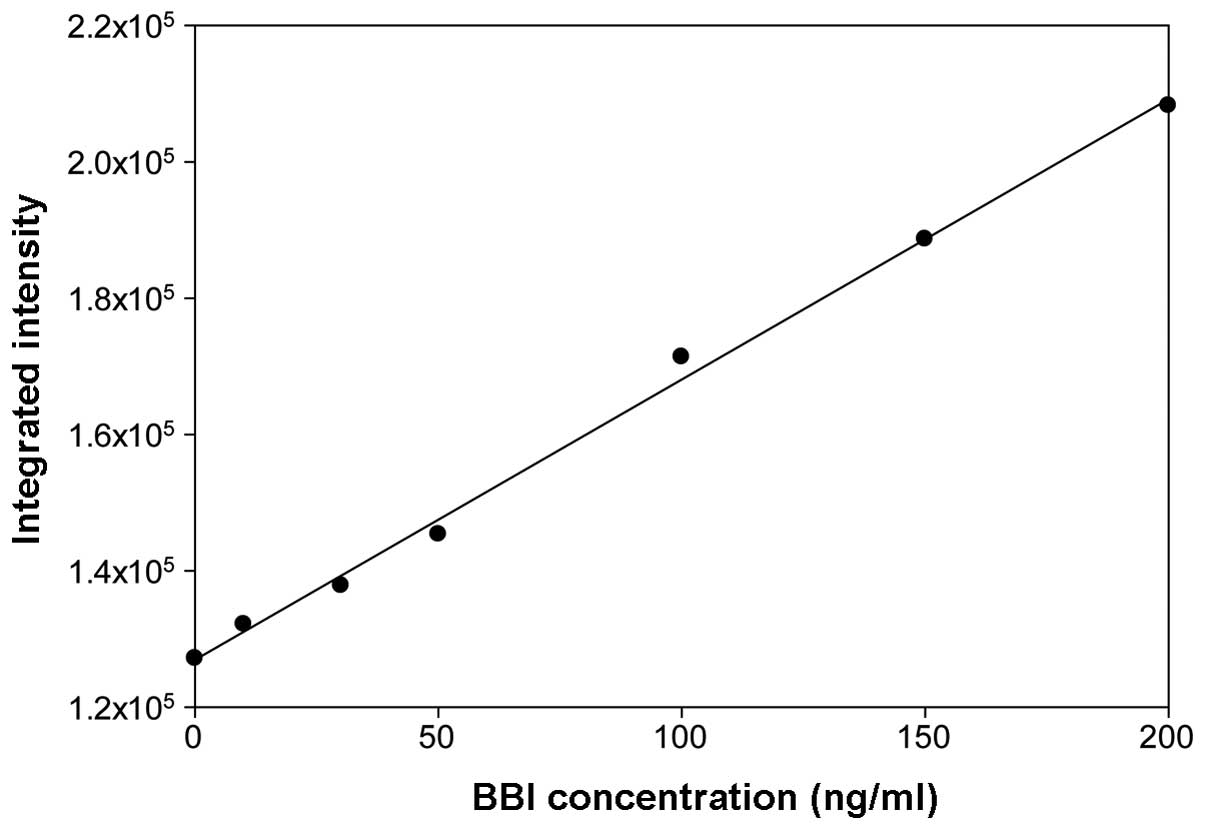
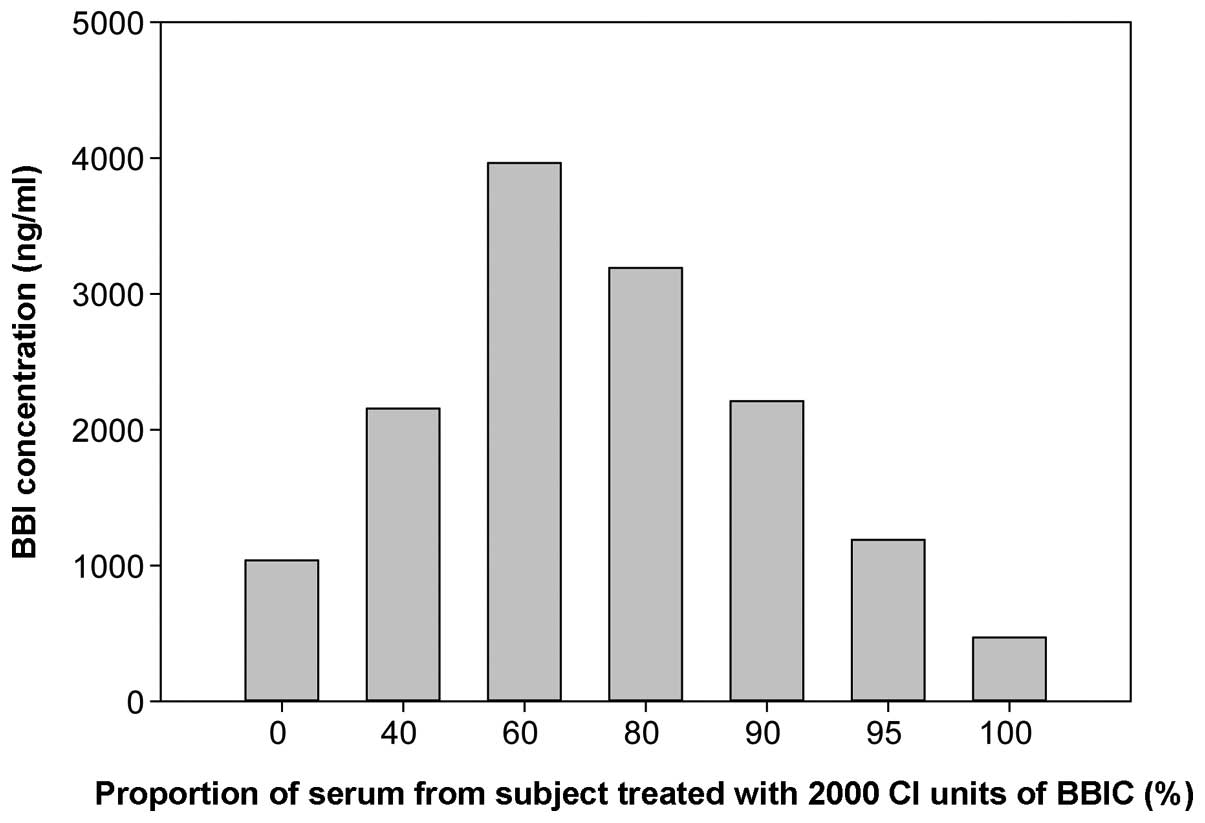
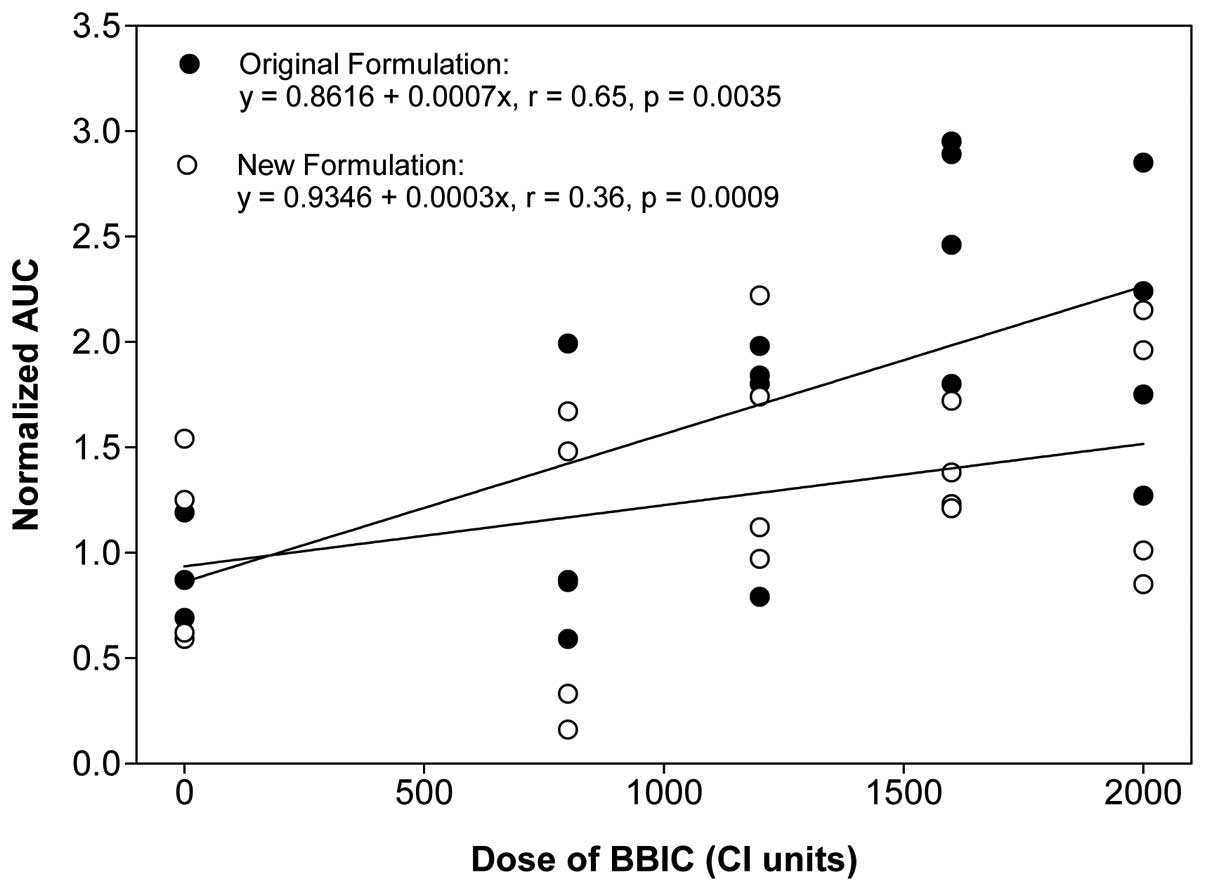
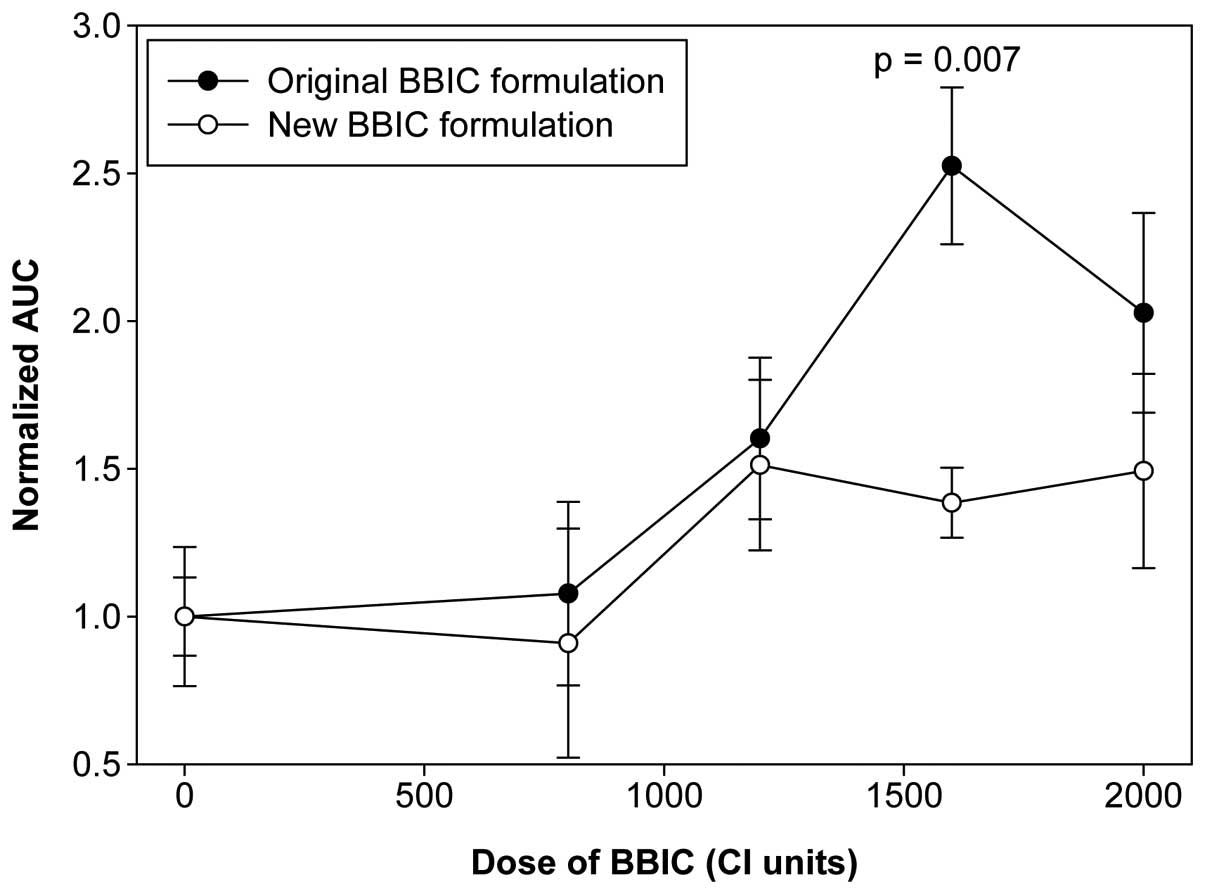
|
1
|
Messina MJ, Persky V, Setchell KD and
Barnes S: Soy intake and cancer risk: a review of the in vitro and
in vivo data. Nutr Cancer. 21:113–131. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kennedy AR: The evidence for soybean
products as cancer preventive agents. J Nutr. 125(3 Suppl):
733S–743S. 1995.PubMed/NCBI
|
|
3
|
Kennedy AR: Chemopreventive agents:
protease inhibitors. Pharmacol Ther. 78:167–209. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Larionova NI, Gladysheva IP, Tikhonova TV
and Kazanskaia NF: Inhibition of cathepsin G and elastase from
human granulocytes by multiple forms of the Bowman-Birk type of soy
inhibitor. Biokhimiia. 58:1437–1444. 1993.(In Russian).
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tikhonova TV, Gladysheva IP, Kazanshaya NF
and Larionova NI: Inhibition of elastin hydrolysis, catalyzed by
human leukocyte elastase and cathepsin G, by the Bowman-Birk type
soy inhibitor. Biokhimiia. 59:1739–1745. 1994.(In Russian).
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gladysheva IP, Larionova NI, Gladyshev DP,
Tikhonova TV and Kazanskaia NF: The classical Bowman-Birk soy
inhibitor is an effective inhibitor of human granulocyte
alpha-chymotrypsin and cathepsin G. Biokhimiia. 59:513–518.
1994.(In Russian).
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ware JH, Wan XS, Rubin H, Schechter NM and
Kennedy AR: Soybean Bowman-Birk protease inhibitor is a highly
effective inhibitor of human mast cell chymase. Arch Biochem
Biophys. 344:133–138. 1997. View Article : Google Scholar
|
|
8
|
Kennedy AR: Cancer prevention by protease
inhibitors. Prev Med. 22:796–811. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kennedy AR: Anticarcinogenic activity of
protease inhibitors: Overview. Protease Inhibitors as Cancer
Chemopreventive Agents. Troll W and Kennedy AR: 1st edition. Plenum
Press; New York: pp. 9–64. 1993, View Article : Google Scholar
|
|
10
|
Kennedy AR: Chemopreventive agents:
protease inhibitors. Pharmacol Ther. 78:167–209. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kennedy AR, Szuhaj BF, Newberne PM and
Billings PC: Preparation and production of a cancer chemopreventive
agent, Bowman-Birk inhibitor concentrate. Nutr Cancer. 19:281–302.
1993. View Article : Google Scholar
|
|
12
|
Kennedy AR: In vitro studies of
anticarcinogenic protease inhibitors. Protease Inhibitors as Cancer
Chemopreventive Agents. Troll W and Kennedy AR: 1st edition. Plenum
Press; New York: pp. 65–91. 1993, View Article : Google Scholar
|
|
13
|
Armstrong WB, Kennedy AR, Wan XS, Atiba J,
McLaren CE and Meyskens FL Jr: Single-dose administration of
Bowman-Birk inhibitor concentrate in patients with oral
leukoplakia. Cancer Epidemiol Biomarkers Prev. 9:43–47.
2000.PubMed/NCBI
|
|
14
|
Malkowicz SB, McKenna WG, Vaughn DJ, et
al: Effects of Bowman-Birk inhibitor concentrate (BBIC) in patients
with benign prostatic hyperplasia. Prostate. 48:16–28. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Armstrong WB, Kennedy AR, Wan XS, et al:
Clinical modulation of oral leukoplakia and protease activity by
Bowman-Birk inhibitor concentrate in a phase IIa chemoprevention
trial. Clin Cancer Res. 6:4684–4691. 2000.
|
|
16
|
Lichtenstein GR, Deren JJ, Katz S, Lewis
JD, Kennedy AR and Ware JH: Bowman-Birk inhibitor concentrate: a
novel therapeutic agent for patients with active ulcerative
colitis. Dig Dis Sci. 53:175–180. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Oken MM, Creech RH, Tormey DC, et al:
Toxicity and response criteria of the Eastern Cooperative Oncology
Group. Am J Clin Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wan XS, Koch CJ, Lord EM, et al:
Monoclonal antibodies differentially reactive with native and
reductively modified Bowman-Birk protease inhibitor. J Immunol
Methods. 180:117–130. 1995. View Article : Google Scholar
|
|
19
|
Furuya Y, Cho S, Ohta S, Sato N, Kotake T
and Masai M: High dose hook effect in serum total and free prostate
specific antigen in a patient with metastatic prostate cancer. J
Urol. 166:2132001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fleseriu M, Lee M, Pineyro MM, et al:
Giant invasive pituitary prolactinoma with falsely low serum
prolactin: the significance of ‘hook effect’. J Neurooncol.
79:41–43. 2006.PubMed/NCBI
|
|
21
|
McCudden CR, Voorhees PM and
Hammett-Stabler CA: A case of hook effect in the serum free light
chain assay using the Olympus AU400e. Clin Biochem. 42:121–124.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Meyskens FL Jr, Armstrong WB, Wan XS, et
al: Bowman-Birk inhibitor concentrate (BBIC) affects oral
leukoplakia lesion size, neu protein levels and proteolytic
activity in buccal mucosal cells. Proc. Am. Assoc. Cancer Res.
40:Abstract #2855. 1999.
|
|
23
|
Wan XS, Meyskens FL Jr, Armstrong WB,
Taylor TH and Kennedy AR: Relationship between protease activity
and neu oncogene expression in patients with oral leukoplakia
treated with the Bowman Birk Inhibitor. Cancer Epidemiol Biomark
Prev. 8:601–608. 1999.
|
|
24
|
Malkowicz SB, Broderick GA, Zoltick B, et
al: Phase I study of oral Bowman-Birk inhibitor concentrate (BBIC),
a soy protein, on patients with lower urinary tract symptoms. J
Urol. 161:2281999. View Article : Google Scholar
|
|
25
|
Billings PC, Brandon DL and Habres JM:
Internalization of the Bowman-Birk protease inhibitor by intestinal
epithelial cells. Eur J Cancer. 27:903–908. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Billings PC, St Clair WH, Maki PA and
Kennedy AR: Distribution of the Bowman Birk protease inhibitor in
mice following oral administration. Cancer Lett. 62:191–197. 1992.
View Article : Google Scholar
|
|
27
|
Yavelow J, Finlay TH, Kennedy AR and Troll
W: Bowman-Birk soybean protease inhibitor as an anticarcinogen.
Cancer Res. 43(5 Suppl): 2454s–2459s. 1983.PubMed/NCBI
|
|
28
|
Brandon DL, Bates AH and Friedman M:
Monoclonal antibody-based enzyme immunoassay of the Bowman-Birk
protease inhibitor of soybeans. J Agric Food Chem. 37:1192–1196.
1989. View Article : Google Scholar
|
|
29
|
Brandon DL, Bates AH and Friedman M: ELISA
analysis of soybean trypsin inhibitors in processed foods. Adv Exp
Med Biol. 289:321–337. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Brandon DL: Antigenicity of soybean
protease inhibitors. Protease inhibitors as Cancer Chemopreventive
agents. Troll W and Kennedy AR: 1st edition. Plenum Press; New
York: pp. 107–129. 1993, View Article : Google Scholar
|
|
31
|
Wan XS, Lu LJ, Anderson KE, Ware JH and
Kennedy AR: Urinary excretion of Bowman-Birk inhibitor in humans
after soy consumption as determined by a monoclonal antibody-based
immunoassay. Cancer Epidemiol Biomark Prev. 9:741–747.
2000.PubMed/NCBI
|
|
32
|
Kennedy AR: The Bowman-Birk inhibitor from
soybeans as an anticarcinogenic agent. Am J Clin Nutr. 68(6 Suppl):
1406S–1412S. 1998.PubMed/NCBI
|