sensitive ovarian cancer
cell line (OV2008) and the resistant variant (C13K), and to
determine the effect Twist2 has on the regulation of cell growth
and cisplatin-induced apoptosis in ovarian cancer cells.
Furthermore, the effect Twist2 has on the protein kinase B
(AKT)/glycogen synthase kinase (GSK)-3β signaling pathway was
investigated.
Materials and methods
Cell lines and culture
A cisplatin-sensitive ovarian cancer cell line
(OV2008) and the resistant variant (C13K) were supplied by Dr
Rakesh Goel from the Ottawa Regional Cancer Center (Ottawa, ON,
Canada). Cells were maintained in RPMI-1640 complete medium
supplemented with 2 mM glutamine and 10% fetal bovine serum (FBS)
at 37°C in a humidified atmosphere containing 5%
CO2.
Chemicals and antibodies
RPMI-1640, FBS, Lipofectamine 2000 and TRIzol
reagent were purchased from Invitrogen Life Technologies (Carlsbad,
CA, USA). Cisplatin was obtained from QiLu Pharmaceutical Co., Ltd.
(Jinan, China) and the phosphoinositide 3-kinase inhibitor,
LY294002, methyltetrazolium (MTT) and dimethyl sulfoxide (DMSO)
were obtained from Sigma-Aldrich (St. Louis, MO, USA). pcDNA3.1(+)
(vector), pcDNA3.1(+)/Twist2 (Twist2), Twist2 siRNA (si-Twist2) and
negative control siRNA (si-NC) were purchased from Guangzhou
RiboBio, Co., Ltd. (Guangzhou, China). Primary antibody against
Twist2 was obtained from Abcam, Inc. (Burlingame, CA, USA), while
those against AKT, AKT Ser-473, GSK-3β and GSK-3β Ser-9 were
obtained from Cell Signaling Technology, Inc. (Beverley, MA, USA).
Polymerase chain reaction (PCR) primers were purchased from
Invitrogen Life Technologies.
Cell transfection
For transient transfection, cells were seeded in
six-well plates at a density of 5×104 cells/well and
incubated at 37°C in an atmosphere with 5% CO2 for 12 h.
When the cells were 30–50% confluent, they were transfected using
Lipofectamine 2000 transfection reagent, according to the
manufacturer’s instructions.
Cell viability assay and cellular growth
rate
Cell viability and IC50 values (drug
concentration causing 50% inhibition of cell growth) were analyzed
with the MTT assay. For transient transfection, cells were seeded
at 5×103 cells/well in 96-well plates one day prior to
transfection. The MTT assay was performed prior to transfection and
then at 24, 48, 72 and 96 h following transfection. For the assay,
20 μl MTT (5 mg/ml) was added to each well and the cells were
incubated for 4 h prior to the addition of 180 μl DMSO for 20 min.
The absorbance of the wells was then measured with a microplate
reader at a test wavelength of 570 nm and a reference wavelength of
630 nm. Appropriate controls lacking cells were included to
determine the background absorbance. The response to drug treatment
was assessed by standardizing the treatment groups to the untreated
control. Cellular growth curves were plotted using the cellular
viability values assessed by the MTS, a colorimetric method used to
determine the number of viable cells using
[3-(4,5-dimethyl-2-yl)-5-(3-carboxymethoxyphenyl)-2(4-sulfophenyl)-2H-tetrazolium,
inner salt; MTS], according to the manufacturer’s instructions
(Promega, Madison, WI, USA).
Quantitative PCR (qPCR)
Total RNA was extracted from cultured cells using
TRIzol reagent. For each sample, 2 μg RNA was reverse transcribed
using a ReverTra Ace qPCR kit (Toyobo, Co., Ltd., Osaka, Japan),
according to the manufacturer’s instructions. qPCR was performed
using SYBR Green qPCR Master Mix (DBI, Inc., Hazleton, PA, USA) on
a CFX Connect real-time system (Bio-Rad Laboratories, Inc.,
Berkeley, CA, USA). The conditions were as follows: 40 cycles of
three-step PCR (95°C for 40 sec, 60°C for 50 sec and 72°C for 30
sec) following initial denaturation (95°C for 5 min). All primers
were supplied by Invitrogen Life Technologies. Primer sequences
were as follows: Forward, 5′-GAGCGACGAGATGGACAATAAGA-3′ and
reverse, 5′-ATGCGCCACACGGAGAA-3′ for Twist2 (product size, 84 bp);
forward, 5′-TGCACCACCAACTGCTTAGC-3′ and reverse
5′-GGCATGGACTGTGGTCATGAG-3′ for GAPDH (housekeeping gene; product
size, 87 bp).
Western blotting
Following transfection, cells were lysed in ice-cold
radioimmunoprecipitation assay lysis buffer containing a protease
inhibitor cocktail. A total of 60 μg protein was separated by 10%
SDS-PAGE and transferred to polyvinylidene fluoride membranes.
Following blocking with Tris-buffered saline containing 5% skimmed
milk at room temperature for 1 h, the membranes were incubated with
primary antibody at 4°C for 12 h. The membranes were then incubated
with horseradish peroxidase-conjugated anti-mouse/rabbit antibodies
at a dilution of 1:3,000 at room temperature for 1 h. Signals were
detected on X-ray film using an enhanced chemiluminescent detection
system (Pierce Biotechnology, Inc., Rockford, IL, USA). Loading
differences were normalized against a monoclonal GAPDH
antibody.
Flow cytometry
All samples were washed in phosphate-buffered saline
and resuspended in 200 μl binding buffer. Next, 5 μl
Annexin-V-fluorescein isothiocyanate and 10 μl propidium iodide
(PI; 1 μg/ml) were added and the cell suspension was incubated in a
dark chamber at room temperature for 1 h. Cell-cycle profiles were
then determined using a FACSCalibur flow cytometer (BD Biosciences,
Franklin Lakes, NJ, USA) and data were analyzed using CellQuest
software (BD Biosciences).
Statistical analysis
All experiments were repeated at least three times
and the data are expressed as the mean ± standard deviation.
P<0.05 was considered to indicate a statistically significant
difference. Statistical analysis was conducted with SPSS 18.0 for
Windows (SPSS, Inc., Chicago, IL, USA) using the Student’s
t-test.
Results
Chemoresistance increases in ovarian
cancer due to high expression levels of Twist2
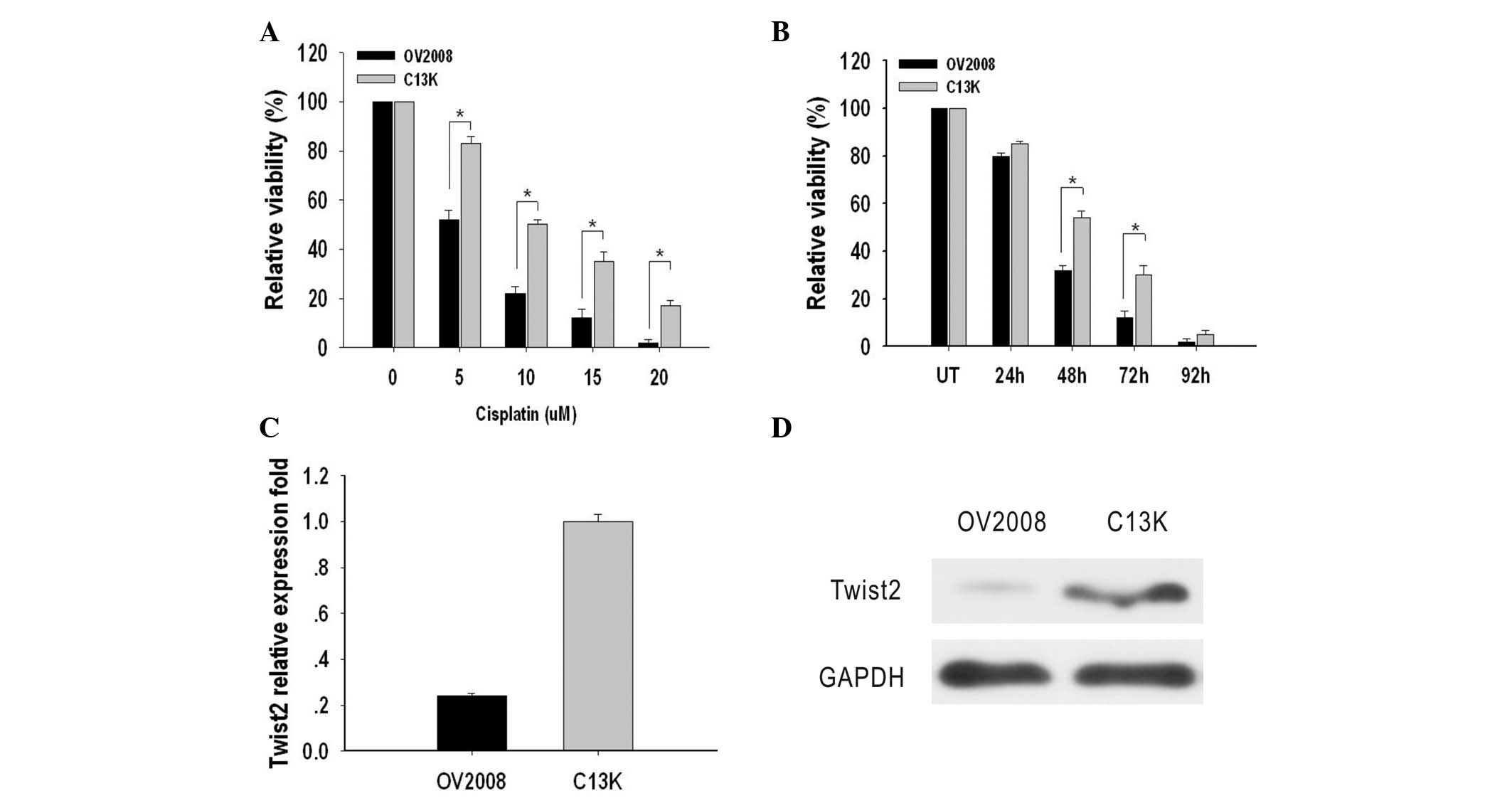
The cytotoxic effect of cisplatin on OV2008 and C13K
cells was determined by an MTT assay. IC50 values were
used to indicate the levels of cytotoxicity. The IC50
values in OV2008 and C13K cells were 5±0.3 μM and 10.0±1.2 μM,
respectively, which indicated that the OV2008 cells were more
sensitive to cisplatin-induced cytotoxicity compared with C13K
cells (Fig. 1A). Next, OV2008 and
C13K cells were treated with 10 μM cisplatin for 24, 48, 72 and 96
h in order to measure cell viability. The results indicated that
C13K cells were significantly more viable after 48 h (1.6 fold) and
72 h (2.5 fold) when compared with OV2008 cells. After 96 h, cell
death had occurred in ~95% of the cells in the two cell types
(Fig. 1B).
Expression levels of Twist2 in these two cell lines
were compared by qPCR and western blotting. The mRNA expression
level of Twist2 in OV2008 and C13K cells was detectable and
relative absorbance values were 0.24±0.012 and 1.00±0.03,
respectively. Protein expression of Twist2 in OV2008 and C13K cells
was assayed and relative absorbance values were 0.15±0.01 and
1.02±0.02, respectively. The mRNA and protein expression levels of
Twist2 in C13K cells were significantly higher compared with those
in OV2008 cells (P<0.05; Fig. 1C and
D).
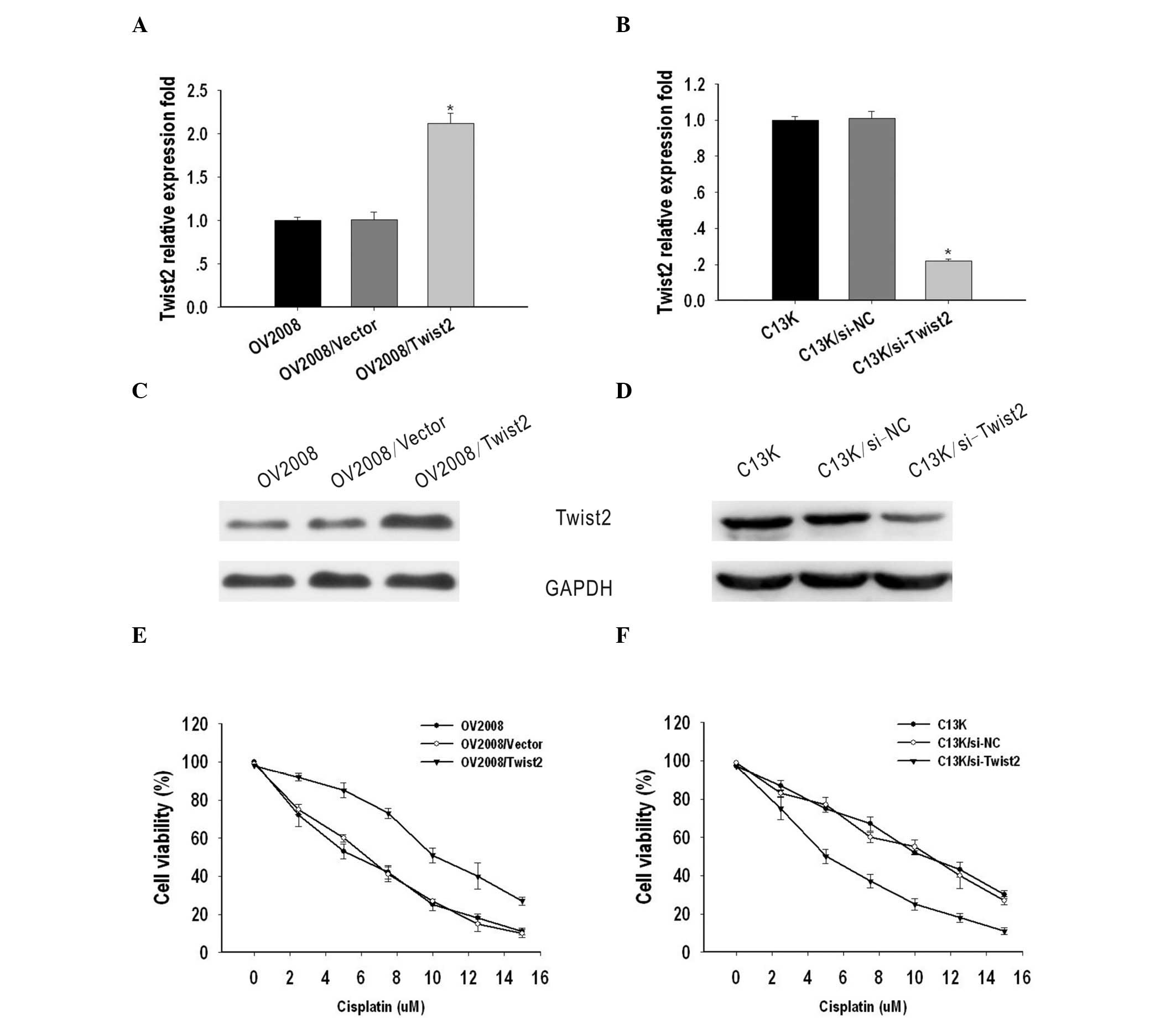
Twist2 confers resistance to cisplatin in
ovarian cancer cells
To investigate the possible role of Twist2 on the
sensitivity of ovarian cancer to cisplatin, OV2008 and C13K cells
were transiently transfected with vector/Twist2 or si-NC/si-Twist2,
respectively. Total RNA and protein were isolated and analyzed by
qPCR and western blotting 48 h after transfection. Compared with
the OV2008 and OV2008/vector cells, the expression of Twist2 was
markedly upregulated in OV2008/Twist2 cells at the mRNA and protein
level (Fig. 2A and C). By contrast,
Twist2 expression was suppressed in C13K cells transfected with
si-Twist2, when compared with C13K and C13K/si-NC cells (Fig. 2B and D).
To evaluate the biological significance of Twist2 on
cell sensitivity to cisplatin, an MTT assay was performed. The
IC50 values of cisplatin for OV2008, OV2008/vector and
OV2008/Twist2 cells were 5±0.12, 6±0.281 and 10±0.193 μM,
respectively, while the IC50 values for C13K, C13K/si-NC
and C13K/si-Twist2 cells were 10.21±0.12, 10.3±0.281 and 5.4±0.193
μM, respectively. These results indicate that Twist2 contributes to
cisplatin-resistance in ovarian cancer cells (Fig. 2E and F).
Twist2 regulates cisplatin-induced
apoptosis and cell growth in ovarian cancer
The effects of apoptosis were examined in Twist2
silenced ovarian cancer cells using Annexin-V and PI
double-staining and measured by flow cytometry. As shown in
Fig. 3A and C, the apoptotic rate
of OV2008/Twist2 (21.7%) was significantly lower than OV2008
(56.95%) and OV2008/vector (60.7%) cells. In addition, Twist2
silencing resulted in 81.01% of C13K cells being apoptotic, while
the apoptotic rate of C13K and C13K/si-NC cells was 15 and 18%,
respectively (P<0.05) (Fig. 3B and
D).
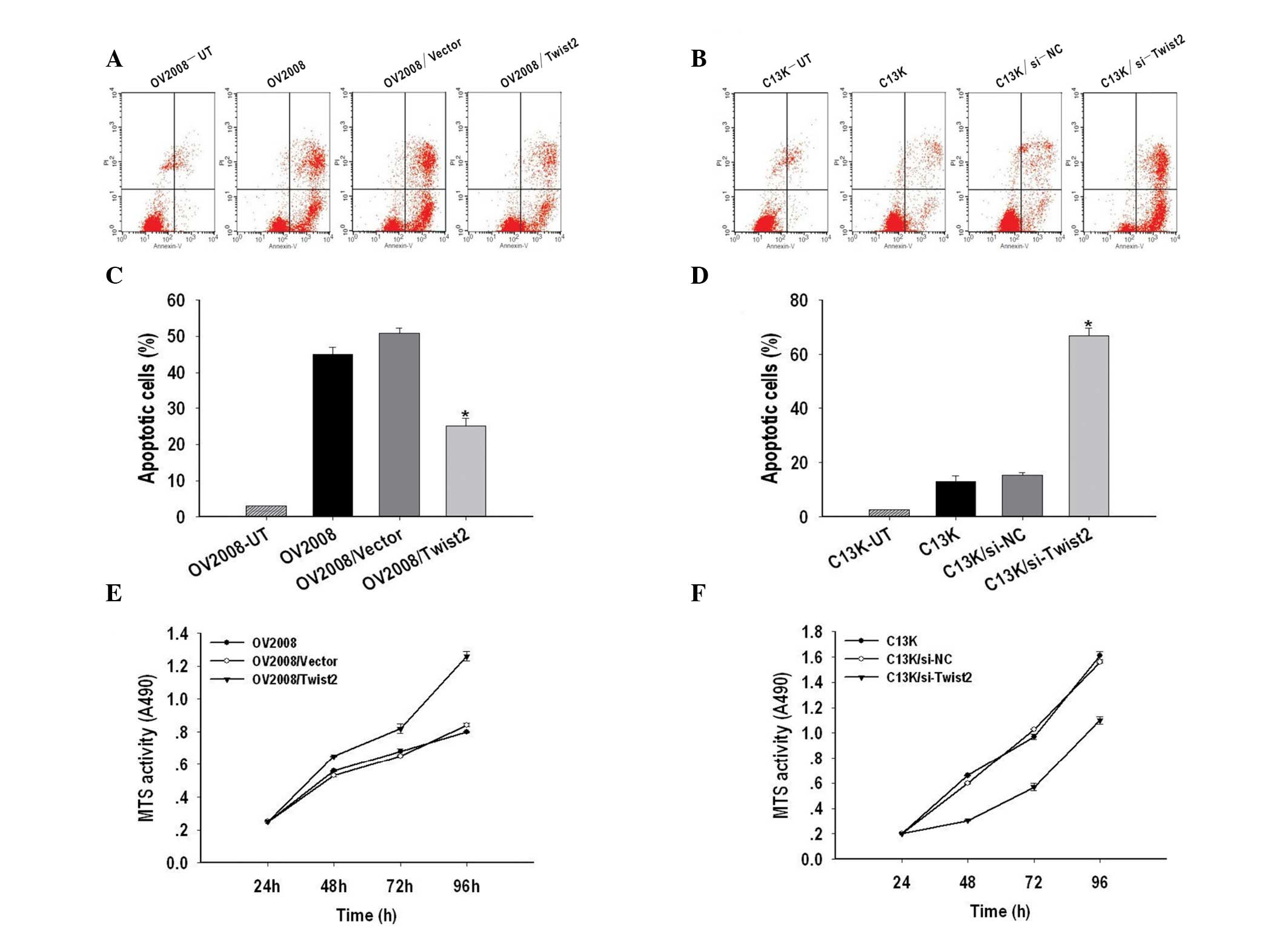 | Figure 3Twist2 regulates cisplatin-induced
apoptosis and cell growth in ovarian cancer. (A) Representative
FACS analyses for the induction of apoptosis in Twist2 on OV2008,
OV2008/Vector and OV2008/Twist2 cells were harvested at 48 h and
stained with Annexin-V-fluorescein isothiocyanate (FITC) and
propidium iodide (PI) followed by FACScan flow cytometric analysis.
(B) Representative FACS analyses for apoptosis induction of C13K,
C13K/si-NC and C13K/si-Twist2 cells were harvested at 48 h and
stained with Annexin-V-FITC and PI followed by FACScan flow
cytometric analysis. (C) Quantitative analysis of the population of
total apoptotic cells. The apoptotic ratio of OV2008/Twist2 was
significantly lower than those of OV2008 and OV2008/Vector cells.
(D) The apoptotic ratio of C13K/si-Twist2 was remarkably higher
than those of C13K and C13K/si-NC cells. Three independent
experiments were conducted and the data shown are the means ± SEM.
(E) The
3-(4,5-dimethyl-2-yl)-5-(3-carboxymethoxyphenyl)-2(4-sulfophenyl)-2H-tetrazolium,
inner salt] MTS assay was performed in order to assess the growth
of OV2008, OV2008/Vector and OV2008/Twist2 cells at 24, 48, 72 and
96 h, revealing a significant increase in the proliferation rate
for OV2008/Twist2 cells. (F) The MTS assay was performed to assess
the growth of C13K, C13K/si-NC and C13K/si-Twist2 cells at 24, 48,
72 and 96 h, showing a significant decrease of proliferation rate
for C13K/si-Twist2 cells. The relative ratio of cell proliferation
to untransfected cells was measured and the data shown are the
means ± SEM of three independent experiments. |
The role of Twist2 in the growth of ovarian cancer
cells was determined by an MTS assay. As shown in Fig. 3E and F, Twist2 upregulation promoted
cell growth of OV2008 cells, while downregulation of Twist2 by
transient transfection of si-Twist2 in C13K cells caused
significant inhibition of cell proliferation. These results
indicate that Twist2 regulates cisplatin-induced apoptosis and cell
growth in ovarian cancer.
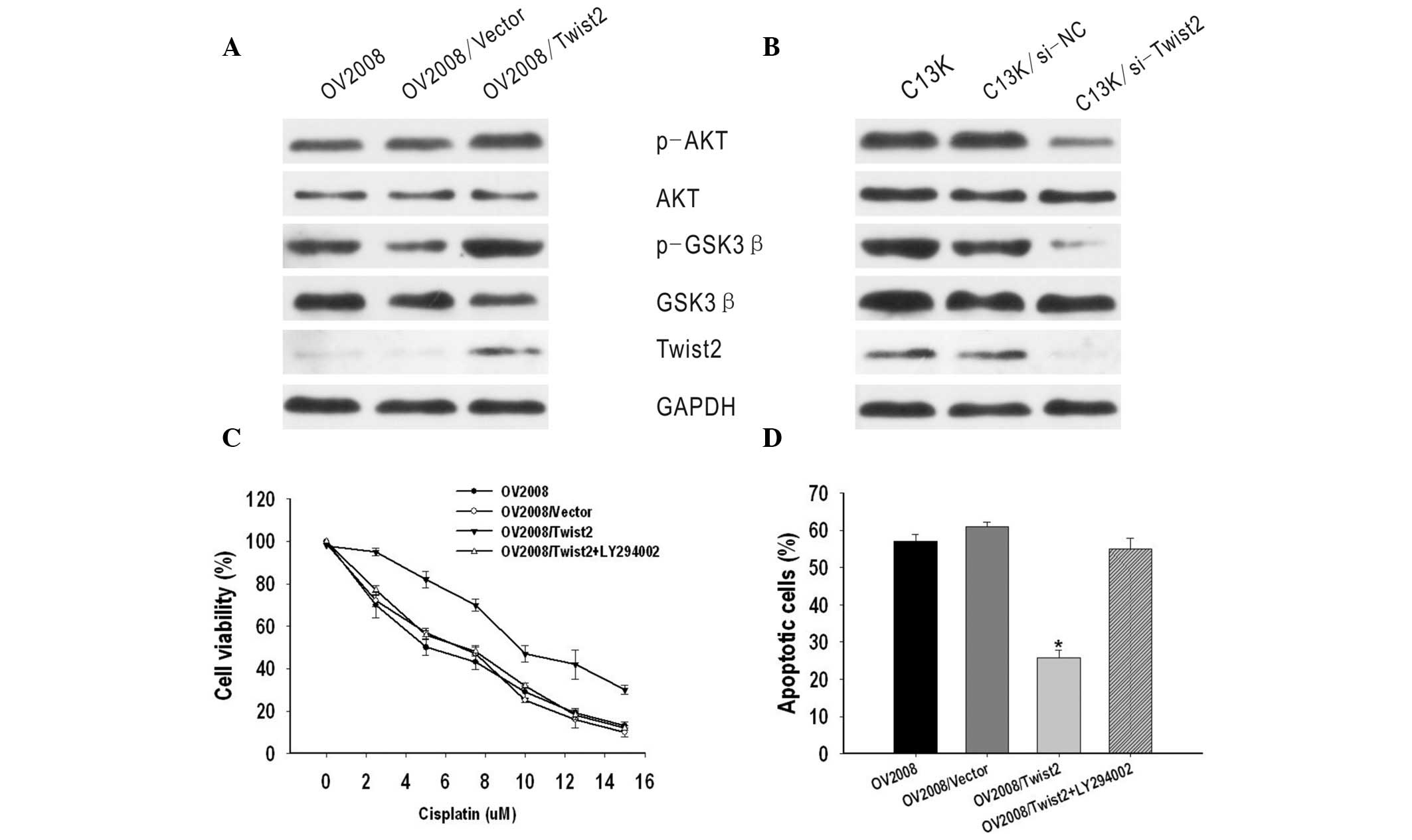
Twist2 mediates cisplatin resistance and
apoptosis via regulating the AKT/GSK-3β pathway
A previous study revealed that AKT/GSK-3β pathway
activation plays an important role in cisplatin resistance of
breast cancer (14). Thus, the
present study investigated the correlation between the AKT/GSK-3β
pathway and Twist2 expression in ovarian cancer. It was identified
that phosphorylated AKT and GSK-3β expression, but not total AKT or
GSK-3β expression, were markedly increased by the upregulation of
Twist2 in OV2008 cells, but decreased in C13K/si-Twist2 cells, as
compared with C13K and C13K/si-NC cells (Fig. 4A and B).
To further investigate the molecular mechanisms, an
inhibitor of PI3K/AKT (LY294002) was used. GSK-3β is the kinase
located downstream of the PI3K/AKT pathway. It was identified that
OV2008/Twist2 cells treated with LY294002 appeared to be more
sensitive to cisplatin than OV2008/Twist2 cells not treated with
the inhibitor. The IC50 value of OV2008/Twist2 +
LY294002 was markedly lower compared with that of OV2008/Twist2
cells (P<0.05) and similar to that of OV2008 and OV2008/vector
cells (P>0.05) (Fig. 4C).
Subsequent cisplatin treatment markedly enhanced apoptotic cell
death. In addition, the apoptotic ratio of OV2008/Twist2 + LY294002
was 54.92±3.01%, which was significantly higher compared with that
of OV2008/Twist2 (25.73±2.2%) (P<0.05) and similar to that of
OV2008 (57.07±1.92%) and OV2008/vector (60.95±1.34%) cells
(P>0.05) (Fig. 4D). These
results indicate that the AKT/GSK-3β signaling pathway may be
involved in Twist2-induced cisplatin-resistance.
Discussion
Ovarian cancer is the leading cause of mortality
among gynecological cancers. A major cause of the high mortality
rates in ovarian cancer is chemotherapy resistance, particularly
cisplatin resistance (15). Cancer
cells develop resistance to chemotherapy by inactivating apoptotic
factors and enhancing survival pathways that antagonize apoptosis
signals (16). The balance between
survival and apoptotic signals determines the sensitivity of cells
to chemotherapy. However, in ovarian cancer, the molecular
mechanisms leading to cisplatin chemoresistance remain poorly
understood.
Twist2 was first identified in 1995 and has been
shown to share high homology and overlapping expression patterns
with Twist1 (8,17). Extensive studies in previous years
have focused on Twist1 and have identified correlations between the
development of acquired metastatic ability, stem cell-like
characteristics and chemoresistance in various human cancers
(18–21). Although gene deletion experiments
have shown that Twist1 and Twist2 have specific functional
similarity and redundancy (22,23),
Tukel et al demonstrated that these two genes exhibit
non-redundant functions in skin and bone development, highlighting
the importance of studying Twist1 and Twist2 as separate entities
(24). Similarly to Twist1, Twist2
has previously been reported to be implicated in cell lineage
determination and differentiation (25). Upregulation of Twist2 expression has
been detected in a wide range of human cancers and Twist2 has
already been shown to be a significant molecule in specific solid
tumors (6). However, the role that
Twist2 plays in drug resistance and the possible underlying
mechanism in ovarian cancer has not yet been established.
In the present study, a pair of chemosensitive
(OV2008) and chemoresistant (C13K) ovarian cancer cell lines were
used to investigate the possible roles of Twist2 in the regulation
of cisplatin-mediated apoptosis and cisplatin resistance in human
ovarian epithelial cancer. C13K cells were found to be more
resistant to cisplatin-induced cytotoxicity than OV2008 cells. In
addition, Twist2 expression in the OV2008 cell line was
significantly lower compared with that in the C13K cell line. These
results indicate that the loss of Twist2 may be involved in
cisplatin sensitivity of ovarian cancer cells and may play an
important role in the chemoresistance of ovarian cancer cells.
However, the mechanism by which Twist2 contributes
to chemoresistance remains unclear. The fate of cancer cells in
response to a chemotherapeutic agent is a consequence of the
overall apoptotic capacity of the cell (26). Successful transfections of OV2008
cells with Twist2 and C13K cells with si-Twist2 were performed with
Lipofectamine 2000. It was found that upregulation of Twist2
expression significantly decreased cisplatin-induced apoptosis and
promoted cell growth. By contrast, downregulation of Twist2
expression was found to be an effective method of reversing the
resistance of C13K cells to cisplatin and sensitizing the cells to
cisplatin-induced apoptosis. Therefore, the results indicate that
combination chemotherapy targeting Twist2 expression is likely to
improve the treatment of ovarian cancer.
The PI3K/AKT antiapoptotic and survival pathway has
been reported to play a crucial role in cisplatin resistance
(27). The activation of AKT
requires phosphorylation at the Ser-473 and Thr-308 sites; thus,
the level of phosphorylated-AKT (Ser-473) represents the activity
of AKT (28). Phosphorylated AKT
promotes survival by phosphorylating and inactivating proapoptotic
factors, including GSK-3β (29). A
number of studies have shown that the activated AKT/GSK-3β pathway
participates in the EMT process and contributes to the aggressive
phenotype and chemoresistance in several human cancers (30–32).
Thus, in the present study, the AKT/GSK-3β pathway was investigated
to determine whether it played a role in Twist2-mediated
cisplatin-resistance in ovarian cancer. The results showed that the
phosphorylation of AKT at Ser-473 and GSK-3β at Ser-9 in
OV2008/Twist2 cells was markedly higher than that in OV2008 and
OV2008/vector cells. However, there was no difference in total AKT
and GSK-3β protein expression. In addition, LY294002, an inhibitor
of PI3K/AKT, effectively reversed Twist2-induced
cisplatin-resistance. Thus, targeting this signaling pathway may be
an effective approach to treating cisplatin-resistance in ovarian
cancer.
In conclusion, the present study demonstrates that
Twist2 plays a crucial role in the chemoresistance of ovarian
cancer. Downregulation of Twist2 expression facilitated apoptosis
and recovered the sensitivity of chemoresistant ovarian cancer
through the AKT/GSK-3β pathway. However, further clarification of
functional characterization is required. The results of the current
study provide support for this potential novel gene therapy with
Twist2 for the treatment of chemoresistant human ovarian
cancer.
Acknowledgements
The study was supported by a grant from the National
Natural Science Foundation of China (no. 81071663).
References
|
1
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar
|
|
2
|
Fraser M, Leung B, Jahani-Asl A, Yan X,
Thompson WE and Tsang BK: Chemoresistance in human ovarian cancer:
the role of apoptotic regulators. Reprod Biol Endocrinol. 1:662003.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jemal A, Siegel R, Ward E, Murray T, Xu J,
Smigal C and Thun MJ: Cancer statistics, 2006. CA Cancer J Clin.
56:106–130. 2006. View Article : Google Scholar
|
|
4
|
Yang X, Xing H, Gao Q, Chen G, Lu Y, Wang
S and Ma D: Regulation of HtrA2/Omi by X-linked inhibitor of
apoptosis protein in chemoresistance in human ovarian cancer cells.
Gynecol Oncol. 97:413–421. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yu H, Jin GZ, Liu K, Dong H, Yu H, Duan
JC, Li Z, Dong W, Cong WM and Yang JH: Twist2 is a valuable
prognostic biomarker for colorectal cancer. World J Gastroenterol.
19:2404–2411. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gasparotto D, Polesel J, Marzotto A,
Colladel R, Piccinin S, Modena P, Grizzo A, Sulfaro S, Serraino D,
Barzan L, Doglioni C and Maestro R: Overexpression of TWIST2
correlates with poor prognosis in head and neck squamous cell
carcinomas. Oncotarget. 2:1165–1175. 2011.PubMed/NCBI
|
|
7
|
Zhou C, Liu J, Tang Y, Zhu G, Zheng M,
Jiang J, Yang J and Liang X: Coexpression of hypoxia-inducible
factor-2α, TWIST2, and SIP1 may correlate with invasion and
metastasis of salivary adenoid cystic carcinoma. J Oral Pathol Med.
41:424–431. 2012.
|
|
8
|
Li Y, Wang W, Wang W, Yang R, Wang T, Su
T, Weng D, Tao T, Li W, Ma D and Wang S: Correlation of TWIST2
up-regulation and epithelial-mesenchymal transition during
tumorigenesis and progression of cervical carcinoma. Gynecol Oncol.
124:112–118. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Koh HS, Lee C, Lee KS, Park EJ, Seong RH,
Hong S and Jeon SH: Twist2 regulates CD7 expression and
galectin-1-induced apoptosis in mature T-cells. Mol Cells.
28:553–558. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ansieau S, Bastid J, Doreau A, Morel AP,
Bouchet BP, Thomas C, Fauvet F, Puisieux I, Doglioni C, Piccinin S,
Maestro R, Voeltzel T, Selmi A, Valsesia-Wittmann S, Caron de
Fromentel C and Puisieux A: Induction of EMT by twist proteins as a
collateral effect of tumor-promoting inactivation of premature
senescence. Cancer Cell. 14:79–89. 2008. View Article : Google Scholar
|
|
11
|
Acloque H, Adams MS, Fishwick K,
Bronner-Fraser M and Nieto MA: Epithelial-mesenchymal transitions:
the importance of changing cell state in development and disease. J
Clin Invest. 119:1438–1449. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: the next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Singh A and Settleman J: EMT, cancer stem
cells and drug resistance: an emerging axis of evil in the war on
cancer. Oncogene. 29:4741–4751. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Johnson-Holiday C, Singh R, Johnson EL,
Grizzle WE, Lillard JW Jr and Singh S: CCR9-CCL25 interactions
promote cisplatin resistance in breast cancer cell through Akt
activation in a PI3K-dependent and FAK-independent fashion. World J
Surg Oncol. 9:462011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ali AY, Farrand L, Kim JY, Byun S, Suh JY,
Lee HJ and Tsang BK: Molecular determinants of ovarian cancer
chemoresistance: new insights into an old conundrum. Ann N Y Acad
Sci. 1271:58–67. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cheng JQ, Jiang X, Fraser M, Li M, Dan HC,
Sun M and Tsang BK: Role of X-linked inhibitor of apoptosis protein
in chemoresistance in ovarian cancer: possible involvement of the
phosphoinositide-3 kinase/Akt pathway. Drug Resist Updat.
5:131–146. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mao Y, Zhang N, Xu J, Ding Z, Zong R and
Liu Z: Significance of heterogeneous Twist2 expression in human
breast cancers. PLoS One. 7:e481782012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tiwari N, Gheldof A, Tatari M and
Christofori G: EMT as the ultimate survival mechanism of cancer
cells. Semin Cancer Biol. 22:194–207. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Smit MA, Geiger TR, Song JY, Gitelman I
and Peeper DS: A Twist-Snail axis critical for TrkB-induced
epithelial-mesenchymal transition-like transformation, anoikis
resistance, and metastasis. Mol Cell Biol. 29:3722–3737. 2009.
View Article : Google Scholar
|
|
20
|
Tsai CC, Chen YJ, Yew TL, Chen LL, Wang
JY, Chiu CH and Hung SC: Hypoxia inhibits senescence and maintains
mesenchymal stem cell properties through down-regulation of E2A-p21
by HIF-TWIST. Blood. 117:459–469. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li QQ, Xu JD, Wang WJ, Cao XX, Chen Q,
Tang F, Chen ZQ, Liu XP and Xu ZD: Twist1-mediated
adriamycin-induced epithelial-mesenchymal transition relates to
multidrug resistance and invasive potential in breast cancer cells.
Clin Cancer Res. 15:2657–2665. 2009. View Article : Google Scholar
|
|
22
|
Bialek P, Kern B, Yang X, Schrock M, Sosic
D, Hong N, Wu H, Yu K, Ornitz DM, Olson EN, Justice MJ and Karsenty
G: A twist code determines the onset of osteoblast differentiation.
Dev Cell. 6:423–435. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tsuji T, Ibaragi S, Shima K, Hu MG,
Katsurano M, Sasaki A and Hu GF: Epithelial-mesenchymal transition
induced by growth suppressor p12CDK2-AP1 promotes tumor cell local
invasion but suppresses distant colony growth. Cancer Res.
68:10377–10386. 2008. View Article : Google Scholar
|
|
24
|
Tukel T, Šošić D, Al-Gazali LI, Erazo M,
Casasnovas J, Franco HL, Richardson JA, Olson EN, Cadilla CL and
Desnick RJ: Homozygous nonsense mutations in TWIST2 cause Setleis
syndrome. Am J Hum Genet. 87:289–296. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Franco HL, Casasnovas J, Rodríguez-Medina
JR and Cadilla CL: Redundant or separate entities? - roles of
Twist1 and Twist2 as molecular switches during gene transcription.
Nucleic Acids Res. 39:1177–1186. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Perez RP: Cellular and molecular
determinants of cisplatin resistance. Eur J Cancer. 34:1535–1542.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Aoki K, Ogawa T, Ito Y and Nakashima S:
Cisplatin activates survival signals in UM-SCC-23 squamous cell
carcinoma and these signal pathways are amplified in
cisplatin-resistant squamous cell carcinoma. Oncol Rep. 11:375–379.
2004.
|
|
28
|
Testa JR and Bellacosa A: AKT plays a
central role in tumorigenesis. Proc Natl Acad Sci USA.
98:10983–10985. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Simon D, Herva ME, Benitez MJ, Garrido JJ,
Rojo AI, Cuadrado A, Torres JM and Wandosell F: Dysfunction of the
PI3K-Akt-GSK-3 pathway is a common feature in cell culture and in
vivo models of prion disease. Neuropathol Appl Neurobiol. Jun
6–2013.(Epub ahead of print). View Article : Google Scholar
|
|
30
|
Arafa el SA, Zhu Q, Barakat BM, Wani G,
Zhao Q, El-Mahdy MA and Wani AA: Tangeretin sensitizes
cisplatin-resistant human ovarian cancer cells through
downregulation of phosphoinositide 3-kinase/Akt signaling pathway.
Cancer Res. 69:8910–8917. 2009.
|
|
31
|
Chen R, Yang Q and Lee JD: BMK1 kinase
suppresses epithelial-mesenchymal transition through the Akt/GSK3β
signaling pathway. Cancer Res. 72:1579–1587. 2012.PubMed/NCBI
|
|
32
|
Maseki S, Ijichi K, Tanaka H, Fujii M,
Hasegawa Y, Ogawa T, Murakami S, Kondo E and Nakanishi H:
Acquisition of EMT phenotype in the gefitinib-resistant cells of a
head and neck squamous cell carcinoma cell line through
Akt/GSK-3β/snail signalling pathway. Br J Cancer. 106:1196–1204.
2012.PubMed/NCBI
|