Introduction
The management of patients with breast carcinoma
currently uses the following prognostic factors: Disease stage
(which takes into account axillary lymph node involvement, tumour
size and distant tumour dissemination), degree of differentiation
(tumour grade), histological type, proliferation index and receptor
status [progesterone receptor (PR), oestrogen receptor (ER) and
receptor 2 of the human epidermal growth factor (HER2)] of the
primary tumours (1–2). Among these features, the expression
levels of hormone receptors appear to best predict the breast
cancer response to different therapeutic strategies (2–3).
Triple-negative breast cancer (TNBC) has been
classified as a breast cancer subgroup that is negative for ER, PR
and HER2 expression. TNBC accounts for 15 to 20% of breast cancer
cases (4). Despite a notably
favourable rate of response to chemotherapy, TNBC patients present
with a higher risk of relapse and a relatively poor outcome
(5).
Mutations in the BRCA1 and BRCA2
tumour suppressor genes have been associated with breast cancer
risk among families with strong recurrence of the disease, whereas
no clear role of the BRCA1/2 mutations has emerged for the
majority of breast cancers occurring sporadically in individuals
with little or no family history (6–8).
Overall, BRCA1/2 mutations have a prevalence of ~5% in the
general population and ~25% in the families with a history of
breast cancer (7–8). Among BRCA1 mutation carriers,
TNBC represents the predominant breast cancer subtype (more than
two-thirds of cases). BRCA1 germline mutations have been
observed in up to one-third of TNBC patients (mainly among those
with an age at diagnosis of <45 years) (9). One possible role for BRCA2
mutations in TNBC has been reported previously (10). Using data from BRCA mutation
analysis in different TNBC cohorts, a low BRCA2 mutation
frequency (5% average) has been indicated among this subset of
patients (9–10).
In Sardinia, breast cancer is the principal
malignancy that causes mortality, with an incidence rate comparable
with that observed in other Western countries (standardized rate,
95 per 100,000 inhabitants per year), and the median age of onset
for breast cancer among Sardinian females is 65 years (11). Among breast cancer patients from
Sardinia, the contribution of BRCA1/2 mutations to the
population incidence of such a disease has been extensively
evaluated by our group in previous years (12–14).
The Sardinian population is considered genetically homogeneous due
to its high rate of inbreeding and the subsequent inheritance of
numerous common genetic traits (15–16).
This may be instrumental in further defining the association
between the germline mutations in the two genes (BRCA1 and
BRCA2) and the TNBC status. The geographical distribution of
the BRCA mutations in the Sardinian breast cancer population
has been demonstrated to be particularly heterogeneous; in the
northern and middle areas of the island, BRCA2 mutations are
the most common genetic variants (with a predominant founder
mutation), while in the southern area, BRCA1 mutations are
largely prevalent instead (14).
In the present study, the association between the
occurrence of BRCA1/2 mutations and the TNBC status among
breast cancer patients from Sardinia was investigated.
Materials and methods
Patient samples
During the period between January 1998 and December
2006, consecutive patients with a histologically-proven diagnosis
of malignant breast cancer were enrolled. To avoid bias, patients
were included regardless of the age of onset, cancer family
history, disease stage or type of treatment. Patients were informed
about the aims and limitations of the study (documentation of
counselling was evaluated prior to genetic testing). Among them,
726 patients provided written consent to undergo genetic analysis
for the detection of BRCA1/2 mutations on germline DNA from
peripheral blood. For the selected patients, the expression levels
of oestrogen, progesterone and HER2 receptors were obtained. Such
pathological features have been carefully verified through analysis
of the hospital medical records and/or pathology reports, and in
certain cases, through review of the pathological material.
Sardinian origin was ascertained in all cases through genealogical
studies; place of birth of all included patients and their parents
was assessed in order to assign their geographical origin within
the island.
The study was reviewed and approved by the ethical
review boards of the main participating Institutions (University of
Sassari, Sassari, Italy and Unit of Medical Oncology, Local Health
Unit 1 (ASL1), Sassari, Italy).
Mutation screening
The genomic DNA was isolated from peripheral blood
using standard methods. Mutation screening in BRCA1 and
BRCA2 genes was performed by a combination of denaturing
high-performance liquid chromatography (DHPLC) analysis using the
Wave® Nucleic Acid Fragment Analysis System
(Transgenomic, Omaha, NE, USA), and a sequencing approach using an
automated fluorescence-cycle sequencer (ABIPRISM 3100; Applied
Biosystems, Foster City, CA, USA). Primer sets and PCR assay
protocols were as previously described (12–14).
Statistical analysis
Univariate analysis of the presence of
BRCA1/2 mutations versus TNBC status was performed by
Pearson’s χ2 test, using the statistical package, SPSS
7.5 for Windows (SPSS, Inc., Chicago, IL, USA).
Results
The germline DNA from 726 consecutive breast cancer
patients, who provided informed consent and were included in the
study, was investigated for mutations in the BRCA1 and
BRCA2 genes, as previously described (14). Briefly, mutation screening was
performed by DHPLC analysis; all PCR products presenting an
abnormal denaturing profile in comparison to the normal controls
were sequenced using an automated approach. Overall, deleterious
BRCA1/2 mutations were detected in 21/726 (2.9%) breast
cancer cases. In particular, BRCA1 mutations were detected
in 7/21 (33.3%) patients, while BRCA2 mutations were
identified in the majority of patients (14/21; 66.7%).
When the mutation status was evaluated according to
the age of breast cancer onset, 11/285 (3.9%) patients with an age
at diagnosis of ≤50 years were found to carry a BRCA1/2
mutation (Table I). No significant
difference was observed between these patients and the remaining
patients with a diagnosis age of >50 years [10/441 (2.3%)
BRCA1/2 mutation-positive (Table
I)]. Of the patients with a BRCA1/2 mutation, 11/21
(52.4%) were ≤50 years old at the time of diagnosis (Table I); no statistical correlation was
detected.
 | Table IDistribution of patients according to
BRCA1/2 mutation status and age at diagnosis. |
Table I
Distribution of patients according to
BRCA1/2 mutation status and age at diagnosis.
| Age, years | BRCA1/2
mutation-positive | % | Total |
|---|
| <20 | 0 | 0.0 | 1 |
| 21–25 | 1 | 16.7 | 6 |
| 26–30 | 1 | 4.8 | 21 |
| 31–35 | 2 | 4.7 | 43 |
| 36–40 | 3 | 4.5 | 66 |
| 41–45 | 2 | 2.7 | 75 |
| 46–50 | 2 | 2.7 | 73 |
| 51–55 | 3 | 3.0 | 99 |
| 56–60 | 2 | 2.4 | 82 |
| 61–65 | 1 | 1.4 | 70 |
| 66–70 | 2 | 2.3 | 86 |
| 71–75 | 1 | 1.8 | 55 |
| 76–80 | 1 | 2.6 | 39 |
| ≥81 | 0 | 0.0 | 10 |
| Total | 21 | 2.9 | 726 |
Using Pearson’s χ2 test, the occurrence
of a BRCA1/2 mutation was evaluated for association with the
expression levels of oestrogen, progesterone and HER2 receptors.
The distribution of BRCA1/2 mutation carriers and
non-carriers was similar in the different subsets of patients,
according to such pathological parameters, and therefore no
statistically significant correlation was observed (Table IIA–C). When the analysis was
focused on patients with TNBC classification, the rate of
BRCA1/2 mutations was significantly higher in the group of
patients with a triple-negative primary tumour compared with those
without such a feature [7/49 (14.3%) vs. 14/677 (2.1%),
respectively; P=0.012; Table
IID].
 | Table IIDistribution of patients according to
BRCA1/2 mutation status and primary tumour receptor
status. |
Table II
Distribution of patients according to
BRCA1/2 mutation status and primary tumour receptor
status.
| A, ER status |
|---|
|
|---|
| BRCA1/2
mutation | Negative, n
(%) | Positive, n
(%) | Total, n (%) |
|---|
| Negative | 177 (95.2) | 528 (97.8) | 705 (97.1) |
| Positive | 9 (4.8) | 12 (2.2) | 21 (2.9) |
| Total | 186 (25.6) | 540 (74.4) | 726 (100) |
|
| B, PR status |
|
| BRCA1/2
mutation | Negative, n
(%) | Positive, n
(%) | Total, n (%) |
|
| Negative | 203 (95.3) | 502 (97.9) | 705 (97.1) |
| Positive | 10 (4.7) | 11 (2.1) | 21 (2.9) |
| Total | 213 (29.3) | 513 (70.7) | 726 (100) |
|
| C, HER2 status |
|
| BRCA1/2
mutation | Negative, n
(%) | Positive, n
(%) | Total, n (%) |
|
| Negative | 547 (97.3) | 158 (96.3) | 705 (97.1) |
| Positive | 15 (2.7) | 6 (3.7) | 21 (2.9) |
| Total | 562 (77.4) | 164 (22.6) | 726 (100) |
|
| D, Triple-negative
(ER−, PR−, HER2−) status |
|
| BRCA1/2
mutation | Absent, n (%) | Present, n (%) | Total, n (%) |
|
| Negative | 663 (97.9) | 42 (85.7) | 705 (97.1) |
| Positive | 14 (2.1) | 7 (14.3) | 21 (2.9) |
| Total | 677 (93.3) | 49 (6.7) | 726 (100) |
As listed in Table
III, the presence of a triple-negative phenotype was strongly
associated with the BRCA1 mutations at a highly significant
level (P<0.001), whereas no association was found with the
BRCA2 mutations (P=0.837). With respect to patient origin
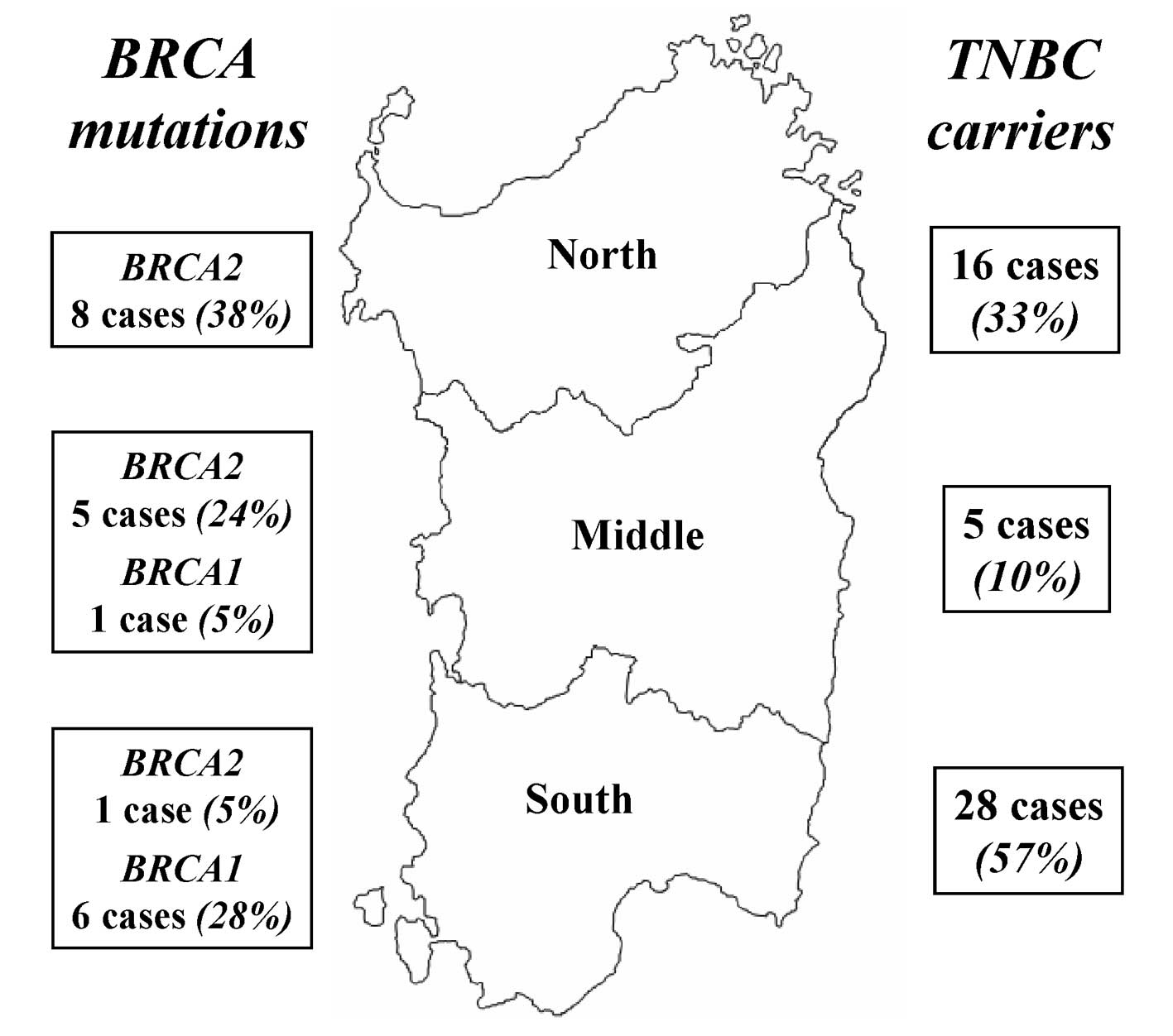
within Sardinia, the distribution of mutations was confirmed as
significantly heterogeneous: 6/7 (85.7%) mutated cases in Southern
Sardinia were represented by BRCA1 mutations, while 13/14
(92.9%) mutated cases in Middle-Northern Sardinia were constituted
by BRCA2 mutations (P<0.001; Table III; Fig. 1). These discrepancies did not result
from incorrect standard sequencing, as confirmed by an independent
duplicate analysis. As a further indication of a strong correlation
between TNBC status and the type of BRCA mutation, patients
from the geographical area with prevalent BRCA1 mutations
presented a frequency of triple-negative tumours much higher than
that observed in cases from the area with prevalent BRCA2
mutations (11.8 vs. 4.3%, respectively; P=0.037).
 | Table IIIComparison between BRCA
mutations and TN status. |
Table III
Comparison between BRCA
mutations and TN status.
| TN status | No. of cases
(%) | BRCA1
mutation cases, n (%) | P-value | BRCA2
mutation cases, n (%) | P-value | BRCA1/2
mutation cases, n (%) | P-value |
|---|
| All cases | 726 | 7 (1.0) | <0.001 | 14 (1.9) | 0.837 | 21 (2.9) | 0.012 |
| Present | 49 (6.7) | 6 (12.2) | | 1 (2.0) | | 7 (14.3) | |
| Absent | 677 (93.3) | 1 (0.1) | | 13 (1.9) | | 14 (2.1) | |
| Southern
Sardinia | 238 | 6 (2.5) | <0.001 | 1 (0.4) | 0.186 | 7 (2.9) | <0.001 |
| TN | 28 (11.8) | 5 (17.9) | | 0 (0.0) | | 5 (17.9) | |
| Non-TN | 210 (88.2) | 1 (0.5) | | 1 (0.5) | | 2 (1.0) | |
| Middle-Northern
Sardinia | 488 | 1 (0.2) | 0.007 | 13 (2.7) | 0.118 | 14 (2.9) | 0.046 |
| TN | 21 (4.3) | 1 (4.8) | | 1 (4.8) | | 2 (9.5) | |
| Non-TN | 467 (95.7) | 0 (0.0) | | 12 (2.6) | | 12 (2.6) | |
Discussion
In the present study, a low prevalence (3%) of
patients with germline mutations in coding regions and splice
boundaries of BRCA1 or BRCA2 was observed. Breast
cancers carrying BRCA1/2 germline mutations often occur in
younger women, and present a lack of ER/PR expression (mostly among
BRCA1-positive tumours) (8,17–19).
In our series, the age at diagnosis was younger in patients
carrying BRCA1/2 mutations [11/21 (52%) <50 years vs.
10/21 (48%) >50 years] than in cases with no detectable mutation
[274/705 (39%) <50 years vs. 431/705 (61%) >50 years]
(Table I); however, such a
difference was not statistically significant.
In Sardinia, the proportion of breast cancer
patients with a diagnosis of TNBC was much lower than expected [7%
instead of 15–20%, as reported in breast cancer worldwide (4)]. Among the TNBC cases, the rate of
BRCA1/2 mutations was significantly higher (14%; P=0.012);
such a significance was due to the occurrence of BRCA1
mutations (Table III). As a
confirmation of this, patients from South Sardinia (the
geographical area with the preponderance of BRCA1 mutations)
presented a significantly higher TNBC frequency compared with that
in breast cancer cases from Middle-North Sardinia (the area with
prevalent BRCA2 mutations); ~12 vs. 4%, respectively
(P=0.037).
In a population sharing a similar lifestyle and diet
habits across the island, these results strongly indicate that a
different ‘genetic background’ may indeed affect the phenotypic
characteristics in the onset of a complex disease such as breast
cancer. Similar data was reported by our group for melanoma and
colorectal carcinoma (20–21); within the island, the geographical
distribution of the genetic variants appears to be correlated to
the specific large areas of Sardinia, which reflect its ancient
history: The northern area, which is linguistically different from
the rest of the island and delineated by the mountain chain
crossing Sardinia, and the middle-southern area, which is the
domain of pastoral culture and the land of the ancient Sardinian
population. Together, these findings clearly indicate that mutation
frequency for candidate cancer genes requires accurate evaluation
in each geographical area within every single population.
Finally, it is noteworthy that the occurrence of
TNBC was significantly associated with the BRCA1 mutation
carrier status (P<0.001), regardless of the geographical origin
of patients in the present series. It may therefore be hypothesized
that the simultaneous lack of expression of ER/PR/HER2, mainly when
associated with an early diagnosis age, is somehow predictive for
the presence of BRCA1 germline mutations. The absence of an
association between TNBC classification and BRCA2 mutations
in the present study was consistent with data previously reported
[reviewed in (4)].
Overall, the prevalence of BRCA1/2 mutations
in TNBC cases remains limited and characterizes only a subset in
this group of malignancies. The present data, along with those from
the literature, support the hypothesis that additional breast
cancer genes should be tested in such patients, in order to improve
the subclassification of the heterogeneous TNBC disease from a
genetic point of view. Further effort is required to reduce the
fraction of patients with a ‘non-mutant’ TNBC status.
Acknowledgements
The authors would like to thank the patients for
their contribution to this study. The study was partially supported
by The Italian Ministry of Health ‘Progetto Ricerca Finalizzata’
and Sardinia Regional Government (Regione Autonoma della
Sardegna).
References
|
1
|
Rakha EA, Reis-Filho JS, Baehner F, Dabbs
DJ, Decker T, Eusebi V, Fox SB, Ichihara S, Jacquemier J, Lakhani
SR, Palacios J, Richardson AL, Schnitt SJ, Schmitt FC, Tan PH, Tse
GM, Badve S and Ellis IO: Breast cancer prognostic classification
in the molecular era: the role of histological grade. Breast Cancer
Res. 12:2072010.
|
|
2
|
Fuksa L, Micuda S, Grim J, Ryska A and
Hornychova H: Predictive biomarkers in breast cancer: their value
in neoadjuvant chemotherapy. Cancer Invest. 30:663–678. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Thibault C, Khodari W, Lequoy M, Gligorov
J and Belkacémi Y: HER2 status for prognosis and prediction of
treatment efficacy in adenocarcinomas: A review. Crit Rev Oncol
Hematol. 88:123–133. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Foulkes WD, Smith IE and Reis-Filho JS:
Triple-negative breast cancer. N Engl J Med. 363:1938–1948. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Metzger-Filho O, Tutt A, de Azambuja E,
Saini KS, Viale G, Loi S, Bradbury I, Bliss JM, Azim HA Jr, Ellis
P, Di Leo A, Baselga J, Sotiriou C and Piccart-Gebhart M:
Dissecting the heterogeneity of triple-negative breast cancer. J
Clin Oncol. 30:1879–1887. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ford D, Easton DF, Stratton M, Narod S,
Goldgar D, Devilee P, Bishop DT, Weber B, Lenoir G, Chang-Claude J,
Sobol H, Teare MD, Struewing J, Arason A, Scherneck S, Peto J,
Rebbeck TR, Tonin P, Neuhausen S, Barkardottir R, Eyfjord J, Lynch
H, Ponder BA, Gayther SA, Zelada-Hedman M, et al: Genetic
heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes
in breast cancer families. The Breast Cancer Linkage Consortium. Am
J Hum Genet. 62:676–689. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dent R and Warner E: Screening for
hereditary breast cancer. Semin Oncol. 34:392–400. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Meads C, Ahmed I and Riley RD: A
systematic review of breast cancer incidence risk prediction models
with meta-analysis of their performance. Breast Cancer Res Treat.
132:365–377. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Peshkin BN, Alabek ML and Isaacs C:
BRCA1/2 mutations and triple negative breast cancers. Breast Dis.
32:25–33. 2010.PubMed/NCBI
|
|
10
|
Meyer P, Landgraf K, Högel B, Eiermann W
and Ataseven B: BRCA2 mutations and triple-negative breast cancer.
PLoS One. 7:e383612012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Budroni M, Cesaraccio R, Pirino D, Sechi
O, Oggiano M, Piras D, Sechi A, Cossu A, Palmieri G and Tanda F:
Cancer incidence in Sassari Province (1998–2002). Cancer Incidence
in Five Continents. IXCurado MP, Edwards B, Shin HR, Storm H,
Ferlay J, Heanue M and Boyle P: (International Agency for Research
on Cancer (IARC) Scientific Publications). (160)Lyon: IARC; pp.
331–332. 2007
|
|
12
|
Palomba G, Pisano M, Cossu A, Budroni M,
Dedola MF, Farris A, Contu A, Baldinu P, Tanda F and Palmieri G:
Spectrum and prevalence of BRCA1 and BRCA2 germline mutations in
Sardinian breast cancer patients through a hospital-based
screening. Cancer. 104:1172–1179. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Palomba G, Cossu A, Friedman E, Budroni M,
Farris A, Contu A, Pisano M, Baldinu P, Sini MC, Tanda F and
Palmieri G: Origin and distribution of the BRCA2-8765delAG mutation
in breast cancer. BMC Cancer. 7:1322007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Palomba G, Loi A, Uras A, Fancello P,
Piras G, Gabbas A, Cossu A, Budroni M, Contu A, Tanda F, Farris A,
Orrù S, Floris C, Pisano M, Lovicu M, Santona MC, Landriscina G,
Crisponi L, Palmieri G and Monne M: A role of BRCA1 and BRCA2
germline mutations in breast cancer susceptibility within Sardinian
population. BMC Cancer. 9:2452009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wright AF, Carothers AD and Pirastu M:
Population choice in mapping genes for complex diseases. Nat Genet.
23:397–404. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Arcos-Burgos M and Muenke M: Genetics of
population isolates. Clin Genet. 61:233–247. 2002. View Article : Google Scholar
|
|
17
|
Mote PA, Leary JA, Avery KA, Sandelin K,
Chenevix-Trench G, Kirk JA and Clarke CL; kConFab Investigators.
Germ-line mutations in BRCA1 or BRCA2 in the normal breast are
associated with altered expression of estrogen-responsive proteins
and the predominance of progesterone receptor A. Genes Chromosomes
Cancer. 39:236–248. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Veronesi A, de Giacomi C, Magri MD,
Lombardi D, Zanetti M, Scuderi C, Dolcetti R, Viel A, Crivellari D,
Bidoli E and Boiocchi M: Familial breast cancer: characteristics
and outcome of BRCA 1–2 positive and negative cases. BMC Cancer.
5:702005.
|
|
19
|
Musolino A, Bella MA, Bortesi B, Michiara
M, Naldi N, Zanelli P, Capelletti M, Pezzuolo D, Camisa R, Savi M,
Neri TM and Ardizzoni A: BRCA mutations, molecular markers, and
clinical variables in early-onset breast cancer: a population-based
study. Breast. 16:280–292. 2007. View Article : Google Scholar
|
|
20
|
Casula M, Colombino M, Satta MP, Cossu A,
Lissia A, Budroni M, Simeone E, Calemma R, Loddo C, Caracò C,
Mozzillo N, Daponte A, Comella G, Canzanella S, Guida M, Castello
G, Ascierto PA and Palmieri G; Italian Melanoma Intergroup. Factors
predicting the occurrence of germline mutations in candidate genes
among patients with cutaneous malignant melanoma from South Italy.
Eur J Cancer. 43:137–143. 2007. View Article : Google Scholar
|
|
21
|
Palomba G, Colombino M, Contu A, Massidda
B, Baldino G, Pazzola A, Ionta MT, Capelli F, Trova V, Sedda T,
Sanna G, Tanda F and Budroni M; Sardinian Translational Oncology
Group (STOG). Palmieri G, Cossu A, Contu M, Cuccu A, Farris A,
Macciò A, Mameli G, Olmeo N, Ortu S, Petretto E, Pusceddu V and
Virdis L: Prevalence of KRAS, BRAF, and PIK3CA somatic mutations in
patients with colorectal carcinoma may vary in the same population:
clues from Sardinia. J Transl Med. 10:1782012. View Article : Google Scholar
|