Introduction
Heterogeneous nuclear RNAs (hnRNAs), from which
mRNAs are generated by RNA processing, associate with specific
nuclear proteins to form large hnRNP complexes (1,2). These
hnRNP proteins bind pre-mRNAs and are considered to be important in
mRNA biogenesis (3,4), nucleocytoplasmic transport of mRNA
(5–7) and cytoplasmic mRNA trafficking
(8). To date, 21 hnRNPs (A through
U) have been identified. Certain family members are emerging as
having an important involvement in tumor development (9).
Heterogeneous ribonucleoprotein K (hnRNP K) is a
464-amino acid protein with three K homology domains that mediate
DNA and RNA binding and contain nuclear localization and nuclear
shuttling domains (10,11). Based on these domains, we presumed
that the hnRNP K protein is involved in multiple steps of gene
expression, including transcription, RNA splicing and translation;
such presumptions were later confirmed. In addition, a hnRNP
protein potentially relevant in tumorigenesis is hnRNP K. In the
nucleus, this protein binds directly to the promoter region of the
human c-myc gene and functions as a transcription factor (12). When localized to the cytoplasm,
hnRNP K inhibits the translation of specific mRNAs, such as
15-lipoxygenase mRNA (13). In
breast cancer cells, hnRNP K significantly enhances cell
proliferation and anchorage-independent growth through a growth
factor dependent mechanism (14).
The present study investigated the effects of hnRNP K siRNA on the
growth of lung cancer cells in vitro.
Materials and methods
Study sample
A total of 70 cases of lung cancer were identified
by pathology at the Department of Thoracic Surgery, West China
Hospital of Sichuan University (Chengdu, China) between 2004 and
2005, in which there were 53 males and 17 females. The average age
was 58.12 years and ranged between 46.2 and 70.04 years. The tumors
from surgical resection included 13 samples with diameters of ≤3
cm, 20 samples with diameters between 3 and 5 cm and 56 samples
with diameters of ≥5 cm. A549 lung cancer cell strains were
obtained from the West China Hospital Respiratory Lab (Chengdu,
China). Institutional review board approval for the present study
was obtained from the Ethics Committee of Sichuan University
(Chengdu, China) and written informed consent was obtained from all
patients.
Materials
Dulbecco’s modified Eagle’s medium, Lipofectamine™
2000, TRIzol reagent and RPMI-1640 were purchased from Invitrogen
Life Technologies (Carlsbad, CA, USA). The T7 RiboMAX™ Express RNAi
system kit and Access reverse transctiption (RT)-polymerase chain
reaction (PCR) introductory system were purchased from Promega
Corporation (Madison, WI, USA). Monoclonal antibodies against hnRNP
K were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz,
CA, USA).
Immunohistochemistry
Tumor tissues were fixed with paraformaldehyde (4%),
paraffin-embedded and sectioned onto Plus slides (Thermo Electron
Corp., Madison, WI, USA). Following antigen retrieval, endogenous
peroxidase activity was inhibited (with 3%
H2O2 in 50% methanol) and sections were
blocked. Sections were then incubated with primary antibody.
Following washing with phosphate-buffered saline (PBS), the
sections were incubated with goat anti-mouse antibody conjugated to
biotin. Then, following avidin-biotin-horseradish peroxidase
amplification (Vectastain ABC Reagent; Vector Laboratories, Inc.,
Burlingame, CA, USA), the sections were incubated with filtered
3,3′-diaminobenzidine until the desired stain intensity had
developed. Following subsequent washing with PBS, the slides were
counterstained with hematoxylin. According to the Matthew
classified criteria and combining the chromogenic strength and
proportion of positive cells, the results were classified into
three degrees between - and ++.
Preparation of siRNA
The sequence data of human hnRNP K mRNA (BC025321;
gi: 19116261) used were collected from GenBank (Bethsda, MD, USA).
siRNA targeting human hnRNP K and one nonsense siRNA were designed
online and obtained by transcription using a kit purchased from the
Shanghai Shen Gong Chemical Co., Ltd. (Shanghai, China). The
following sequences of hnRNP K siRNA were used: Sense:
5′-gatccccCTATTCCCAAAGATTTGGCttcaagagaGCCAAATCTTTGGGAATAGtttttggaaa-3′,
anti-sense:
5′-agcttttccaaaaaCTATTCCCGATTTGGCtctcttgaaGCCAAATCTTTGGGAATAGggg-3′.
The basic GC composition of the following nonsense siRNA was the
same as for the siRNA targeting human hnRNP K: Sense,
5′-gatcccTATGGCGTACGTTATAATttcaagagaATTATCAACGTACGCCATAtttttggaaa-3′
and antisense:
5′-agcttttccaaaaaTATGGCGTACGTTGATAATtctcttgaaATTATCAACGTACGCCATAggg-3′,
but had no distinguished homology with human hnRNP K RNA and was
used as a negative control.
Cell culture
The A549 cell line was maintained in RPMI-1640
supplemented with 10% fetal calf serum and incubated at 37°C in a
humidified incubator containing 5% CO2.
Cell cycle and apoptosis assay
Cells (1.5×105/l) were suspended in
RPMI-1640 and then plated onto 25-cm culture flasks. After gene
transferring for 24 h, cells were collected, suspended in 0.01
mol/l PBS and fixed in 70% ethanol for 24 h. Cells were washed once
with PBS, digested by RNase A (60 μg/ml) at 37°C for 30 min and
stained with 1 ml propidium iodide (50 μg/ml) at 4°C for 30 min.
DNA histograms were assayed by flow cytometry. In each sample, a
minimum of 2.5×105 cells were counted and stored in list
mode. Data analysis was performed using standard CellQuest software
(Becton-Dickinson, Franklin Lakes, NJ, USA).
Semi-quantitative RT-PCR analysis
The following sequences of PCR primers were used for
hnRNP K amplification: 5′-ctgcttcagagcaagaatgct-3′ and
5′-aactgcaggccctcttcca-3′. The predicted size of the PCR products
was 200 bp. GAPDH served as a positive control. The following
sequences of PCR primers were used for GAPDH amplification: are
5′-cctcaagatcatcagcaat-3′ and 5′-ccatccacagtcttctgggt-3′. The
predominant cDNA amplification product was 141 bp in length. Cells
(1.5×105/l) were suspended in RPMI-1640, plated onto
six-well culture plates and incubated in a humidified incubator
containing 5% CO2 for 24 h at 37°C. Following
transfection with siRNAs for 24 h, cells were collected and total
RNA was extracted.
In total, 35 cycles of PCR were performed at 94°C
for 3 min, 53°C for 30 sec, 72°C for 60 sec and a final extension
at 72°C for 5 min. Following electrophoresis and ethidium bromide
staining, DNA bands were visualized using an ultraviolet
transilluminator (Ultra-Violet Products Ltd., Cambridge, UK). The
results were scanned onto a computer to measure DNA band
intensities.
Western blot analysis
Cytoplasmic and nuclear protein fractions were
prepared as previously described. For whole cell protein
extraction, cells were lysed in 1 ml radioimmunoprecipitation assay
lysis buffer. A total of 20 μg of protein was separated by 10%
SDS-PAGE gel and subsequently transferred onto polyvinylidene
fluoride membranes for western blot analysis. The following
antibodies were used: Anti-hnRNP K (1:500); and HRP-conjugated
secondary antibody (1:5,000).
Determination of A549 cell growth and MTT
viability
Each group of cells was transfected for 0, 24, 48,
72 and 96 h, to measure MTT absorbance and for cell growth curve
mapping. The cell growth curve was produced with time (h) as the
horizontal axis and the light absorption value as the vertical
axis.
Statistical analysis
Statistically significant differences were
determined by one-way analysis of variance and the independent
samples t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
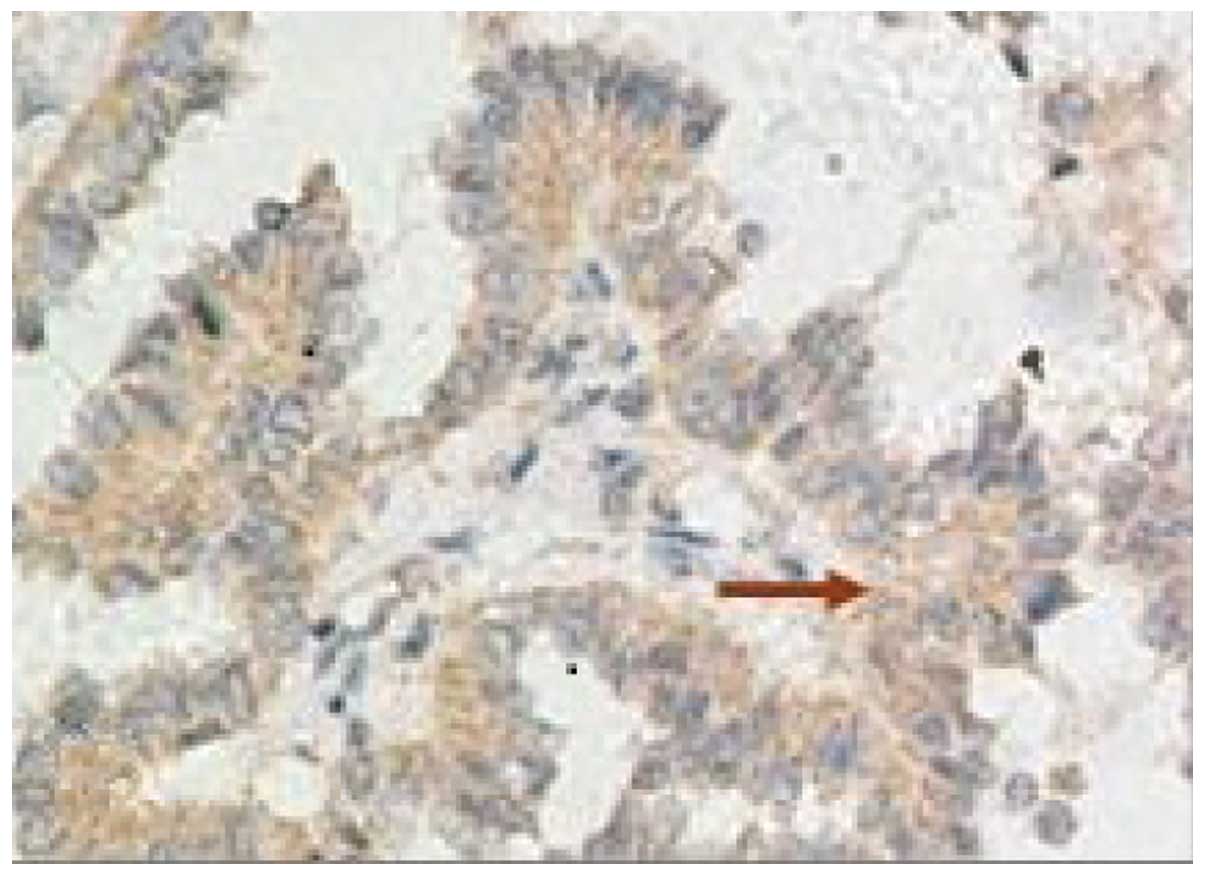
Expression of hnRNP K in lung cancer
tumors
The rates of positive hnRNP K expression in lung
tumors with diameters of ≤3, 3–5 and ≥5 cm, were 38.5, 95.2 and
91.7%, respectively (P<0.01; Fig.
1 and Table I).
 | Table IExpression of hnRNP K in tumors of
different diameters. |
Table I
Expression of hnRNP K in tumors of
different diameters.
| Tumor diameter,
cm | n | hnRNP K
expression | Positive rate, % |
|---|
|
|---|
| − | + | ++ |
|---|
| ≤3 | 13 | 8 | 4 | 1 | 38.5 |
| 3–5 | 21 | 1 | 20 | 0 | 95.2a |
| ≥5 | 36 | 3 | 25 | 8 | 91.7a |
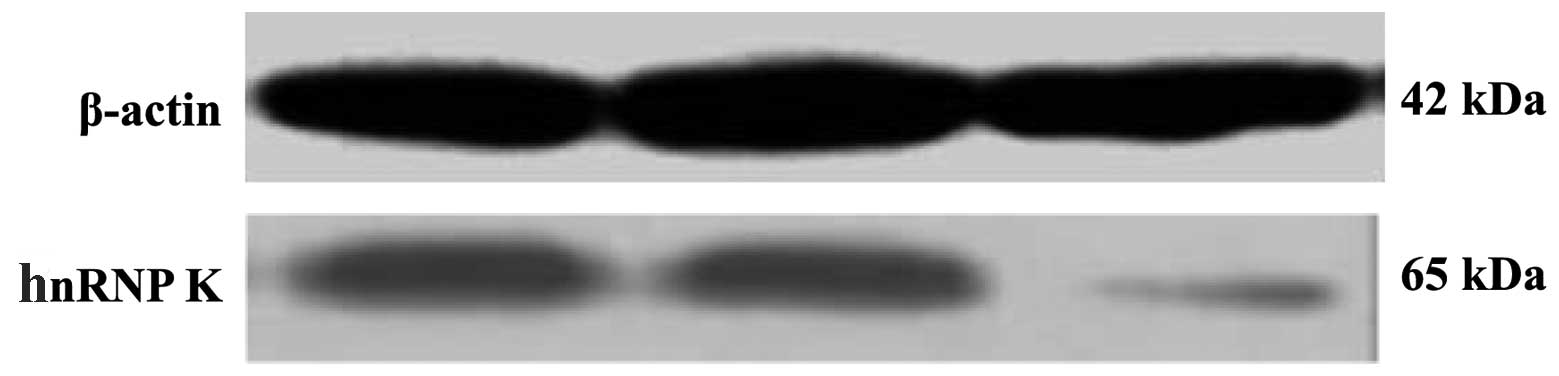
Expression of hnRNP K in A549 cells
To examine the specific effect of hnRNP K siRNA
treatment on hnRNP K expression in the A549 cell line, hnRNP K mRNA
and protein expression levels were determined quantitatively using
RT-PCR and western blot analyses, respectively (Fig. 2 and Table II). hnRNP K mRNA and protein were
markedly expressed in lung cancer A549 cells as reflected by RT-PCR
and western blot analysis. hnRNP K expression was decreased
significantly at 24 h following transfection with specific hnRNP K
siRNA.
 | Table IIExpression of hnRNP K mRNA and protein
in different groups of A549 cells. |
Table II
Expression of hnRNP K mRNA and protein
in different groups of A549 cells.
| Group | n | hnRNP K mRNA,
2−ΔΔCt | Expression ratio of
hnRNP K proteins |
|---|
| hnRNP K siRNA | 6 | 0.24±0.53a | 0.23±0.12a |
| siRNAn | 6 | 1.00 | 0.87±0.17 |
| Controls | 6 | 1.14±0.97 | 1.00±0.03 |
Effects of hnRNPK siRNA on cell cycle
distributions and apoptosis rates
Compared with the hnRNPK siRNAn and untreated
groups, specific hnRNP K siRNA caused an accumulation of cells in
the G1 and S phases, decreased the number of cells in the G2/M
phase and increased the hypodiploid DNA content (P<0.01) at 24 h
following transfection (Table
III). In the groups of specific hnRNP K siRNA, the apoptosis
rate was 4.79% (Table III).
 | Table IIIEffects of treatment with hnRNP K
siRNA on cell cycle distribution and apoptosis rate (%). |
Table III
Effects of treatment with hnRNP K
siRNA on cell cycle distribution and apoptosis rate (%).
| Group | Cell cycles | Apoptosis rate,
% |
|---|
|
|---|
| G0/G1 | S | G2/M |
|---|
| hnRNP K |
| siRNA | 85.60±3.94a | 13.50±3.02a | 0.32±0.07a | 4.79±1.03a |
| siRNAn | 42.53±2.78 | 43.28±4.12 | 14.20±2.90 | 1.05±0.43 |
| Controls | 37.21±3.39 | 47.71±2.73 | 13.00±0.92 | 0.86±0.14 |
hnRNP K siRNA on the proliferation of
A549 cells
Following transfection at 0, 24, 48, 72 and 96 h, at
each time point the cell growth activity of cells was determined.
The results showed that compared with the control and siRNAn
groups, the proliferation rate minimized (P<0.01) in the hnRNP K
siRNA group of A549 cells at 48 h (Table IV).
 | Table IVChanges in the light absorption of
cells in each group. |
Table IV
Changes in the light absorption of
cells in each group.
| Time periods, h | hnRNP K siRNA | siRNAn | Controls |
|---|
| 0 | 0.487±0.014a | 0.499±0.035 | 0.501±0.004 |
| 24 | 0.193±0.003b | 0.598±0.003 | 0.617±0.004 |
| 48 | 0.017±0.004b | 0.690±0.011 | 0.720±0.004 |
| 72 | 0.30±0.007 | 0.739±0.011 | 0.751±0.015 |
| 96 | 0.504±0.004 | 0.768±0.011 | 0.776±0.007 |
Discussion
The balance between the apoptosis and antiapoptosis
signaling pathways is involved in the pathogenesis of a variety of
cancers. It has been previously demonstrated that the inhibition of
apoptosis promotes the mitotic progression in cancer cells
(15).
hnRNP K protein is an abundant factor involved in
transcription, mRNA processing and other events that compose gene
expression. It is likely that the increased K protein levels
observed in the nuclei of the proliferating cells serve to support
nuclear processes that not only compose the inducible expression of
a large number genes, but also maintains conducive chromatin
topology in growing cells (16).
hnRNP K may promote cell proliferation and have a
negative effect against the promotion of differentiation (17); in several states of enhanced cell
proliferation, increased K protein levels have been identified in
the nucleus. Induction of cell proliferation results in the
activation of a large repertoire of genes (18). A previous study identified increased
K protein expression in breast cancer cells. The authors provided
evidence that the increased K protein levels contribute to the
enhanced c-myc gene expression in such tumors (14).
In the present study, the expression and strength of
hnRNP K was found to closely correlate with the size of tumor,
specifically in the group of tumors with diameters of >3 cm, in
which the positive rate of hnRNP K was >90%, a significant
statistical difference compared with the group of tumors with
diameters of ≤3 cm (the positive rate was 38.5%). This implied that
hnRNP K may promote the growth and proliferation of lung cancer
cells.
The c-Src interaction and activation domains in
hnRNP K may be separated in vitro and in transfected cells.
In the cellular context, the respective domains are present in
hnRNP K. Therefore, the protein not only interacts with c-Src, as
it does with lymphocyte-specific protein tyrosine kinase, but is
also capable of activating c-Src in a specific manner. This
supports the function of hnRNP K as a multifunctional scaffold
protein that mediates the cross-talk between signaling pathways
that controls cell differentiation and maturation (19). By interfering with the expression of
hnRNP K in A549 lung cancer cell strains, the present study
identified that the expression of the hnRNP K protein and mRNA
decreased evidently following interference with hnRNP K siRNA
compared with the non-interference group. This indicated that the
hnRNP K gene is inhibited in transcription and at the protein
expression level. The results obtained from flow cytometry
indicated that hnRNP K siRNA inhibits the growth of A549 lung
cancer cell strains, in which the cells remain in the G0/G1 stage.
In addition, the increase of subdiploid DNA implied that hnRNP K
siRNA induces cell apoptosis.
A major consequence of p53 activation following DNA
damage is the induction of cell-cycle arrest at the G1/S or G2/M
transition stages. This is achieved primarily through the
p53-induced expression of target genes that encode factors, such as
p21WAF/CIP, a negative regulator of cyclin-dependent kinases (CDKs)
that induces G1/S arrest (20) and
proteins, such as GADD45, 14-3-3s and Reprimo, which are required
for an efficient G2/M arrest following DNA damage (21). The hnRNP K protein is required for
p53-mediated transcription of cell cycle checkpoint genes (22). It enhances the transcription of
oncogenes, such as c-myc and c-src and is considered to promote
cell proliferation, survival and migration. hnRNP K has also been
implicated in chromatin remodeling, mRNA splicing, export and
translation (10). hnRNP K
depletion abrogates the transcriptional induction of p53 target
genes and causes defects in DNA damage-induced cell cycle
checkpoint arrests. As a cofactor for p53, hnRNP K is key in
coordinating transcriptional responses to DNA damage (22). hnRNP K is a multifunctional protein
that has been studied primarily in cancer cells and has been
suggested to be involved in cell cycle progression (23).
The results of the present study identified the
significant increase of G0/G1 stage cells and significant decrease
of G2/M stage cells in A549 lung cancer strains following
transfection with hnRNP K siRNA. Previously, an inability of hnRNP
K-depleted cells to induce p21, which normally mediates G1 arrest
by inhibiting CDKs and by preventing the involvement of the
proliferating cell nuclear antigen in DNA replication with DNA
polymerase, has been identified (24,25).
This result is consistent with the results of the current study,
that the G0/G1 stage cells significantly increase in A549 strains
following transfection with hnRNP K siRNA.
The present study supports that hnRNP K promotes the
growth and proliferation of lung cancer cells and interfering with
the hnRNP K expression may promote the apoptosis of lung cancer
cells. Through the further investigation of the mechanism of hnRNP
K promoting the growth and proliferation of lung cancer cells, new
target sites of lung cancer therapy may be provided.
Acknowledgements
The authors would like to thank the patients and
their families for participating in the study with patience and
cooperation. The present study was supported by the National
Natural Science Foundation of China (grant nos. 81241068 and
81201851) and the Sichuan Provincial Science and Technology
Department programs, including the Science and Technology Support
Program (grant no. 2011SZ0194), International Cooperation Program
(grant no. 2011HH0051) and Application Foundation Program (grant
no. 2013JY0012).
References
|
1
|
Krecic AM and Swanson MS: hnRNP complexes:
composition, structure, and function. Curr Opin Cell Biol.
11:363–371. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dreyfuss G, Matunis GM, Piñol-Roma S and
Burd CG: hnRNP proteins and the biogenesis of mRNA. Annu Rev
Biochem. 62:289–321. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Swanson MS: Functions of nuclear
pre-mRNA/mRNA binding proteins. Pre-mRNA Processing. Lamond AL:
Springer-Verlag GmbH; Berlin: pp. 17–33. 1995, View Article : Google Scholar
|
|
4
|
Nigg EA: Nucleocytoplasmic transport:
signals, mechanisms and regulation. Nature. 386:779–787. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nakielny S and Dreyfuss G: Nuclear export
of proteins and RNAs. Curr Opin Cell Biol. 9:420–429. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Piñol-Roma S and Dreyfuss G:
Transcription-dependent and transcription independent nuclear
transport of hnRNP proteins. Science. 253:312–314. 1991.PubMed/NCBI
|
|
7
|
Piñol-Roma S and Dreyfuss G: Shuttling of
pre-mRNA binding proteins between nucleus and cytoplasm. Nature.
355:730–732. 1992.PubMed/NCBI
|
|
8
|
Carson JH, Kwon S and Barbarese E: RNA
trafficking in myelinating cells. Curr Opin Neurobiol. 8:607–612.
1998. View Article : Google Scholar
|
|
9
|
Carpenter B, Mackay C, Alnabulsi A, et al:
The roles of heterogeneous nuclear ribonucleoproteins in tumour
development and progression. Biochim Biophys Acta. 1765:85–100.
2006.PubMed/NCBI
|
|
10
|
Bomsztyk K, Denisenko O and Ostrowski J:
hnRNP K: one protein multiple processes. Bioessays. 26:629–638.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bomsztyk K, Van Seuningen I, Suzuki H, et
al: Diverse molecular interactions of the hnRNP K protein. FEBS
Lett. 403:113–115. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Michelotti EF, Michelotti GA, Aronsohn AI
and Levens D: Heterogeneous nuclear ribonucleoprotein K is a
transcription factor. Mol Cell Biol. 16:2350–2360. 1996.
|
|
13
|
Kühn H, Heydeck D, Brinckman R and Trebus
F: Regulation of cellular 15-lipoxygenase activity on
pretranslational, translational, and posttranslational levels.
Lipids. 34(Suppl): S273–S279. 1999.PubMed/NCBI
|
|
14
|
Mandal M, Vadlamudi R, Nguyen D, et al:
Growth factors regulate heterogeneous nuclear ribonucleoprotein K
expression and function. J Biol Chem. 276:9699–9704. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kawamura K, Sato N, Fukuda J, et al:
Survivin acts as an antiapoptotic factor during the development of
mouse preimplantation embryos. Dev Biol. 256:331–341. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ostrowski J and Bomsztyk K: Nuclear shift
of hnRNP K protein in neoplasms and other states of enhanced cell
proliferation. Br J Cancer. 89:1493–1501. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yano M, Okano HJ and Okano H: Involvement
of Hu and heterogeneous nuclear ribonucleoprotein K in neuronal
differentiation through p21 mRNA post-transcriptional regulation. J
Biol Chem. 280:12690–12699. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Iyer VR, Eisen MB, Ross DT, et al: The
transcriptional program in the response of human fibroblasts to
serum. Science. 283:83–87. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Adolph D, Flach N, Mueller K, et al:
Deciphering the cross talk between hnRNP K and c-Src: the c-Src
activation domain in hnRNP K is distinct from a second interaction
site. Mol Cell Biol. 27:1758–1770. 2007. View Article : Google Scholar
|
|
20
|
Bartek J and Lukas J: Pathways governing
G1/S transition and their response to DNA damage. FEBS Lett.
490:117–122. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Taylor WR and Stark GR: Regulation of the
G2/M transition by p53. Oncogene. 20:1803–1815. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Moumen A, Masterson P, O’Connor MJ and
Jackson SP: hnRNP K: an HDM2 target and transcriptional coactivator
of p53 in response to DNA damage. Cell. 123:1065–1078. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Laury-Kleintop LD, Tresini M and Hammond
O: Compartmentalization of hnRNP-K during cell cycle progression
and its interaction with calponin in the cytoplasm. Cell Biochem.
95:1042–1056. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Harris SL and Levine AJ: The p53 pathway:
positive and negative feedback loops. Oncogene. 24:2899–2908. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Waga S, Hannon GJ, Beach D and Stillman B:
The p21 inhibitor of cyclin-dependent kinases controls DNA
replication by interaction with PCNA. Nature. 369:574–578. 1994.
View Article : Google Scholar : PubMed/NCBI
|