Introduction
Increasing evidence indicates that individuals with
a high dietary intake of soybean-derived products have low
incidence and mortality rates from common cancers in the Western
hemisphere, including cancers of the colon, breast and prostate
(1). A number of different agents
in soybeans may act as cancer chemopreventive agents in human
populations (2). These agents
include the soybean-derived protease inhibitor, Bowman-Birk
inhibitor (BBI), inositol hexaphosphate (phytic acid), the sterol,
β-sitosterol, and the isoflavone, genistein, which have been
demonstrated to suppress the development of cancer in animal
carcinogenesis assay systems. BBI has been shown to have the
strongest anticarcinogenic activity in animal carcinogenesis model
systems in comparison to other potential cancer chemopreventive
agents in soybeans (2). BBI, as a
purified compound and an extract of soybeans in which BBI has been
concentrated, has been shown to suppress carcinogenesis in a wide
variety of in vivo and in vitro carcinogenesis assay
systems (3).
BBI is an 8-kDa soybean-derived protein containing
71 amino acids with two functional domains. One domain inhibits
trypsin, the other inhibits chymotrypsin and several other serine
proteases with chymotrypsin-like specificity, including elastase
(4,5), cathepsin G (5,6) and
chymase (7). BBI has been shown to
have several therapeutic activities (reviewed in 8–10). BBI
concentrate (BBIC) is a soybean extract enriched in BBI (11). It is believed that the chymotrypsin
inhibitory activity of BBI conveys these therapeutic activities,
therefore, the potency of BBIC is measured in chymotrypsin
inhibitor (CI) units. One CI unit is defined as the amount of a
substance required to inhibit 1 mg of bovine pancreatic
chymotrypsin (11). Like BBI, BBIC
inhibits trypsin and chymotrypsin and is anticarcinogenic, as
measured by its ability to prevent malignant transformation in
vitro and suppress carcinogenesis in vivo (reviewed in
3,9,11,12).
In phase I clinical trials performed previously, no toxicity was
observed when BBIC was orally administered in a single dose of up
to 800 CI units in patients with premalignant lesions known as oral
leukoplakia (13) or in daily doses
of up to 800 CI units for 6 months in patients with benign
prostatic hyperplasia (14). A
subsequent phase IIa clinical trial in patients with oral
leukoplakia demonstrated a dose-dependent reduction in oral lesion
size after a one-month treatment with BBIC at doses of up to 1,066
CI units (15). In the clinical
trial with benign prostatic hyperplasia patients, statistically
significant decreases were observed in the serum prostate-specific
antigen (PSA) level, serum triglyceride level and prostate volume
following a 6-month treatment period with BBIC at doses of up to
800 CI units (14). BBIC tablets
have also been administered to patients with active ulcerative
colitis at a dose of 800 CI units per day for 12 weeks (16). In this study, the Sutherland Disease
Activity Index (SDAI) was used to assess disease activity, response
(index decrease >3) and remission (index <1 with no rectal
bleeding). Favorable trends were observed in the rates of remission
and clinical response, and no severe adverse events were observed.
The results of the trial indicated a potential advantage over the
placebo for achieving a clinical response and the induction of
remission in patients with active ulcerative colitis, without
apparent toxicity.
Based on the non-toxicity and positive clinical
responses observed in the previous clinical trials, two additional
clinical trials were performed for the present study, using single
BBIC doses of up to 2,000 CI units to determine the
pharmacokinetics and safety of BBIC administered orally as a
suspension in orange juice (OJ). Males were chosen for these trials
as it was predicted that this would be the beginning of a prostate
cancer prevention program utilizing BBI as the prostate cancer
chemopreventive agent. One of these trials used the original
formulation of BBIC and the other trial used a new formulation of
BBIC. The primary objectives were to determine i) the dose-limiting
toxicities for single doses of BBIC and expansion of the range of
doses tested in humans, ii) the recommended doses of BBIC for a
subsequent phase I multiple-dose study, and iii)
pharmacokinetic characterization of the original and new BBIC
formulations.
Materials and methods
Two sequential randomized, double-blind,
placebo-controlled trials were performed in healthy male subjects
(NCT00287833 and NCT00679094). The trials were approved by the
Institutional Review Board at the University of Pennsylvania
(Philadelphia, PA, USA) and all subjects provided informed consent.
The subjects were male, aged 18–65 years and assessed to be in good
health by physical exam, electrocardiography and standard
hematological tests. The inclusion criteria consisted of an Eastern
Cooperative Oncology Group performance status (17) of 0–2, the lack of chronic medical
conditions, no evidence of psychiatric problems and a weight within
15% of the ideal body weight. The subjects were excluded if they
had a history of heart disease, chemotherapy in the last 12 months,
tobacco smoking, allergies or prior adverse reactions to soybeans
or if they had a prior diagnosis of pancreatic or other
gastrointestinal diseases. Those who reported taking more than two
vitamin supplements or non-steroidal anti-inflammatory drug on a
regular basis, and vegetarians and others with a large soy
component to their diet were also excluded.
BBIC
The original BBIC formulation was manufactured by
Central Soya (Fort Wayne, IN, USA) according the procedure
described previously (11). The new
BBIC formulation was manufactured originally by Central Soya, but
purified by Dynamic Extractions Ltd., (DE; Slough, Berkshire, UK)
under direction from the National Cancer Institute (NCI) Division
of Cancer Prevention (DCP) and supplied to the University of
Pennsylvania School of Medicine Investigational Drug Service by the
NCI Repository (McKesson Bioservices, Rockville, MD, USA). The
contents of the original BBIC formulation have been described in
detail previously (11). While the
original BBIC formulation did contain low levels of normal food
bacteria that are deemed non-pathogenic and are not expected to
lead to adverse health effects, the NCI DCP produced a more
purified formulation of BBIC that would not contain the normal food
bacteria and represented a more concentrated version of BBIC. A
more concentrated form of BBIC was expected to be considerably more
useful in future human trials in which the total BBIC doses could
be increased substantially. The company chosen to purify BBIC was
DE, which is a UK specialist chromatography company. The DE product
was called freeze-dried BBIC (BBIC-700). The final CI activity of
the DE product was 562 CI units/g, while the original BBIC product
was approximately 100 CI units/g (11). The microbiological content of the DE
product was as follows: Aerobes <1 cfu/g (upper limit, 100
cfu/g); yeasts and molds <1 cfu/gram (upper limit, 100 cfu/g);
E. coli and Salmonella were absent. The methods used in the
preparation of freeze-dried BBIC were consistent with current good
manufacturing principles. The new BBIC formulation from DE was
stored in the refrigerator at 2 to 8°C. The original BBIC
formulation was stored at room temperature.
Study design and endpoints
Each study aimed to enroll a total of 20 healthy
male volunteers, who were sequentially assigned to four different
cohorts (drug levels), with five subjects per cohort. Subjects were
recruited from the city of Philadelphia using print advertisements
in local newspapers and posters around the campus of the University
of Pennsylvania. One subject per cohort was assigned to receive a
placebo instead of the active medication. Assignment to placebo or
active medication was performed in a random and double-blind
manner, the study pharmacist used a random number generator to
assign treatments. The assigned treatment group was revealed at the
completion of the trial. The following drug levels were
investigated: 800, 1,200, 1,600 and 2,000 CI units. The first and
second BBIC trials used the original formulation and the new
formulation of BBIC, respectively. The subjects assigned to receive
the placebo received 11.5 fl oz of OJ (Minute Maid Original 100%
Orange Juice from Concentrate; Minute Maid, Sugar Land, TX, USA)
with no additives. For subjects receiving BBIC, the measured dose
of study medication was suspended in 11.5 fl oz of OJ from a single
container. Both of the above ingredients were added to a
subsequently sealed container and agitated until the suspension
mixed uniformly. The total volume of the preparation was
approximately 12 fl oz (350 ml). BBIC was administered orally to
subjects in the form of a suspension at a concentration of 6% (w/v)
in OJ. The CI activity is preserved in this formulation at this
medication concentration for at least 3 h after suspension in the
OJ, so the study medication was administered immediately after
suspension in OJ.
Subjects and sampling schedule
The subjects arrived at the Clincial and
Translational Research Center (Philadelphia, USA) after fasting
overnight. The subjects swallowed a single dose of BBIC suspension
or placebo and immediately ate a defined low-fat breakfast. The
subjects remained in the clinical research facility for the first
48 h of the study to facilitate the required frequent blood draws
and to ensure that all subjects consumed the same low-fat diet.
Subsequent to the first 48 h, the subjects returned to their homes,
ate their normal diets without restrictions and then returned to
the clinic for the required blood draws.
Blood and urine samples were obtained for BBI
pharmacokinetic evaluation at the following times following
completion of drug ingestion: 0 (immediately prior to BBIC
administration), 0.5, 1, 2, 3, 4, 5, 6, 8, 10, 12, 24 and 48 h.
Additional blood samples were obtained at 12, 24 and 48 h, and on
or around days 7, 14, 21 and 28 for clinical blood chemistry and
toxicity evaluation.
Serum for pharmacokinetic analysis was separated
from blood cells and frozen at −80°C in 1-ml aliquots. Urine
samples were obtained at the same times as blood samples. At each
collection time, the subjects were directed to void their bladder.
All urine collected during that time interval was pooled, total
volume recorded and a sample was frozen at −20°C for
pharmacokinetic analysis.
Amylase, lipase and low density lipoprotein/high
density lipoprotein (LDL/HDL) were also assessed at times 0h, 12 h,
1 week and 4 weeks. Safety and toxicity were scored using the NCI
Common Toxicity Criteria scoring system version 2.0. Statistical
analysis consisted of tabulation of graded toxicities by dose
level.
Reagents used for BBI measurement
A mouse monoclonal antibody, designated 5G2, was
used as a primary antibody for BBI measurement by a dot-blot
analysis in this study. The 5G2 antibody was produced and
characterized as previously described (18). A horseradish peroxidase-conjugated
goat anti-mouse IgG2b antibody was purchased from Southern
Biotechnology Associates (Birmingham, AL, USA) and used as a
secondary antibody for the BBI measurement. BBI was purchased from
Sigma Chemicals (St. Louis, MO, USA) and radio-chemically reduced
by exposure to 137Cs γ-rays under anoxic conditions in a
buffer containing 100 mM formate and 10 mM phosphate buffer (PB; pH
5.5), as previously described (18). The reduced BBI was diluted to a
concentration of 50 μg/ml and used as a stock solution of BBI
standard antigen for BBI quantitation. Enhanced chemiluminescence
(ECL) reagent was purchased from GE Healthcare (Piscataway, NJ,
USA) for dot visualization in the dot-blot analysis.
BBI measurement by dot-blot analysis
The BBI in serum samples was measured using a
dot-blot analysis procedure. The serum samples were diluted in a
buffer containing 70% 10 mM sodium PB (pH 7.5) and 30% absolute
ethyl alcohol. For each dot-blot analysis, the BBI standard was
serially diluted in a buffer containing 70% 10 mM PB (pH 7.5) and
30% absolute ethyl alcohol to BBI concentrations of 0 (baseline),
10, 30, 50, 100, 150 and 200 ng/ml and analyzed along with the
serum samples to generate a standard curve for BBI
quantitation.
To perform the dot-blot analysis, three 10-μl
aliquots of each sample or BBI standard were spotted onto an
immobilion-PSQ membrane (Millipore, Billerica, MA, USA), with each
membrane containing the entire set of serum samples from two or
three subjects in addition to the BBI standards. The membranes were
allowed to dry completely at room temperature. The membranes were
rinsed with 10 mM PB (pH 7.5) for 2 min, blocked with 5% milk for
30 min, rinsed with PB three times for 5 min each and incubated
with the primary antibody for 1 h. Following incubation, the
membranes were rinsed again with PB three times for 5 min each and
incubated with the secondary antibody for 1 h. The membranes were
washed with PB and incubated with ECL reagent for 1 min, and then
exposed to X-ray film.
The integrated density of each spot on the membrane
was obtained by scanning the X-ray films using ImageJ software
(Sigmaplot version 12.0) from the National Institutes of Health
(http://rsbweb.nih.gov/ij/index.html)
following background subtraction. For each dot-blot analysis,
triplicate values of each BBI standard were averaged and a standard
curve was established by a linear regression analysis using the BBI
concentration as the independent variable and the averaged
integrated density as the dependent variable. The BBI concentration
in each serum sample was determined using the standard curve
generated on the same dot-blot membrane.
Pharmacokinetic and statistical
analysis
The serum BBI levels were analyzed using
pharmacokinetic function macros developed for Microsoft Excel
(http://www.boomer.org/pkin/soft.html). The data for
the area under the curve (AUC) in each clinical trial were analyzed
by linear regression analyses and compared among different
treatment groups by one-way ANOVA followed by Tukey’s test. The AUC
data from the two clinical trials were further analyzed by two-way
ANOVA using the BBIC dose and BBIC formulation (original vs. new)
as the independent variables. P<0.05 was considered to indicate
a statistically significant result.
Results
First BBIC study
A total of 37 patients were screened and a total of
20 subjects were enrolled in the initial BBIC study between
December 2005 and March 2007. The characteristics of the patients
enrolled are listed in Table I. No
subject was lost to follow-up. Adverse events were observed in
placebo- and BBIC-treated subject groups at approximately equal
frequency. A total of 50 adverse events were observed in the 16
BBIC-treated subjects (mean, 3.125 per subject) vs. 13 in the 4
subjects receiving the placebo (3.25 per subject). The rates of
adverse events did not increase with dose, and in the majority of
cases were lower in the highest dose group than in the other
groups. The exceptions to this were incidents of grade 1
hyperglycemia [6 reported incidents in the 16 treated subjects (3
in subjects receiving 2,000 CI units) vs. 1 incident in a subject
receiving placebo] and a single report of hyperkalemia in a subject
receiving 2,000 CI units (Tables
II and III). The only grade 3
adverse event was a high alanine aminotransferase (ALT) level in a
subject who received 800 CI units. The subject’s ALT levels were
observed to be normal during the inpatient portion of the study (48
h post-ingestion time period). During the subject’s one week
follow-up visit, the grade 3 high ALT was observed, but by the
second week, the ALT had resolved to a normal level.
 | Table ICharacteristics of patients in each
BBIC study. |
Table I
Characteristics of patients in each
BBIC study.
| First BBIC study | Second BBIC
study |
|---|
|
|
|
|---|
| Characteristic | BBIC | Placebo | BBIC | Placebo |
|---|
| Caucasian, n | 13 | 4 | 9 | 3 |
| African descent,
n | 1 | 0 | 5 | 0 |
| Asian or Pacific
Islander, n | 1 | 0 | 2 | 1 |
| Hispanic, n | 1 | 0 | 0 | 0 |
| Mixed ethnicity,
n | 0 | 0 | 1 | 0 |
| Mean age, years | 27.9 | 33.3 | 30.2 | 34.9 |
 | Table IIMaximum toxicity in BBIC-treated
subjects (combined) in the first BBIC trial (n=16). |
Table II
Maximum toxicity in BBIC-treated
subjects (combined) in the first BBIC trial (n=16).
| Maximum toxicity
grade |
|---|
|
|
|---|
| Toxicity type | 0 | 1 | 2 | 3 | 4 |
|---|
| ALT, n | 14 | 1 | 0 | 1 | 0 |
| AST, n | 12 | 3 | 1 | 0 | 0 |
| Bilirubin,
serum-high (hyperbilirubinemia), n | 15 | 1 | 0 | 0 | 0 |
| Calcium, serum-low
(hypocalcemia), n | 14 | 2 | 0 | 0 | 0 |
| Cholesterol,
serum-high (hypercholesteremia), n | 14 | 2 | 0 | 0 | 0 |
| Glucose, serum-low
(hypoglycemia), n | 2 | 10 | 4 | 0 | 0 |
| Glucose, serum-high
(hyperglycemia), n | 10 | 6 | 0 | 0 | 0 |
| Headache, n | 14 | 2 | 0 | 0 | 0 |
| Hemoglobin, n | 15 | 1 | 0 | 0 | 0 |
| Infection,
cold-sore (lip), n | 15 | 0 | 1 | 0 | 0 |
| Leukocytosis,
n | 12 | 4 | 0 | 0 | 0 |
| Potassium,
serum-high (hyperkalemia), n | 15 | 1 | 0 | 0 | 0 |
| Sodium, serum-high
(hypernatremia), n | 11 | 5 | 0 | 0 | 0 |
| Triglyceride,
serum-high (hypertriglyceridemia), n | 14 | 2 | 0 | 0 | 0 |
 | Table IIIMaximum toxicity in placebo-treated
subjects in the first BBIC trial (n=4). |
Table III
Maximum toxicity in placebo-treated
subjects in the first BBIC trial (n=4).
| Maximum toxicity
grade |
|---|
|
|
|---|
| Toxicity type | 0 | 1 | 2 | 3 | 4 |
|---|
| Albumin, n | 3 | 1 | 0 | 0 | 0 |
| ALT, n | 4 | 0 | 0 | 0 | 0 |
| AST, n | 3 | 1 | 0 | 0 | 0 |
| Bilirubin,
serum-high (hyperbilirubinemia), n | 2 | 2 | 0 | 0 | 0 |
| Calcium, serum-low
(hypocalcemia), n | 2 | 2 | 0 | 0 | 0 |
| Cholesterol,
serum-high (hypercholesteremia), n | 3 | 1 | 0 | 0 | 0 |
| Glucose, serum-low
(hypoglycemia), n | 1 | 2 | 1 | 0 | 0 |
| Glucose, serum-high
(hyperglycemia), n | 3 | 1 | 0 | 0 | 0 |
| Headache, n | 4 | 0 | 0 | 0 | 0 |
| Hemoglobin, n | 3 | 1 | 0 | 0 | 0 |
| Infection,
cold-sore (lip), n | 4 | 0 | 0 | 0 | 0 |
| Leukocytosis,
n | 4 | 0 | 0 | 0 | 0 |
| Potassium,
serum-high (hyperkalemia), n | 4 | 0 | 0 | 0 | 0 |
| Sodium, serum-high
(hypernatremia), n | 4 | 0 | 0 | 0 | 0 |
| Triglyceride,
serum-high (hypertriglyceridemia), n | 3 | 1 | 0 | 0 | 0 |
Second BBIC study
A total of 34 subjects were screened and a total of
21 subjects were enrolled in the second BBIC trial between June
2007 and March 2009. No subject was lost to follow-up. A dosing
error occurred for one subject, who received 1,500 CI units BBIC
instead of 1,200 CI units BBIC. This subject’s data were reported
separately in this analysis and the subject was replaced by an
additional patient in the 1,200 CI units BBIC dose cohort. Hence,
there were a total of 21 subjects in the second study instead of
the initially intended 20 subjects. No other dosing anomalies
occurred.
No serious adverse events were reported during the
study. The only grade 2 toxicities reported were abnormal
triglyceride and aspartate aminotransferase (AST) levels and
hypoglycemia. The single occurrence of a grade 2 abnormal
triglyceride level occurred in a patient receiving the placebo
(Table IV). The single occurrence
of a grade 2 abnormal AST level occurred in a patient in the 1,600
CI units BBIC dose level (Table V). Ten occurrences of grade 2
hypoglycemia were experienced at 800 CI units BBIC (3 subjects),
1,200 CI units BBIC (3 subjects), 1,600 CI units BBIC (2 subjects)
and 2,000 CI units BBIC (2 subjects), and therefore did not appear
to be correlated with dose.
 | Table IVMaximum toxicity in placebo-treated
control subjects for the second BBIC trial (n=4). |
Table IV
Maximum toxicity in placebo-treated
control subjects for the second BBIC trial (n=4).
| Maximum toxicity
grade |
|---|
|
|
|---|
| Toxicity type | 0 | 1 | 2 | 3 | 4 |
|---|
| Alkaline
phosphatase, n | 3 | 1 | 0 | 0 | 0 |
| AST, n | 3 | 1 | 0 | 0 | 0 |
| Calcium, serum-low
(hypocalcemia), n | 4 | 0 | 0 | 0 | 0 |
| Calcium, serum-high
(hypercalcemia), n | 4 | 0 | 0 | 0 | 0 |
| Creatinine, n | 4 | 0 | 0 | 0 | 0 |
| Dizziness, n | 4 | 0 | 0 | 0 | 0 |
| Cholesterol,
serum-high (hypercholesteremia), n | 2 | 2 | 0 | 0 | 0 |
| Glucose, serum-low
(hypoglycemia), n | 0 | 4 | 0 | 0 | 0 |
| Glucose, serum-high
(hyperglycemia), n | 1 | 3 | 0 | 0 | 0 |
| Hemoglobin, n | 3 | 1 | 0 | 0 | 0 |
| Hemorrhage, GU -
Bladder, n | 3 | 1 | 0 | 0 | 0 |
| Hemorrhage, GU -
Urinary NOS, n | 4 | 0 | 0 | 0 | 0 |
| Lipase, n | 4 | 0 | 0 | 0 | 0 |
|
Neutrophils/granulocytes (ANC/AGC), n | 4 | 0 | 0 | 0 | 0 |
| Potassium,
serum-low (hypokalemia), n | 4 | 0 | 0 | 0 | 0 |
| Potassium,
serum-high (hyperkalemia), n | 4 | 0 | 0 | 0 | 0 |
| Sodium, serum-high
(hypernatremia), n | 4 | 0 | 0 | 0 | 0 |
| Triglyceride,
serum-high (hypertriglyceridemia), n | 1 | 2 | 1 | 0 | 0 |
Pharmacokinetic characterization of the
original and new BBIC formulations
The BBI concentration in the serum samples collected
from subjects orally administered with BBIC at doses of up to 2,000
CI units was analyzed by a dot-blot analysis procedure using the
5G2 monoclonal antibody, which is specific for reduced BBI. Based
on the signal responses of the BBI standards included in the assay,
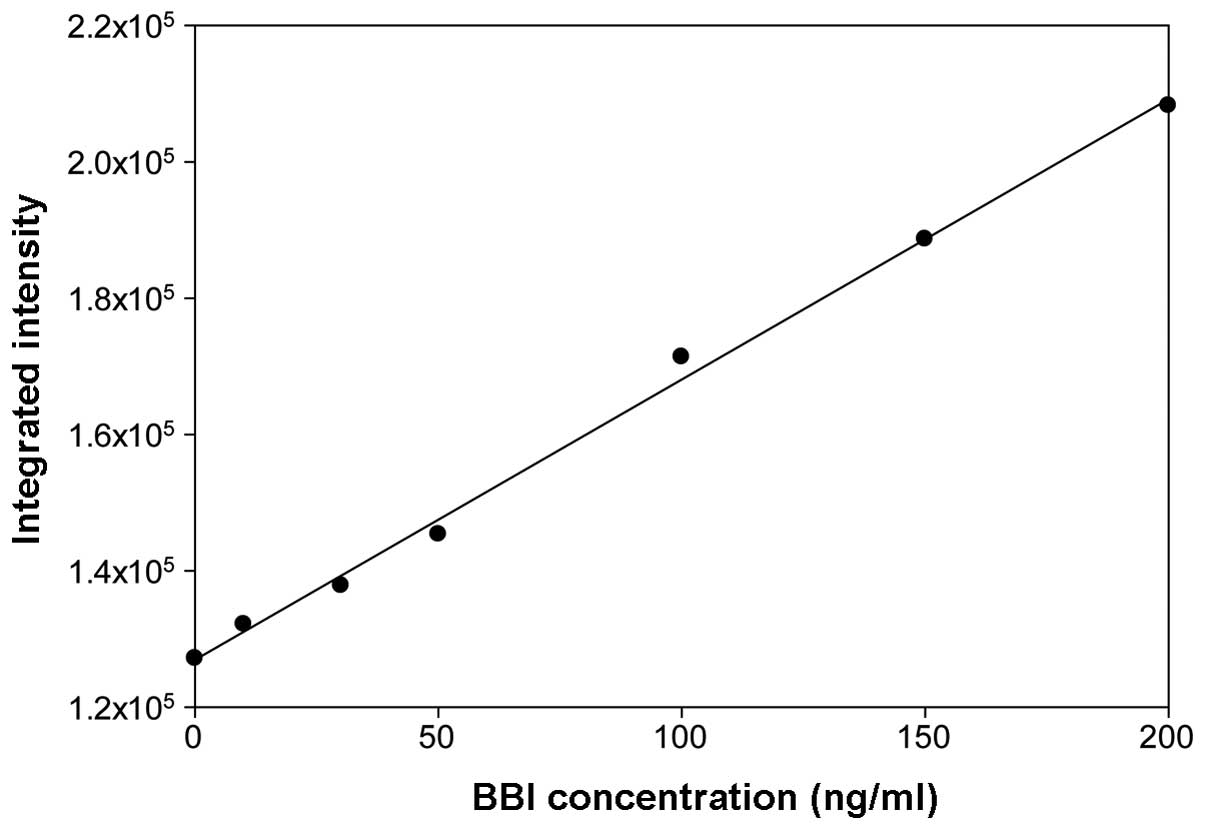
the dot-blot analysis was linear in a BBI concentration range of
0–200 ng/ml (Fig. 1). Preliminary
analyses of 1:200 diluted serum samples produced lower BBI results
in the dot-blot analyses for the subjects who received the highest
dose of BBIC than for the subjects in the other treatment groups
(data not shown), which indicated the presence of a hook effect of
falsely low values in immunoassays when an excess of antigen
affects the binding capacity of the detection antibody (18–20).
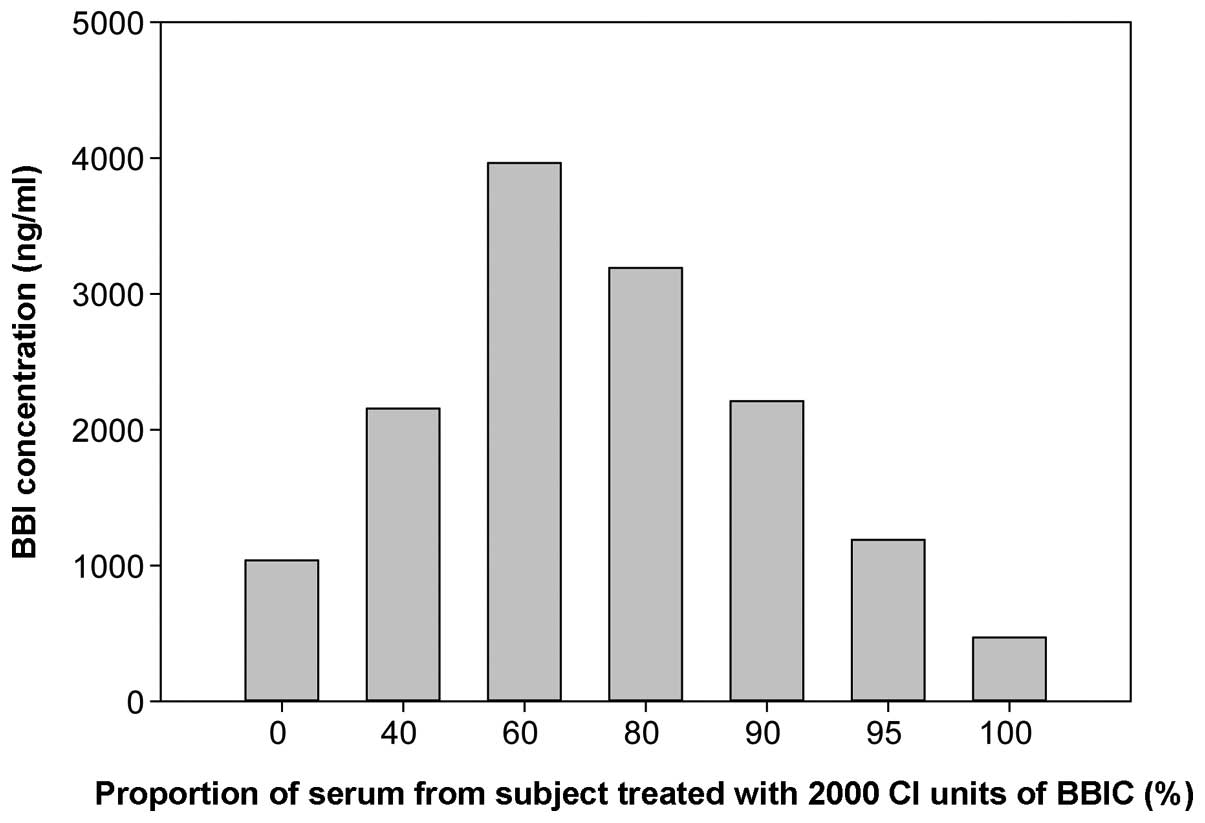
To confirm the presence of the hook effect, a serum sample from a
subject treated with the highest dose of BBIC (2,000 CI units) was
mixed with a serum sample from a subject treated with placebo in
various proportions and subjected to the dot-blot analysis. The
results indicated that the measured BBI values increased with the
proportion of serum from the BBIC treated subject up to 60% and
then declined (Fig. 2). The results
confirmed the presence of a hook effect in the dot-blot analysis
for the 1:200 diluted serum samples from the subject in the highest
BBIC dose group.
To avoid a possible hook effect on the serum BBI
measurement, the serum samples from subjects in the two highest
BBIC dose groups were diluted 1:500 for the dot-blot analysis. The
serum samples from subjects treated with placebo or BBIC at the two
low doses (800 and 1,200 CI units) were diluted at 1:200 for the
dot-blot analysis, since the BBI concentration in these samples was
not predicted to be high enough to cause a hook effect.
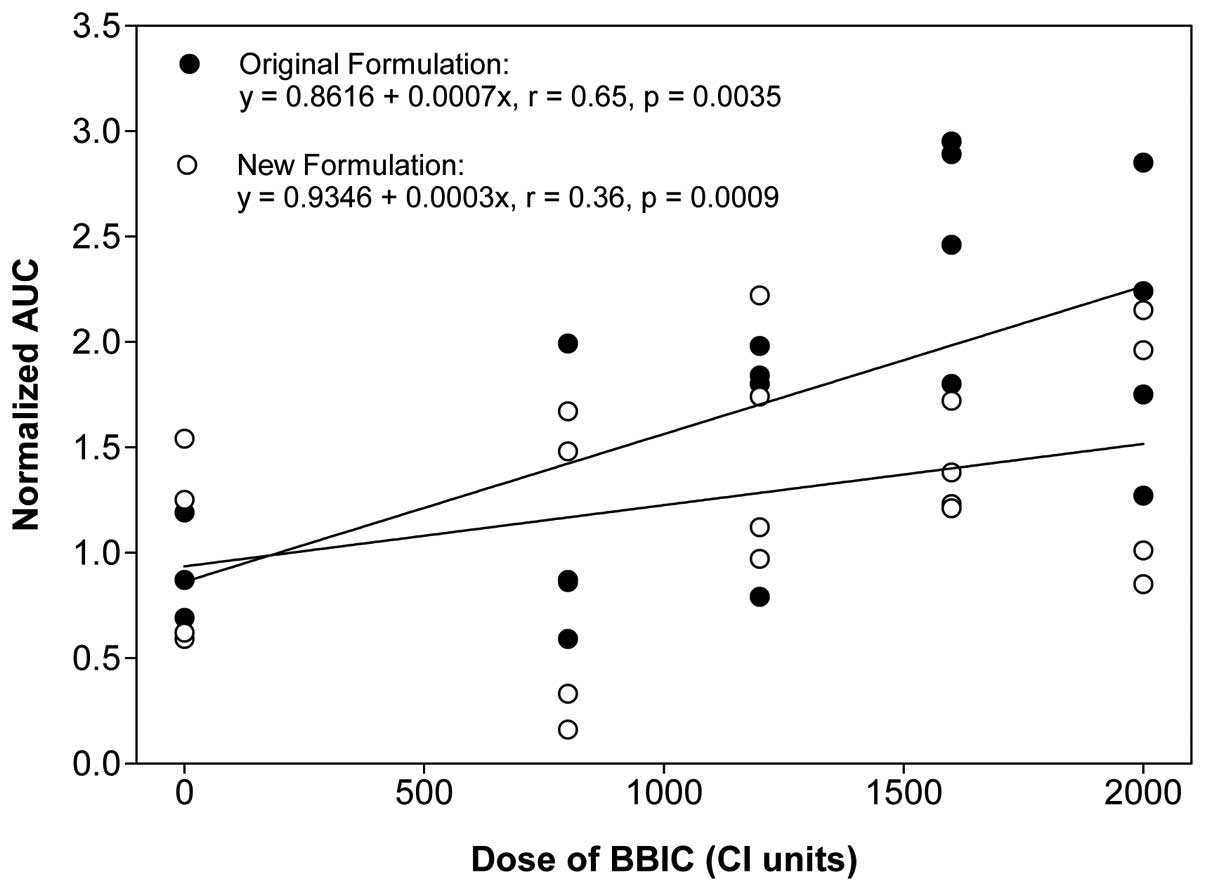
For the first phase I trial, the normalized AUC for
the serum BBI level was moderately correlated with the BBIC dose,
with a correlation coefficient of 0.65 and a slope value of 0.0007
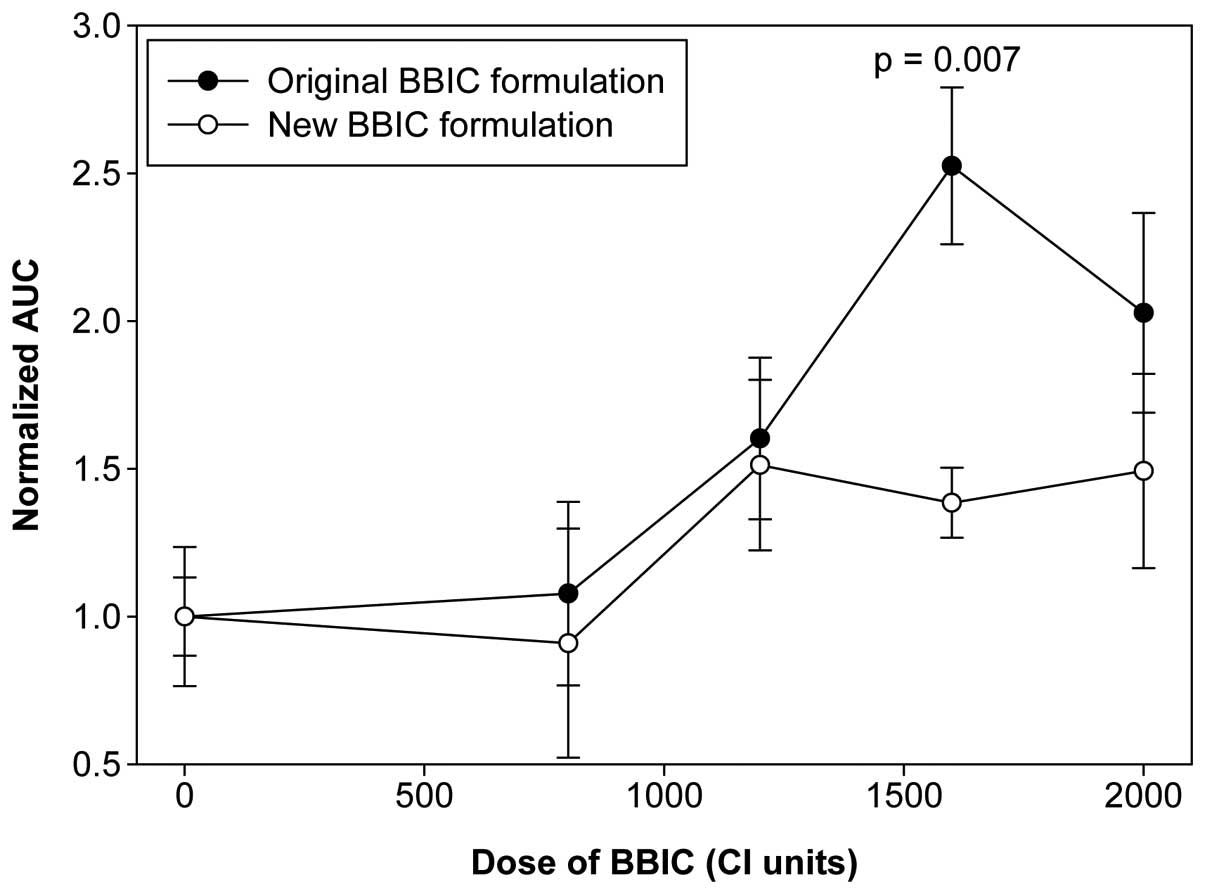
(Fig. 3). The mean normalized AUC
values for subjects treated with placebo or BBIC at doses of 800,
1,200, 1,600 or 2,000 CI units were 1.00±0.13, 1.08±0.31,
1.60±0.27, 2.53±0.27 and 2.03±0.34, respectively (Fig. 4). The difference among the treatment
groups was statistically significant (P=0.006 by one-way
ANOVA).
For the second phase I trial, the normalized AUC for
the serum BBI level was only weakly correlated with the BBIC dose,
with a correlation coefficient of 0.36 and a slope value of 0.0003
(Fig. 3). The mean normalized AUC
values for the subjects treated with placebo or BBIC at doses of
800, 1,200, 1,600 or 2,000 CI units were 1.00±0.24, 0.91±0.39,
1.51±0.29, 1.39±0.12 and 1.49±0.33, respectively (Fig. 4), and the difference was not
statistically significant (P=0.446 by one-way ANOVA).
To determine whether the dose-responses of the serum
BBI levels were different between the first clinical trial, which
used the original BBIC formulation, and the second clinical trial,
which used the new BBIC formulation, the AUC data obtained in the
two clinical trials were analyzed by a two-way ANOVA. The results
indicate that the serum BBI level expressed as the AUC was
significantly affected by the BBIC dose (P=0.022) and the BBIC
formulation (P=0.031) received by the subjects. The mean normalized
AUC value for BBIC-treated subjects (all doses combined) was 1.808
and 1.325 for the first and second clinical trials, respectively,
and the difference was statistically significant (P=0.031). After
subtracting the baseline normalized AUC value of 1 for the placebo
treatment group, the net increases in the mean normalized AUC value
for BBIC treated subjects in the first and second clinical trials
were 0.808 and 0.325, respectively.
Discussion
Two phase I randomized double-blind pharmacokinetic
and safety trials were conducted using two different formulations
of BBIC, a candidate chemopreventive agent, administered orally as
a suspension in OJ. These clinical trials were initiated in
preparation for a prostate chemoprevention program in prostate
cancer at the University of Pennsylvania. The main objectives of
the pharmacokinetic analyses were to measure and characterize the
levels of BBI in serum and urine following a single dose of BBIC or
placebo in healthy male subjects; these analyses will be reported
separately. No serious adverse events were observed. No clinically
significant laboratory abnormalities were reported for any dose
level.
Four human trials utilizing BBIC have been completed
previously (13–15,22–24).
Phase I and phase IIa studies of BBIC in patients with oral
leukoplakia were also performed (13,15,22,23).
The results from these trials indicated no toxicity from BBIC at
any dose level studied. Over the dose range of 200–1,000 CI units
per day, BBIC caused a reduction of total oral leukoplakia lesion
size that was linearly correlated with increase in dose. The
compound was well-tolerated with no evidence of laboratory,
symptomatic or clinical side-effects (23). A randomized, double-blind trial of
BBIC in patients with benign prostatic hyperplasia (BPH) was also
performed (14,24). This trial involved 6 months of BBIC
treatment involving dosage levels of 100–800 CI units per day in
oral tablet form. There was no dose-limiting toxicity of BBIC
observed in this study. For the BBIC single-dose phase I trials
reported in the present study, the dose range chosen was higher
than any that had been used previously in BBIC human trials
(800–2,000 CI units). No dose-limiting toxicity was observed for
BBIC, even at the highest dose evaluated (2,000 CI units), and the
results from the two trials were comparable for the studies
involving two different formulations of BBIC. Thus, the results
from the two trials indicate that a multidose trial of BBIC may be
performed with doses of up to 2,000 CI units per day.
The pharmacokinetic studies using urine samples from
previously completed clinical trials have shown that BBI is
excreted rapidly in the urine between 3 and 12 h after BBIC
administration (13). Linear
regression analyses of the BBI results demonstrated dose-dependent
increases in mean BBI concentration, peak BBI concentration, peak
minus mean BBI concentration and the peak to mean ratio of BBI
concentration in the urine samples of subjects following BBIC
treatment (14). These results
indicate that BBI is absorbed systemically in human subjects
following oral administration of BBIC. In the present study, serum
BBI levels were determined for subjects enrolled in two new
clinical trials using the original and new BBIC formulations. The
results demonstrate that the AUC value for the serum BBI level was
significantly correlated with the dose of BBIC received by the
subjects in the two trials, however, the slope of the linear
regression line for the first trial (slope=0.0007) was more than
twice as steep as the slope of the linear regression line for the
second clinical trial (slope=0.0003). In addition, the mean
normalized AUC value for the serum BBI level in the BBIC-treated
subjects was significantly higher in the first clinical trial than
in the second clinical trial. Based on the ratio of regression line
slopes between the two clinical trials (0.0003/0.0007=0.4286) and
the ratio of the net increase in the mean normalized AUC values
(0.325/0.808=0.4022), the bioavailability of BBI in the second
clinical trial was approximately 40 to 43% of the BBI
bioavailability reached in the first clinical trial. Since the two
clinical trials were performed using the same experimental design,
with the exception of the BBIC formulation, the differences
observed are attributable to the different BBIC formulations used
in these two clinical trials.
While the CI activity in the original and new
formulations of BBIC appeared to be comparable, there are numerous
variables that may affect the biological activities of BBI/BBIC, as
previously discussed (11). Two of
these factors are the refrigeration and freezing of BBIC samples,
which result in a reduced ability of the samples to affect
radiation-induced transformation in vitro (11). The new formulation of BBIC was
exposed and maintained under refrigerated and frozen conditions
during the purification procedure used by DE or the subsequent
storage of the new product, while it is recommended that the
original formulation of BBIC should not be exposed to either
refrigerated or frozen conditions. It is likely that the
temperatures, or the new solvents (ethyl acetate and methanol) used
by DE in the production of the new formulation of BBIC, may have
altered the bioavailability of BBI compared to the BBI in the
original formulation of BBIC.
Prior to the BBIC clinical trials in humans,
information about the absorption, distribution and excretion of BBI
was primarily based on animal studies utilizing radiolabeled BBI.
These studies indicated that approximately half of the BBI
administered orally is excreted in the feces in an unaltered form,
whereas the remainder enters intestinal epithelial cells (25) or crosses the intestinal lumen via a
paracellular mechanism (26). In
animal studies, BBI is able to survive the digestive process, reach
the colon in an active form and is capable of interacting with
proteases in the same manner as expected for BBI (26,27).
The measurement of BBI in biological samples by immunoassay has
proven to be technically challenging. BBI is readily detectable
using monoclonal antibodies that have been generated by Brandon
et al (28) in food samples
(29) or in human serum and urine
samples spiked with purified BBI in its native form (30). Oral administration of BBI results in
a form of BBI in the bloodstream and urine that cannot be detected
with the antibodies against BBI in its native form (18), despite the fact that the BBI
appearing in blood and urine following oral intake has the same
molecular weight and the same ability to inhibit trypsin and
chymotrypsin as BBI (26). Since it
is necessary to use antibodies reactive with reduced BBI to detect
BBI in blood and urine samples from subjects following BBI oral
intake (18,31), it is assumed that BBI is present in
a reduced form in body fluids. Pharmacokinetic studies of BBI have
previously been performed in rodents, dogs and humans with
antibodies that react with reduced BBI (reviewed in 32). As part of subchronic toxicity
studies of BBIC in rats and dogs, serum concentrations of BBI were
measured using one of the antibodies that reacts specifically with
reduced BBI, known as 5G2 (18), by
a dot-blot method. As summarized previously (32), the serum BBI level was 32–48% higher
in rats treated with oral daily doses of 500 or 1,000 mg/kg BBIC
for 3 months and 35–50% higher in dogs treated with oral daily
doses of 500 or 1,000 mg/kg BBIC for 45 or 89 days compared to
their respective control groups. The highest dose of BBIC
administered to human subjects in the present study was at least
one order of magnitude below the dose used in the previous rat and
dog toxicity studies on a kg-body weight basis. However, the
magnitude of increase in the mean normalized AUC value for the
serum BBI level of the highest BBIC dose groups in the present
study was of the same order of magnitude as previously observed in
animal toxicity studies, indicating that there may be an upper
limit for BBI absorption following oral administration. Based on
the shape of the dose-response curves of the serum BBI level
(Fig. 4), the serum BBI level
appeared to reach a plateau at the dose of 1,600 CI units, and
further increases in the BBIC dose did not result in an additional
increase in the BBI level in the circulation. This may have
practical implications for future clinical trials using BBIC or
other soybean-based dietary supplements with BBI as the main active
ingredient.
The results from the first and second trials of BBIC
utilizing the original and new BBIC formulations indicate that a
dose-limiting toxicity for BBIC was not observed up to a dose of
2,000 CI units. Therefore, it is proposed that a multidose BBIC
study may be extended up to a high dose of 2,000 CI units per
day.
Acknowledgements
This study was sponsored by NCI Contract/Grant
Number: NO1-CN-25118.
References
|
1
|
Messina MJ, Persky V, Setchell KD and
Barnes S: Soy intake and cancer risk: a review of the in vitro and
in vivo data. Nutr Cancer. 21:113–131. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kennedy AR: The evidence for soybean
products as cancer preventive agents. J Nutr. 125(3 Suppl):
733S–743S. 1995.PubMed/NCBI
|
|
3
|
Kennedy AR: Chemopreventive agents:
protease inhibitors. Pharmacol Ther. 78:167–209. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Larionova NI, Gladysheva IP, Tikhonova TV
and Kazanskaia NF: Inhibition of cathepsin G and elastase from
human granulocytes by multiple forms of the Bowman-Birk type of soy
inhibitor. Biokhimiia. 58:1437–1444. 1993.(In Russian).
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tikhonova TV, Gladysheva IP, Kazanshaya NF
and Larionova NI: Inhibition of elastin hydrolysis, catalyzed by
human leukocyte elastase and cathepsin G, by the Bowman-Birk type
soy inhibitor. Biokhimiia. 59:1739–1745. 1994.(In Russian).
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gladysheva IP, Larionova NI, Gladyshev DP,
Tikhonova TV and Kazanskaia NF: The classical Bowman-Birk soy
inhibitor is an effective inhibitor of human granulocyte
alpha-chymotrypsin and cathepsin G. Biokhimiia. 59:513–518.
1994.(In Russian).
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ware JH, Wan XS, Rubin H, Schechter NM and
Kennedy AR: Soybean Bowman-Birk protease inhibitor is a highly
effective inhibitor of human mast cell chymase. Arch Biochem
Biophys. 344:133–138. 1997. View Article : Google Scholar
|
|
8
|
Kennedy AR: Cancer prevention by protease
inhibitors. Prev Med. 22:796–811. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kennedy AR: Anticarcinogenic activity of
protease inhibitors: Overview. Protease Inhibitors as Cancer
Chemopreventive Agents. Troll W and Kennedy AR: 1st edition. Plenum
Press; New York: pp. 9–64. 1993, View Article : Google Scholar
|
|
10
|
Kennedy AR: Chemopreventive agents:
protease inhibitors. Pharmacol Ther. 78:167–209. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kennedy AR, Szuhaj BF, Newberne PM and
Billings PC: Preparation and production of a cancer chemopreventive
agent, Bowman-Birk inhibitor concentrate. Nutr Cancer. 19:281–302.
1993. View Article : Google Scholar
|
|
12
|
Kennedy AR: In vitro studies of
anticarcinogenic protease inhibitors. Protease Inhibitors as Cancer
Chemopreventive Agents. Troll W and Kennedy AR: 1st edition. Plenum
Press; New York: pp. 65–91. 1993, View Article : Google Scholar
|
|
13
|
Armstrong WB, Kennedy AR, Wan XS, Atiba J,
McLaren CE and Meyskens FL Jr: Single-dose administration of
Bowman-Birk inhibitor concentrate in patients with oral
leukoplakia. Cancer Epidemiol Biomarkers Prev. 9:43–47.
2000.PubMed/NCBI
|
|
14
|
Malkowicz SB, McKenna WG, Vaughn DJ, et
al: Effects of Bowman-Birk inhibitor concentrate (BBIC) in patients
with benign prostatic hyperplasia. Prostate. 48:16–28. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Armstrong WB, Kennedy AR, Wan XS, et al:
Clinical modulation of oral leukoplakia and protease activity by
Bowman-Birk inhibitor concentrate in a phase IIa chemoprevention
trial. Clin Cancer Res. 6:4684–4691. 2000.
|
|
16
|
Lichtenstein GR, Deren JJ, Katz S, Lewis
JD, Kennedy AR and Ware JH: Bowman-Birk inhibitor concentrate: a
novel therapeutic agent for patients with active ulcerative
colitis. Dig Dis Sci. 53:175–180. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Oken MM, Creech RH, Tormey DC, et al:
Toxicity and response criteria of the Eastern Cooperative Oncology
Group. Am J Clin Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wan XS, Koch CJ, Lord EM, et al:
Monoclonal antibodies differentially reactive with native and
reductively modified Bowman-Birk protease inhibitor. J Immunol
Methods. 180:117–130. 1995. View Article : Google Scholar
|
|
19
|
Furuya Y, Cho S, Ohta S, Sato N, Kotake T
and Masai M: High dose hook effect in serum total and free prostate
specific antigen in a patient with metastatic prostate cancer. J
Urol. 166:2132001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fleseriu M, Lee M, Pineyro MM, et al:
Giant invasive pituitary prolactinoma with falsely low serum
prolactin: the significance of ‘hook effect’. J Neurooncol.
79:41–43. 2006.PubMed/NCBI
|
|
21
|
McCudden CR, Voorhees PM and
Hammett-Stabler CA: A case of hook effect in the serum free light
chain assay using the Olympus AU400e. Clin Biochem. 42:121–124.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Meyskens FL Jr, Armstrong WB, Wan XS, et
al: Bowman-Birk inhibitor concentrate (BBIC) affects oral
leukoplakia lesion size, neu protein levels and proteolytic
activity in buccal mucosal cells. Proc. Am. Assoc. Cancer Res.
40:Abstract #2855. 1999.
|
|
23
|
Wan XS, Meyskens FL Jr, Armstrong WB,
Taylor TH and Kennedy AR: Relationship between protease activity
and neu oncogene expression in patients with oral leukoplakia
treated with the Bowman Birk Inhibitor. Cancer Epidemiol Biomark
Prev. 8:601–608. 1999.
|
|
24
|
Malkowicz SB, Broderick GA, Zoltick B, et
al: Phase I study of oral Bowman-Birk inhibitor concentrate (BBIC),
a soy protein, on patients with lower urinary tract symptoms. J
Urol. 161:2281999. View Article : Google Scholar
|
|
25
|
Billings PC, Brandon DL and Habres JM:
Internalization of the Bowman-Birk protease inhibitor by intestinal
epithelial cells. Eur J Cancer. 27:903–908. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Billings PC, St Clair WH, Maki PA and
Kennedy AR: Distribution of the Bowman Birk protease inhibitor in
mice following oral administration. Cancer Lett. 62:191–197. 1992.
View Article : Google Scholar
|
|
27
|
Yavelow J, Finlay TH, Kennedy AR and Troll
W: Bowman-Birk soybean protease inhibitor as an anticarcinogen.
Cancer Res. 43(5 Suppl): 2454s–2459s. 1983.PubMed/NCBI
|
|
28
|
Brandon DL, Bates AH and Friedman M:
Monoclonal antibody-based enzyme immunoassay of the Bowman-Birk
protease inhibitor of soybeans. J Agric Food Chem. 37:1192–1196.
1989. View Article : Google Scholar
|
|
29
|
Brandon DL, Bates AH and Friedman M: ELISA
analysis of soybean trypsin inhibitors in processed foods. Adv Exp
Med Biol. 289:321–337. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Brandon DL: Antigenicity of soybean
protease inhibitors. Protease inhibitors as Cancer Chemopreventive
agents. Troll W and Kennedy AR: 1st edition. Plenum Press; New
York: pp. 107–129. 1993, View Article : Google Scholar
|
|
31
|
Wan XS, Lu LJ, Anderson KE, Ware JH and
Kennedy AR: Urinary excretion of Bowman-Birk inhibitor in humans
after soy consumption as determined by a monoclonal antibody-based
immunoassay. Cancer Epidemiol Biomark Prev. 9:741–747.
2000.PubMed/NCBI
|
|
32
|
Kennedy AR: The Bowman-Birk inhibitor from
soybeans as an anticarcinogenic agent. Am J Clin Nutr. 68(6 Suppl):
1406S–1412S. 1998.PubMed/NCBI
|