Introduction
Esophageal squamous cell carcinoma (ESCC) is one of
the most aggressive types of gastrointestinal cancer, due to the
relatively high risk of metastasis even in the early stage. In
particular, lymph node metastasis is one of the most important
prognostic factors (1). Tumor cells
take advantage of the lymphatic vascular system to promote
metastasis to the lymph nodes and beyond (2). Tumor-induced lymphangiogenesis
promotes metastasis to regional lymph nodes and often represents
the first step in tumor dissemination. Lymph node metastasis offers
a major prognostic indicator for the progression of types of human
cancer. Two members of the vascular endothelial growth factor
(VEGF) family, VEGF-C and VEGF-D, reportedly induce not only
angiogenesis, but also lymphangiogenesis via VEGF receptor
(VEGFR)-2 and VEGFR-3 on lymphatic endothelial cells (3,4). These
receptors not only regulate lymphangiogenesis, but also enhance
lymphatic metastasis (5). In
addition, VEGF-C and VEGFR-3, which together have been proposed as
a marker for lymphatic endothelial cells, have recently been
reported to be expressed by tumor cells in correlation with the
invasion, metastasis and progression of cancer cells (6–8).
Several studies have previously examined the roles
of the VEGF-C/VEGFR-3 axis and lymphangiogenesis. Lymphangiogenesis
is a key factor in nodal metastasis and a prognostic factor for
various carcinomas of the esophagus (9), stomach (10–12),
colorectum (13), lung (14), cervix (15,16)
and prostate (17,18).
The present study aimed to clarify whether
expression of VEGF-C and VEGFR-3 in the tumor cells of ESCC
correlates with tumor lymphangiogenesis, lymph node metastasis and
other clinicopathological factors. In addition, it was examined
whether VEGF-C and VEGFR-3 have potential as targets of molecular
therapies.
Materials and methods
Patients
In total, 119 patients with ESCC (108 males and 11
females) who underwent curative esophagectomy with lymph node
dissection between 1996 and 2003 at the Kagoshima University
Hospital (Kagoshima, Japan) were enrolled. Patient ages ranged
between 38 and 86 years (mean, 65.3 years). Transthoracic
esophagectomy by right and left thoracotomy was performed in 89
(74.8%) and six patients (4.2%), respectively. In addition,
transhiatal esophagectomy without thoracotomy was performed in 21
patients (17.6%) and abdominal lower esophagectomy was performed in
three patients (3.4%). Three-field lymphadenectomy (cervical,
mediastinal and abdominal regions) was performed in 42 patients
(35.3%), two-field lymphadenectomy (mediastinal and abdominal
regions) in 74 patients (62.2%) and one-field (abdominal region)
lymphadenectomy in the remaining three patients. The median number
of removed lymph nodes was 42 (range, 5–136) and the number of
patients with R0 and R1 resection was 107 and 12, respectively.
None of these patients underwent endoscopic mucosal or palliative
resection, preoperative chemotherapy or radiotherapy, or exhibited
synchronous or metachronous cancer in other organs. Specimens of
cancer and non-cancerous adjustment tissues were collected from the
patients after informed written consent had been obtained in
accordance with the institutional guidelines of the hospital.
Clinicopathological observations were based on the
criteria of the TNM classification for esophageal carcinoma of the
International Union Against Cancer (19). In total, 29 of the ESCCs were
classified as well-differentiated, 68 as moderately differentiated
and 22 as poorly differentiated. In addition, 26 of the tumors were
located in the upper third of the esophagus, 60 in the middle third
and 33 in the lower third. Overall, 40 patients exhibited pT1
tumors, 18 exhibited pT2 tumors and 61 exhibited pT3 tumors. Lymph
node metastasis was found in 76 of the 119 patients (63.9%) and
lymphatic and venous invasion was identified in 74.8% (89/119) and
66.4% (79/119) of patients, respectively. All the M1 tumors
exhibited distant lymph node metastases. Each patient was followed
up after discharge with a chest X-ray every 1 to 3 months, computed
tomography every 3 to 6 months and ultrasonography every 6 months.
Bronchoscopy and endoscopy were performed when necessary.
Postoperative follow-up data were available for all patients with a
median follow-up period of 39 months (range, 1–137 months).
Consequently, 51 patients exhibited relapsed disease in the
follow-up period.
Immunohistochemistry
Once the primary lesions had been fixed in 10%
formaldehyde and routinely embedded in paraffin, 3-μm-thick
sections were prepared for immunohistochemistry. Sections were
deparaffinized in xylene, rehydrated in graded ethanol and
incubated in 0.3% H2O2 solution in methanol
for 30 min to block endogenous peroxidases. All sections were
autoclaved in 10 mM sodium citrate (pH 6.0) for 10 min and allowed
to cool at room temperature. Following washing three times with
phosphate-buffered saline for 5 min each, sections were treated
with 1% bovine serum albumin (Sigma-Aldrich, St Louis, MO, USA) for
30 min at room temperature.
Sections were incubated overnight at 4°C with the
following three antibodies: Mouse anti-VEGF-C monoclonal (1:50;
Santa Cruz Biotechnology, Santa Cruz, CA, USA), goat anti-VEGFR-3
polyclonal (1:200; R&D Systems, Wiesbaden, Germany) and mouse
anti-D2-40 monoclonal (1:50; Dako, Carpinteria, CA, USA). These
reactions were developed using an avidin-biotin immunoperoxidase
technique (ABC method). The reaction was visualized using the
Vectastain Elite ABC kit and 3,3′-diaminobenzidine solution (Vector
Laboratories, Burlingame, CA, USA). Sections were then slightly
counterstained with hematoxylin.
Expression of VEGF-C and VEGFR-3 in >30% of the
cells examined was considered to represent a positive result
(9). Expression of VEGF-C and
VEGFR-3 was evaluated in 10 fields of ≥100 cells each using
high-power (magnification, ×200) light microscopy (BX50, Olympus,
Tokyo, Japan). All immunostained slides were evaluated by two
independent observers (I.O. and M.M.).
Evaluation of microlymphatic vessel
density (MLVD)
Vessel count was assessed by light microscopy in
areas of tumor containing the highest numbers of capillaries at the
invasive edge. Highly vascular areas were identified by scanning
tumor sections at low power (magnification, ×40 and ×100; DP71,
Olympus). In total, six areas showing the highest degree of
neovascularization were identified, vessel count was performed in a
×200 field (x20 objective and ×10 ocular) and the mean count for
the six fields was determined as MLVD. As previously described by
Weidner et al, identification of a vessel lumen was not
necessary for a structure to be defined as a vessel (20).
Statistical analysis
Statistical analysis was performed using
JMP® 5.0.1 (SAS Institute Inc., Cary, NC, USA),
Student’s t-test, χ2 test, Kaplan-Meier method and
log-rank test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Expression of VEGF-C, VEGFR-3 and D2-40
in esophageal carcinoma tissue
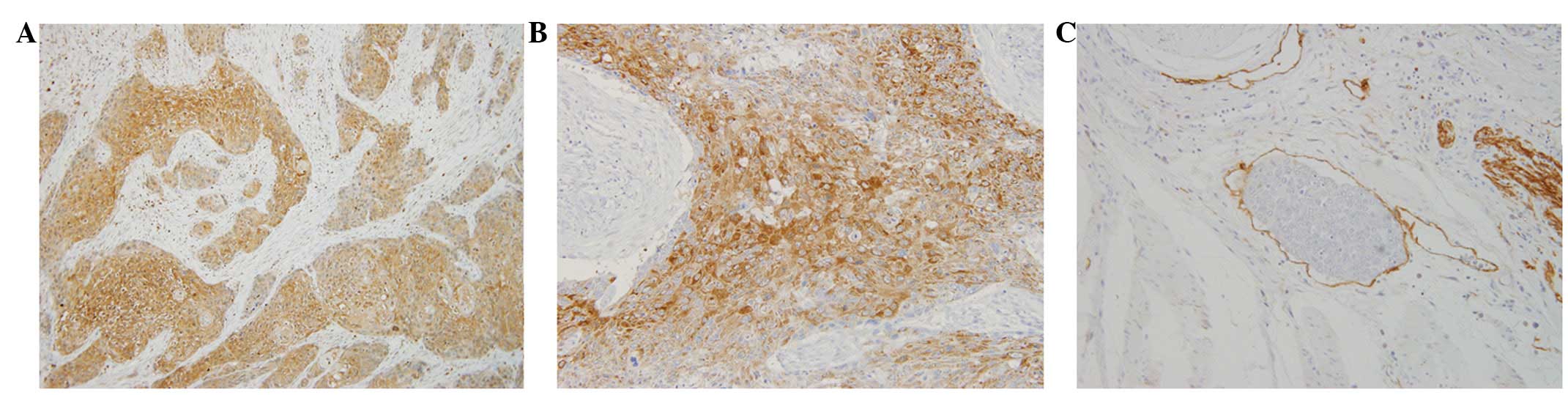
Expression of VEGF-C (Fig. 1A) and VEGFR-3 (Fig. 1B) was distributed throughout the
cytoplasm of cancer cells. Rates of positive VEGF-C and VEGFR-3
expression were 42.9% (51/119) and 28.6% (34/119), respectively.
D2-40 expression was detected in lymphatic endothelial cells
(Fig. 1C) and the mean MLVD was
25.8±13.4/field (range, 0–68/field).
Correlation between clinicopathological
factors and expression of VEGF-C and VEGFR-3
Table I shows the
correlation between VEGF-C expression and pathological
observations. VEGF-C expression was found to correlate
significantly with tumor depth, presence of lymph node metastasis
and lymphatic invasion (P<0.0001 each). Table I also shows the correlation between
VEGFR-3 expression and pathological observations. VEGFR-3
expression was found to correlate significantly with tumor depth
and lymphatic invasion (P=0.01 and P=0.032, respectively).
Although, the incidence of lymph node metastasis tended to occur in
patients with positive expression of VEGFR-3; however, the
correlation was not significant.
 | Table ICorrelation between VEGF-C and VEGFR-3
expression and clinicopathological factors in 119 ESCC
patients. |
Table I
Correlation between VEGF-C and VEGFR-3
expression and clinicopathological factors in 119 ESCC
patients.
| Factors | VEGF-C-positive
expression (n=51), n (%) | P-value | VEGFR-3-positive
expression (n=34), n (%) | P-value |
|---|
| Histopathological
grading | | 0.4954 | | 0.0859 |
| Grade 1–2
(n=97) | 43 (44) | | 31 (32) | |
| Grade 3 (n=22) | 8 (36) | | 3 (14) | |
| Depth of tumor
invasion | | <0.0001 | | 0.0140 |
| T1 (n=40) | 7 (18) | | 5 (13) | |
| T2 (n=18) | 6 (33) | | 5 (28) | |
| T3 (n=61) | 38 (62) | | 24 (39) | |
| Lymphatic
invasion | | <0.0001 | | 0.0327 |
| Negative (n=30) | 2 (6) | | 5 (16) | |
| Positive (n=89) | 49 (55) | | 30 (33) | |
| Lymph node
metastasis | | <0.0001 | | 0.3343 |
| Negative (n=43) | 6 (14) | | 10 (23) | |
| Positive (n=76) | 45 (58) | | 24 (32) | |
Correlation between MLVD and expression
of VEGF-C and VEGFR-3
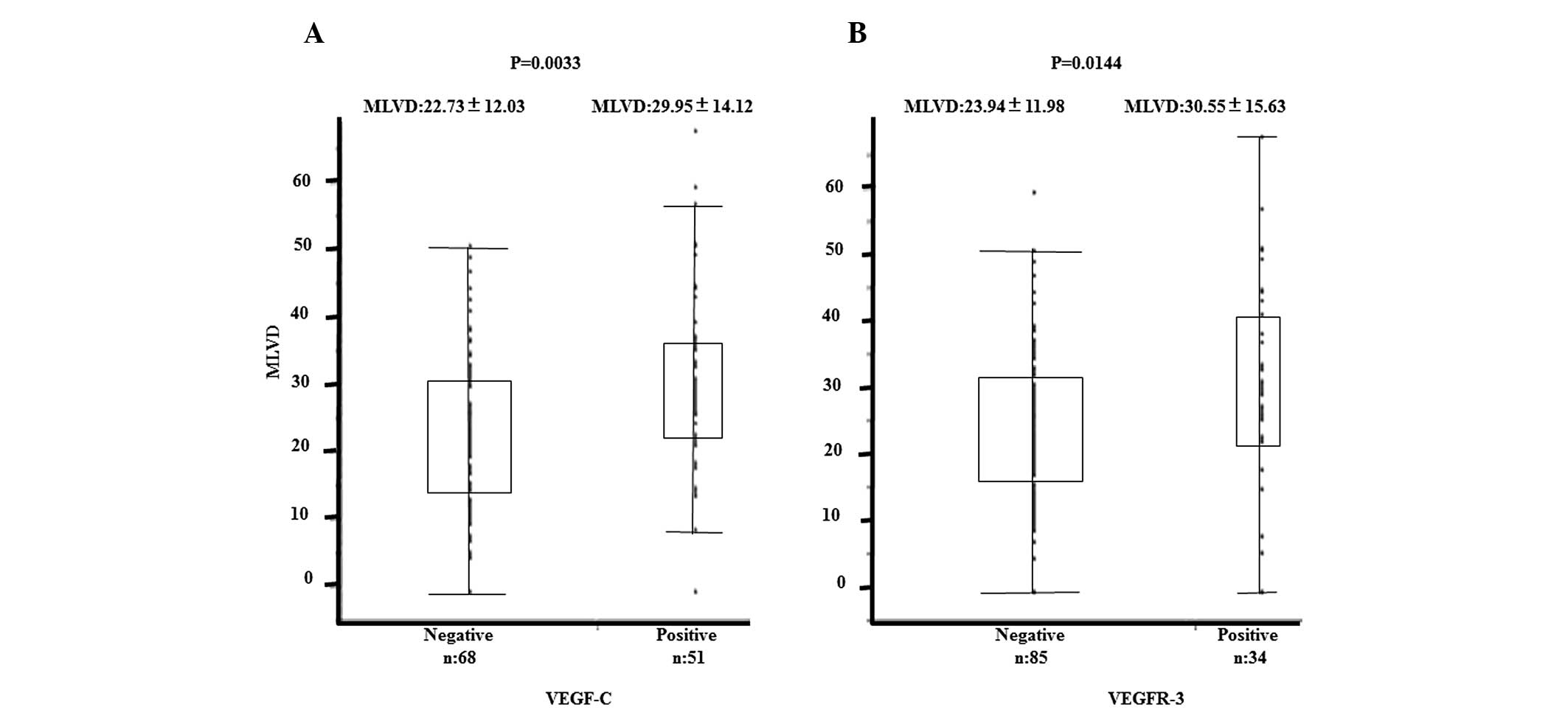
Correlations between the expression of VEGF-C and
VEGFR-3 and MLVD are shown in Figs. 2A
and B. VEGF-C and VEGFR-3 expression was found to correlate
significantly with high MLVD (P=0.0033 and P=0.014, respectively).
Mean MLVD was 29.95±14.12/field in the VEGF-C-positive group,
22.73±12.03 in the VEGF-C-negative group, 30.55±15.63/field in the
VEGFR-3-positive group and 23.94±11.98 in the VEGFR-3-negative
group.
Correlation between prognosis and
expression of VEGF-C and VEGFR-3
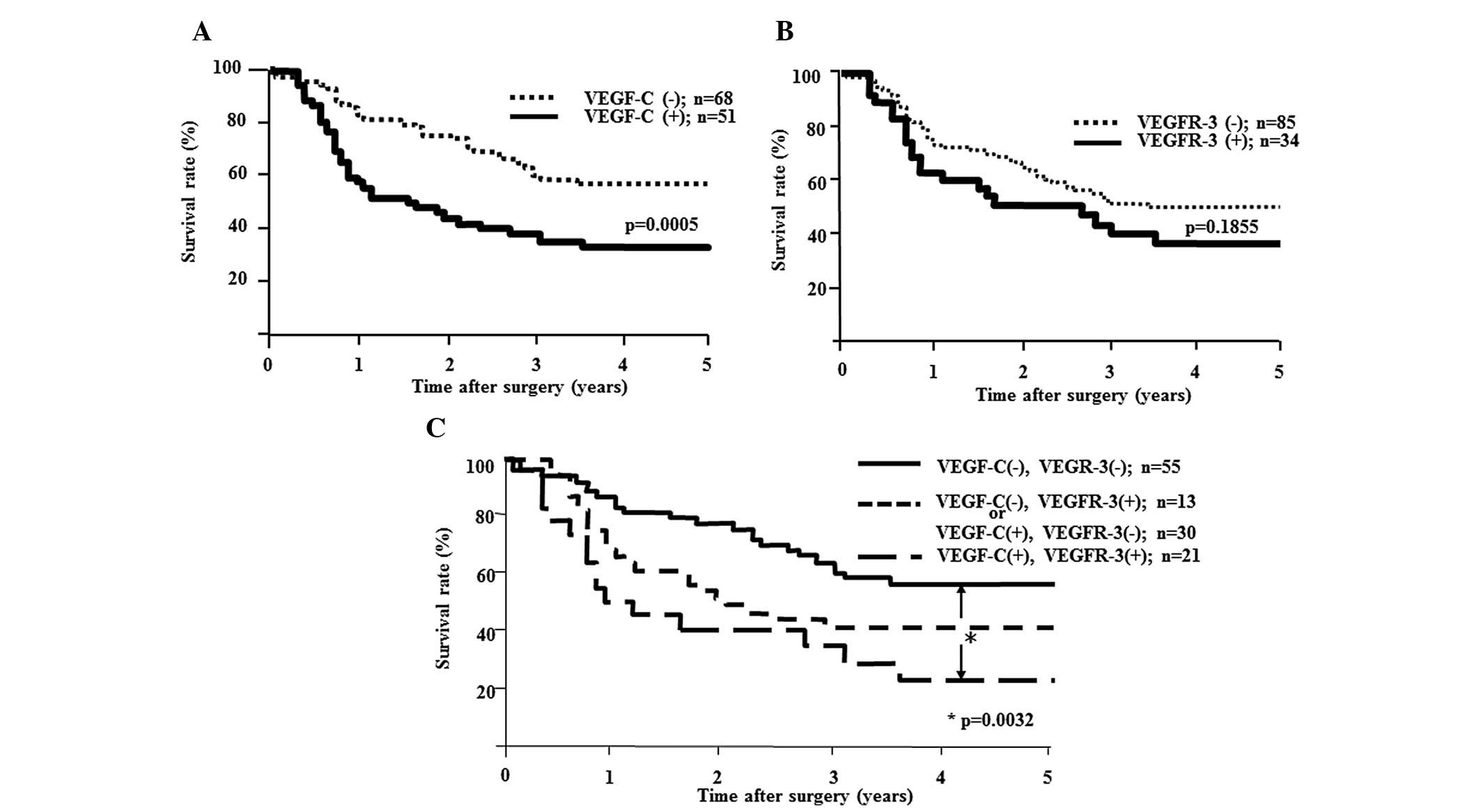
Five-year survival rates were analyzed according to
the expression of VEGF-C and VEGFR-3. The 5-year survival rate was
significantly higher in VEGF-C-negative patients (55%) than in
patients with positive expression (31%; P=0.0006; Fig. 3A). No significant difference in
5-year survival rate was found according to the expression of
VEGFR-3 (Fig. 3B).
Prognosis according to the expression of
VEGF-C and VEGFR-3
The 5-year survival rate was significantly higher in
the double-negative group than in the double-positive group
(P=0.0032; Fig. 3C).
Uni- and multivariate analyses of
survival
Univariate analysis showed that the following
factors were significantly associated with postoperative survival:
Tumor depth, lymph node metastasis, VEGF-C expression, and
coexpression of VEGF-C and VEGFR-3 (P<0.05). Multivariate
regression analysis indicated depth of tumor invasion and lymph
node metastasis as independent prognostic factors (Table II).
 | Table IIUni- and multivariate analyses of
prognostic factors. |
Table II
Uni- and multivariate analyses of
prognostic factors.
| Factors | Univariate
P-value | Multivariate
P-value | 95% confidence
interval | Hazard ratio |
|---|
| pT1b/pT2-3 | <0.0001 | 0.0017 | 1.188–2.256 | 1.610 |
|
pN−/+ | 0.0002 | 0.0095 | 1.095–2.031 | 1.473 |
|
VEGF-C−/+ | 0.0005 | 0.1567 | 0.919–1.649 | 1.237 |
| VEGF-C+,
VEGFR-3+ and other patterns | 0.0210 | 0.7295 | 0.760–1.498 | 0.061 |
Discussion
Lymphangiogenesis represents an important step in
tumor progression and metastasis. Previous studies have revealed
that tumors actively induce their own networks of lymphatics that
connect with surrounding lymphatic vessels (21–25).
The transport of tumor cells by lymphatic vessels represents the
most common pathway for initial dissemination, with cancer spread
by afferent lymphatics following routes of natural drainage
(26–29). Previously, two members of the VEGF
family, VEGF-C and VEGF-D, have been associated with
lymphangiogenesis and are known as natural ligands for VEGFR-3
(30,31). The present study focused on the
expression of VEGF-C and VEGFR-3 and MLVD in ESCC, and evaluated
the involvement of the VEGF-C/VEGFR-3 signaling pathway on
lymphangiogenesis in ESCC.
In the present study, D2-40 antibody, which reacts
with an oncofetal antigen present in fetal germ cells, is a highly
reliable lymphatic endothelial marker (32), was first used to detect
microlymphatic vessels. Numerous studies have previously indicated
that the immunostaining for D2-40 allows specific evaluation of
lymphatic invasion and MLVD in types of human cancer (10,33).
In the present study, D2-40-expressing microvessels were found in
carcinoma tissues, particularly ESCC with lymph node
metastases.
With regard to the correlations with
clinicopathological features, VEGF-C expression was found to
correlate well with several factors, including tumor depth,
lymphatic invasion, lymph node metastasis and MLVD, while close
correlations with VEGFR-3 expression were limited to tumor depth
and MLVD. This may suggest the existence of other pathways for
lymphatic spread, but the two molecules were found to closely
correlate with each other. These observations suggested that VEGF-C
is the most important factor in lymphatic spread and that
overexpression of VEGF-C and VEGFR-3 facilitates tumor
lymphangiogenesis, resulting in the proliferation of lymphatic
vessels. In other words, VEGF-C induces tumor lymphangiogenesis by
stimulating VEGFR-3 expression on lymphatic endothelial cells.
Next, the prognosis of ESCC patients was analyzed
and patients with overexpression of VEGF-C showed poorer outcomes
than those without overexpression, while VEGFR-3 expression was not
found to correlate significantly with survival rate. However,
expression of VEGF-C and VEGFR-3 resulted in poorer outcomes than
other combinations. These results suggested that VEGFR-3 expression
in ESCC may have effects only in the presence of sufficient VEGF-C.
As previously described in several reports, the VEGF-C/VEGFR-3 axis
is critical in cancer progression by inducing lymphangiogenesis and
facilitating the mobility of several types of cancer cells. The
results of the present study support these previous observations
with regard to the role of the VEGF-C/VEGFR-3 axis in the induction
of lymphangiogenesis that results in the lymphatic spread of ESCC.
MLVD was found to significantly correlate with the VEGF-C/VEGFR-3
system and may present a risk factor for lymph node metastasis and
a prognostic factor in ESCC.
Previously, various anti-angiogenic treatments have
been applied in clinical situations. VEGF-A and VEGFR-2 are
currently the main focus of study. Bevacizumab is a humanized
monoclonal antibody against VEGF-A and aflibercept (VEGF-Trap) is a
soluble fusion protein for the extracellular domain of VEGFR-1 and
VEGFR-2 and the Fc region of immunoglobulin G. These agents
neutralize VEGF-A, preventing tumor angiogenesis. VEGFR tyrosine
kinase inhibitors, such as sunitinib and sorafenib, are also
effective in anti-angiogenic tumor therapy by inhibiting VEGFR
signaling. Anti-VEGF drugs currently appear promising as therapies
for various cancer patients.
Conversely, lymphangiogenesis shows similar
biological mechanisms to angiogenesis. VEGF-C and VEGFR-3
expression, as well as MLVD, may serve as prognostic biomarkers in
patients with ESCC (34).
Lymphangiogenesis is activated in cancer and inflammation, but is
largely inactive in normal physiology, suggesting the therapeutic
potential of targeting the underlying mechanisms. As demonstrated
in the results of the current study, VEGF-C and VEGFR-3 signaling
appear essential for the development of lymphatic vessels and,
thus, provide a promising target for the inhibition of tumor
lymphangiogenesis. Previously, Burton et al (35) emphasized the importance of
inhibiting prostate cancer by blockade of the VEGF-C/VEGFR-3 axis.
The authors used a VEGF-C ligand trap and antibody directly against
VEGFR-3, which significantly reduced tumor lymphangiogenesis and
metastasis to regional lymph nodes and distal vital organs without
influencing tumor growth.
An additional potential application to clinical
situations is the early detection of cancer spread. Previously,
Mumprecht et al (36)
applied immune-positron emission tomography with a
lymphatic-specific antibody, LYVE-1, to detect metastases in the
early stage. The resulting images suggested the usefulness of this
approach in determining the progression of diseases with a marked
lymphangiogenic component. In the present study, overexpression of
VEGF-C and VEGFR-3 was suggested to induce lymphatic proliferation
of the tumor. Obtaining information predictive of lymphatic spread
and lymph node metastases must be useful for selecting appropriate
strategies for ESCC treatment.
The VEGF-C/VEGFR-3 axis is important in tumor
lymphangiogenesis. Targeting the VEGF-C/VEGFR-3 axis may be
therapeutically important for cancer metastasis (28,37).
The results of the present study may be beneficial for the
treatment of patients with ESCC, and new drugs aimed at blocking
the VEGF-C/VEGFR-3 axis may be useful for limiting lymph node
metastasis. However, several issues remain with regard to the
frequency, mechanisms and biological importance of lymphatic
metastases. Numerous growth factors appear to be important in
determining the lymph node metastatic potential of ESCC. Future
study is necessary to clarify the molecular pathways and introduce
novel therapeutic options.
Acknowledgements
The authors would like to thank the laboratory
assistants for their technical support.
References
|
1
|
Daly JM, Fry WA, Little AG, et al:
Esophageal cancer: results of an American College of Surgeons
Patient Care Evaluation Study. J Am Coll Surg. 190:562–573. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Plate K: From angiogenesis to
lymphangiogenesis. Nat Med. 7:151–152. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dumont DJ, Jussila L, Taipale J, et al:
Cardiovascular failure in mouse embryos deficient in VEGF
receptor-3. Science. 282:946–949. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Joukov V, Pajusola K, Kaipainen A, et al:
A novel vascular endothelial growth factor, VEGF-C, is a ligand for
the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases.
Embo J. 15:290–298. 1996.
|
|
5
|
Jeltsch M, Kaipainen A, Joukov V, et al:
Hyperplasia of lymphatic vessels in VEGF-C transgenic mice.
Science. 276:1423–1425. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Su JL, Yang PC, Shih JY, et al: The
VEGF-C/Flt-4 axis promotes invasion and metastasis of cancer cells.
Cancer Cell. 9:209–223. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Su JL, Yen CJ, Chen PS, et al: The role of
the VEGF-C/VEGFR-3 axis in cancer progression. Br J Cancer.
96:541–545. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Su JL, Chen PS, Chien MH, et al: Further
evidence for expression and function of the VEGF-C/VEGFR-3 axis in
cancer cells. Cancer Cell. 13:557–560. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kitadai Y, Amioka T, Haruma K, et al:
Clinicopathological significance of vascular endothelial growth
factor (VEGF)-C in human esophageal squamous cell carcinomas. Int J
Cancer. 93:662–666. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Arigami T, Natsugoe S, Uenosono Y, et al:
Lymphatic invasion using D2–40 monoclonal antibody and its
relationship to lymph node micrometastasis in pN0 gastric cancer.
Br J Cancer. 93:688–693. 2005.
|
|
11
|
Han FH, Li HM, Zheng DH, He YL and Zhan
WH: The effect of the expression of vascular endothelial growth
factor (VEGF)-C and VEGF receptor-3 on the clinical outcome in
patients with gastric carcinoma. Eur J Surg Oncol. 36:1172–1179
|
|
12
|
Kodama M, Kitadai Y, Tanaka M, et al:
Vascular endothelial growth factor C stimulates progression of
human gastric cancer via both autocrine and paracrine mechanisms.
Clin Cancer Res. 14:7205–7214. 2008. View Article : Google Scholar
|
|
13
|
Witte D, Thomas A, Ali N, Carlson N and
Younes M: Expression of the vascular endothelial growth factor
receptor-3 (VEGFR-3) and its ligand VEGF-C in human colorectal
adenocarcinoma. Anticancer Res. 22:1463–1466. 2002.PubMed/NCBI
|
|
14
|
Arinaga M, Noguchi T, Takeno S, Chujo M,
Miura T and Uchida Y: Clinical significance of vascular endothelial
growth factor C and vascular endothelial growth factor receptor 3
in patients with nonsmall cell lung carcinoma. Cancer. 97:457–464.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Botting SK, Fouad H, Elwell K, et al:
Prognostic significance of peritumoral lymphatic vessel density and
vascular endothelial growth factor receptor 3 in invasive squamous
cell cervical cancer. Transl Oncol. 3:170–175. 2010. View Article : Google Scholar
|
|
16
|
Van Trappen PO, Steele D, Lowe DG, et al:
Expression of vascular endothelial growth factor (VEGF)-C and
VEGF-D, and their receptor VEGFR-3, during different stages of
cervical carcinogenesis. J Pathol. 201:544–554. 2003.
|
|
17
|
Li R, Younes M, Wheeler TM, et al:
Expression of vascular endothelial growth factor receptor-3
(VEGFR-3) in human prostate. Prostate. 58:193–199. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jennbacken K, Vallbo C, Wang W and Damber
JE: Expression of vascular endothelial growth factor C (VEGF-C) and
VEGF receptor-3 in human prostate cancer is associated with
regional lymph node metastasis. Prostate. 65:110–116. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sobin LH, Gospodarowicz MK and Wittekind
C: TNM Classification of Malignant Tumours. 7th Edition.
International Union Against Cancer; 2009
|
|
20
|
Weidner N, Semple JP, Welch WR and Folkman
J: Tumor angiogenesis and metastasis - correlation in invasive
breast carcinoma. N Engl J Med. 324:1–8. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liang P, Hong JW, Ubukata H, et al:
Increased density and diameter of lymphatic microvessels correlate
with lymph node metastasis in early stage invasive colorectal
carcinoma. Virchows Arch. 448:570–575. 2006. View Article : Google Scholar
|
|
22
|
Tomita N, Matsumoto T, Hayashi T, et al:
Lymphatic invasion according to D2–40 immunostaining is a strong
predictor of nodal metastasis in superficial squamous cell
carcinoma of the esophagus: algorithm for risk of nodal metastasis
based on lymphatic invasion. Pathol Int. 58:282–287. 2008.
|
|
23
|
Liu B, Ma J, Wang X, et al:
Lymphangiogenesis and its relationship with lymphatic metastasis
and prognosis in malignant melanoma. Anat Rec (Hoboken).
291:1227–1235. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Saad RS, Lindner JL, Liu Y and Silverman
JF: Lymphatic vessel density as prognostic marker in esophageal
adenocarcinoma. Am J Clin Pathol. 131:92–98. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhou M, He L, Zu X, Zhang H, Zeng H and Qi
L: Lymphatic vessel density as a predictor of lymph node metastasis
and its relationship with prognosis in urothelial carcinoma of the
bladder. BJU Int. 107:1930–1935. 2011. View Article : Google Scholar
|
|
26
|
Skobe M, Hawighorst T, Jackson DG, et al:
Induction of tumor lymphangiogenesis by VEGF-C promotes breast
cancer metastasis. Nat Med. 7:192–198. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Podgrabinska S, Braun P, Velasco P, Kloos
B, Pepper MS and Skobe M: Molecular characterization of lymphatic
endothelial cells. Proc Natl Acad Sci USA. 99:16069–16074. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wissmann C and Detmar M: Pathways
targeting tumor lymphangiogenesis. Clin Cancer Res. 12:6865–6868.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hirakawa S: From tumor lymphangiogenesis
to lymphvascular niche. Cancer Sci. 100:983–989. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ferrara N and Davis-Smyth T: The biology
of vascular endothelial growth factor. Endocr Rev. 18:4–25. 1997.
View Article : Google Scholar
|
|
31
|
Ferrara N: Vascular endothelial growth
factor: basic science and clinical progress. Endocr Rev.
25:581–611. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ordonez NG: D2-40 and podoplanin are
highly specific and sensitive immunohistochemical markers of
epithelioid malignant mesothelioma. Hum Pathol. 36:372–380. 2005.
View Article : Google Scholar
|
|
33
|
Franchi A, Gallo O, Massi D, Baroni G and
Santucci M: Tumor lymphangiogenesis in head and neck squamous cell
carcinoma: a morphometric study with clinical correlations. Cancer.
101:973–978. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yonemura Y, Endou Y, Sasaki T, et al:
Surgical treatment for peritoneal carcinomatosis from gastric
cancer. Eur J Surg Oncol. 36:1131–1138. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Burton JB, Priceman SJ, Sung JL, et al:
Suppression of prostate cancer nodal and systemic metastasis by
blockade of the lymphangiogenic axis. Cancer Res. 68:7828–7837.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mumprecht V, Honer M, Vigl B, et al: In
vivo imaging of inflammation- and tumor-induced lymph node
lymphangiogenesis by immuno-positron emission tomography. Cancer
Res. 70:8842–8851. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zehnder-Fjällman AH, Marty C, Halin C, et
al: Evaluation of anti-VEGFR-3 specific scFv antibodies as
potential therapeutic and diagnostic tools for tumor
lymph-angiogenesis. Oncol Rep. 18:933–941. 2007.PubMed/NCBI
|