Introduction
Lung cancer is the leading cause of
cancer-associated mortality worldwide. For patients with
early-stage (stage I and II) non-small cell lung cancer (NSCLC) and
for appropriately selected patients with locally advanced disease
(stage IIIA), complete surgical resection is the optimal treatment.
Although advancements in early diagnosis and treatment have been
made in the hope of improving survival, recurrence remains a major
problem. Reported recurrence rates following complete surgical
resection range between 30 and 75% (1–6) [~15%
for pathological stage (p-stage) I cases (7,8)]. The
majority of recurrent tumors are distant and >80% of recurrences
occur within the first 2 years after resection.
Several previous studies have evaluated the
prognostic factors associated with survival following recurrence of
NSCLC. Chemotherapy and radiation therapy are generally accepted
treatment options for recurrent NSCLC. Encouraging new treatments
[including epidermal growth factor receptor-tyrosine kinase
inhibitors (EGFR-TKIs), anaplastic lymphoma kinase inhibitors,
pemetrexed and bevacizumab for adenocarcinomas] have benefited
specific patients with advanced or recurrent NSCLC (9–15).
Identification of activating mutations of EGFR is one of the most
important developments in the field of NSCLC. EGFR mutations are
present predominantly in females, in never-smokers and in Asian
individuals, and are sensitive to EGFR-targeted therapy, including
gefitinib (16–18). The response rate to gefitinib is
almost 75% in patients with tumors harboring EGFR mutations in
Asian clinical trials (9–11,17,18).
These advances in post-recurrence therapy may improve overall
survival among patients who undergo surgery. The present study
investigated the clinicopathological factors influencing
post-recurrence survival and the effect of post-recurrence therapy
on Japanese NSCLC since 2002.
Patients and methods
Patients
The study group included 637 patients with NSCLC:
435 were diagnosed as having adenocarcinomas, 147 had squamous cell
carcinomas, 20 had adenosquamous carcinomas and 10 had large-cell
carcinomas. All had undergone complete resection at the Department
of Surgery, Nagoya City University Hospital (Nagoya, Japan) between
2002 and 2012. Patients who succumbed to disease without
identifiable recurrence were excluded from the study. The lung
tumors were classified according to the general rules for clinical
and pathological diagnosis of lung cancer in Japan (19).
The clinical and pathological characteristics of the
637 lung cancer patients were as follows: 435 cases at stage I, 93
at stage II and 109 at stage III. The mean age was 66.8±9.7 years
(range, 22–87 years). Males accounted for 401 cases and 236 were
female. Non-smokers numbered 232. Regarding the mutation of EGFR,
the majority of samples from these patients had been analyzed
previously (16,17,20,21).
EGFR mutations were present in 215 cases and 366 were wild type.
The remaining 56 were unknown. This study was approved by the
ethics committee of Nagoya City University (Nagoya, Japan). Written
informed consent was obtained from the patient.
Post-recurrence survival analysis
Clinical characteristics were retrieved from
available clinical records. The following clinicopathological
factors were assessed in the post-recurrence analysis: Age, gender,
smoking status, pathological stages and histology (adenocarcinoma
vs. others). The length of the recurrence-free period was
calculated in months between the date of resection and the date of
initial recurrence or the date of the last follow-up without
recurrence. To calculate the recurrence-free proportion, patients
known to have no recurrence at the date of last contact were
excluded from this study. The length of post-recurrence survival
was measured between the date of initial recurrence and the date on
which the patient succumbed to disease or the date on which the
patient was last known to be alive.
Statistical analysis
The overall survival of patients with lung cancers
was examined by the Kaplan-Meier methods and differences were
examined by the log-rank test. The other clinicopathological
characteristics were examined using Student’s t-test and
χ2 tests as appropriate. Analyses were performed using
Statview version 5.0 (Abacus Concepts Inc., Berkeley, CA, USA) or
Excel (Microsoft Corporation, Redmond, WA, USA) software and
P<0.05 was considered to indicate a statistically significant
differences.
Results
Of the 637 patients, 127 (19.9%) had recurrent
diseases, with a median age of 66.6±9.3 years (range, 29–85) at the
initial surgery. Median follow-up time for the patients from the
initial surgery was 38.3±24.5 months (range, 4–127 months). Median
post-recurrence survival time for these patients was 21.9±18.4
months (range, 1–98 months). The 1- and 2-year post-recurrence
survival proportions were 69.8 and 44.4%, respectively. The
recurrence rate was 12.6% in stage I cases, 37.6% in stage II and
33.9% in stage III.
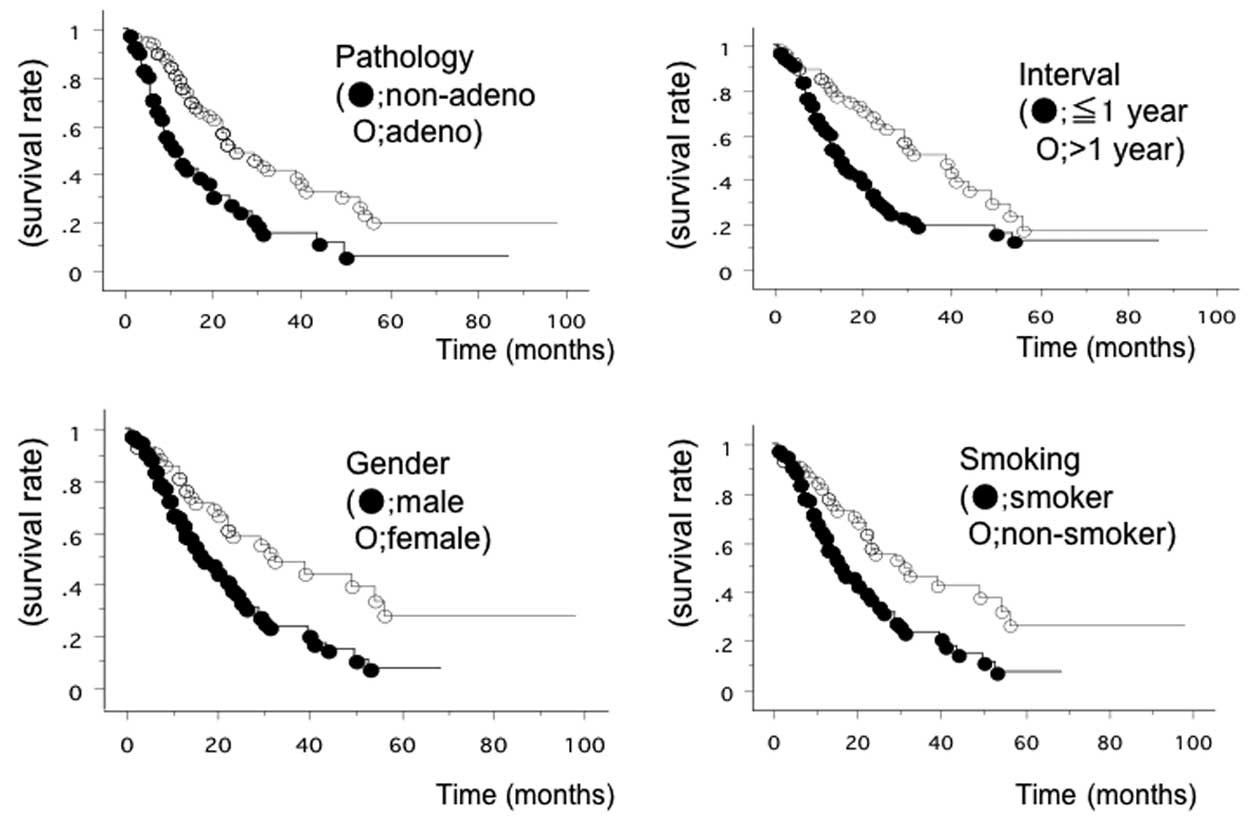
As shown in Table I,
univariate and multivariate analyses of recurrence, according to
the clinicopathological characteristics of NSCLC patients, were
performed. Univariate analysis identified 5 significant risk
factors: Male gender (P=0.0024), a history of smoking (P=0.0026),
non-adenocarcinoma histology (P=0.0002), an age of ≥65 years old at
recurrence (P=0.0060) and a recurrence interval of ≤1 year
(P=0.0016) (Fig. 1). Multivariate
analysis demonstrated that below 65 years old [hazard ratio (HR),
2.299; 95% confidence interval (CI), 1.374–3.846; P=0.0015) and a
recurrence interval of <1 year (HR, 2.119; 95% CI, 1.296–3.464;
P=0.027) were statistically significant predictors of shorter
survival. EGFR mutation itself was not a prognostic factor.
 | Table IClinicopathological data of 127 lung
cancer patients. |
Table I
Clinicopathological data of 127 lung
cancer patients.
| Prognostic
analysis |
|---|
|
|
|---|
| Univariate | Multivariate |
|---|
|
|
|
|---|
| Factors | Patients, n | MST, months | P-value | HR (P-value) |
|---|
| Age, years | | | 0.0060 | 2.299 (0.0015) |
| <65 | 41 | 34.2 | | |
| ≥65 | 86 | 23.2 | | |
| Smoking history | | | 0.0026 | 1.378 (0.4548) |
| Non-smoker | 81 | 22.2 | | |
| Smoker | 46 | 34.0 | | |
| Pathological
subtypes | | | 0.0002 | 1.541 (0.0752) |
| Adenocarcinoma | 86 | 31.6 | | |
|
Non-adenocarcinoma | 41 | 17.9 | | |
| Gender | | | 0.0024 | 1.201 (0.6771) |
| Male | 83 | 22.3 | | |
| Female | 44 | 34.5 | | |
| Recurrence interval,
years | | | 0.0016 | 2.119 (0.0027) |
| <1 | 70 | 21.4 | | |
| >1 | 57 | 34.1 | | |
| Brain metastasis | | | 0.2596 | |
| Yes | 18 | 14.5 | | |
| No | 109 | 27.7 | | |
| Bone metastasis | | | 0.4111 | |
| Yes | 28 | 24.1 | | |
| No | 99 | 27.8 | | |
| Liver metastasis | | | 0.0910 | |
| Yes | 4 | 14.5 | | |
| No | 123 | 24.8 | | |
Initial recurrence sites are shown in Table I and post-recurrent treatment is
shown in Table II. Liver
metastases were associated with a trend towards poorer prognosis
(P=0.091). However, none of the involved organs, including the
bones and brain were significant prognostic factors.
Post-recurrence treatment was administered in 114 patients (89.8%),
including chemotherapy for 99 patients and radiotherapy for 82.
Univariate analysis revealed the administration of chemotherapy,
but not radiotherapy, to be a prognostic factor. EGFR-TKIs were
used in 39 cases and such therapy was revealed to be a marginal
prognostic factor by univariate analysis. However, these were not
significant prognostic factors according to multivariate analysis.
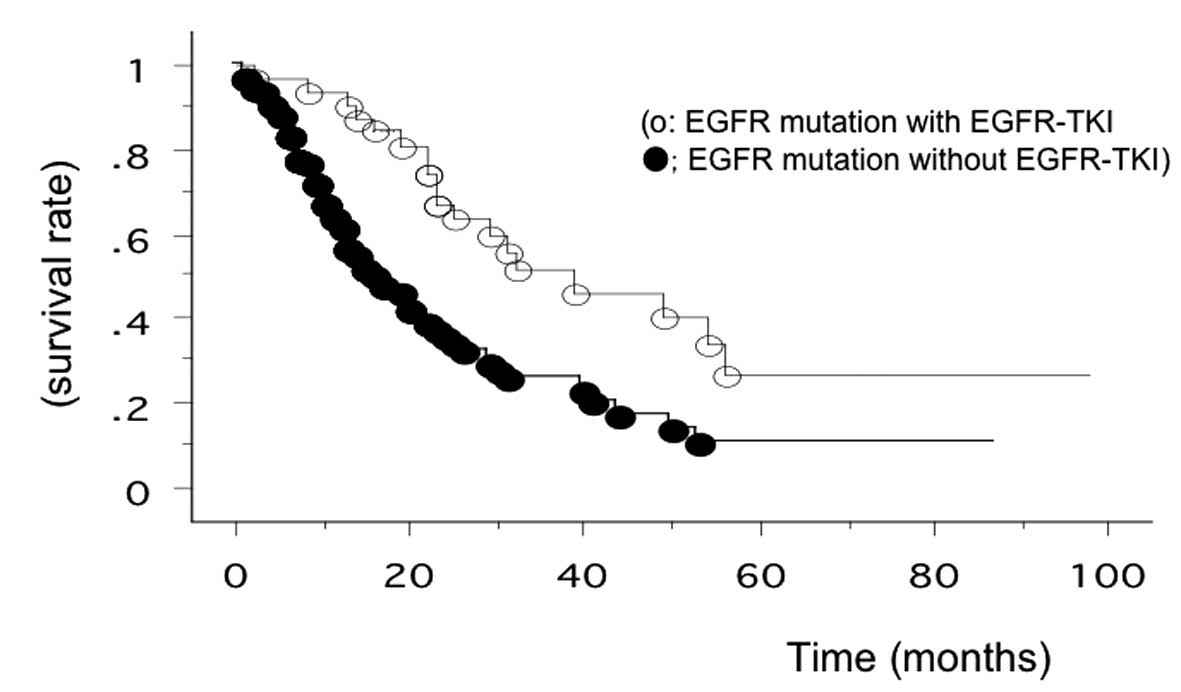
Among the 39 patients treated with EGFR-TKIs, 29 had EGFR
mutations. Subgroup analysis placed the overall survival of the 29
EGFR-mutant patients treated with EGFR-TKIs at 37.4 months (range,
2–98 months after recurrence), while that of other patients was
22.5 months. The overall survival of EGFR-TKI-treated patients with
EGFR mutation was the longest in the present study (Fig. 2).
 | Table IITreatment of 127 patients with
recurrent lung cancers. |
Table II
Treatment of 127 patients with
recurrent lung cancers.
| | Prognosis | |
|---|
| |
| |
|---|
| Treatment | Samples, n | MST, months | Two-year survival, n
(%) | P-value |
|---|
| Chemotherapy | | | | 0.0007 |
| Yes | 99 | 29.6 | 37 (49.5) | |
| No | 28 | 16.3 | 4 (23.2) | |
| EGFR TKIs | | | | 0.0315 |
| Yes | 39 | 32.8 | 19 (55.0) | |
| No | 88 | 23.6 | 23 (39.2) | |
| EGFR mutations | | | | 0.0563 |
| Yes | 40 | 31.6 | 20 (50.0) | |
| No | 87 | 24.2 | 22 (37.8) | |
| Radiotherapy | | | | 0.3832 |
| Yes | 82 | 25.4 | 27 (42.8) | |
| No | 45 | 29.8 | 15 (47.8) | |
Discussion
In a previous study, Yoshino et al reported
that post-recurrence survival of NSCLC patients who underwent
complete resection was significantly affected by gender, age at
recurrence, p-stage, pulmonary metastasis and recurrence interval
(3). In this study, further
analysis was conducted with special reference to organ and therapy.
Therefore, patients with recurrence were selected between 2002 and
2012 when gefitinib had become a key drug for NSCLC. The results of
the present study are similar in part.
The prognostic effect of initial lung cancer stage
on survival following recurrent cancer has been investigated.
Advanced stages of the initially resected NSCLC have been shown to
be associated with increased rates of recurrence and shortened
recurrence-free intervals (3,22–24),
suggesting the utility of p-stage as a marker of tumor
aggressiveness or occult disease at resection. The association of
stage with post-recurrence survival has been demonstrated
previously, with advanced stages accompanying a 30–90% increased
risk of mortality (3,22,23).
Post-operative recurrence is understood as the reappearance of
latent cancer cells known as micrometastases. Therefore, the
positive correlation between advanced p-stage and high recurrence
rate or a short disease-free interval is well understood. However,
in the present study p-stage was not a prognostic factor.
Appropriately staging and chemotherapy, including EGFR TKI therapy,
may overcome the initial pathological stages. However, disease-free
interval was a prognostic factor. Walsh et al (23) characterized disease-free interval as
an “indirect measure of a patient’s tumor biology and
aggressiveness”. Thus, longer disease-free interval has been
reported to be associated with prolonged survival following
recurrence in several studies (24–27).
Major advances in NSCLC treatment have resulted from
the understanding of the molecular biology of the disease, the
development of molecule-targeting agents and the identification of
biomarkers for targeted treatment. Since 2002, gefitinib therapy
has been approved for the treatment of inoperable or recurrent
NSCLC in Japan, hence the focus of the present study on cases
subsequent to 2002. EGFR-TKIs have been proven to improve the
survival of certain advanced NSCLC patients (28–30),
with the overall benefit being determined primarily by the EGFR
mutation subgroup (9–11,16,17,31).
EGFR-TKIs have also improved endurance and health-related quality
of life compared with platinum-based doublet chemotherapy (9–11).
EGFR-TKIs are therefore good candidates for first-line
post-recurrence treatment in resected adenocarcinoma patients with
distant metastases, but only in those with EGFR mutations (11,28).
There are several limitations in the present study.
This study is retrospective and bias may exist. Patient selection
bias regarding post-recurrence treatment was unavoidable. Curative
intent therapy or systematic treatment is difficult to perform in
patients with poor performance status and therefore younger
patients had better prognoses. In addition, complete follow-up was
not available for all eligible patients. One challenge for the
future is to create systematic treatment strategies for recurrent
NSCLC according to the individual patient’s recurrent disease
characteristics, including the initial recurrence site,
recurrence-free interval and original tumor characteristics.
Acknowledgements
This study was supported by Grants-in-Aid for
Scientific Research, Japan Society for the Promotion of Science
(nos. 23659674, 24592097 and 25293303).
References
|
1
|
Martini N, Bains MS, Burt ME, et al:
Incidence of local recurrence and second primary tumors in resected
stage I lung cancer. J Thorac Cardiovasc Surg. 109:120–129. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
al-Kattan K, Sepsas E, Fountain SW and
Townsend ER: Disease recurrence after resection for stage I lung
cancer. Eur J Cardiothorac Surg. 12:380–384. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yoshino I, Yohena T, Kitajima M, et al:
Survival of non-small cell lung cancer patients with postoperative
recurrence at distant organs. Ann Thorac Cardiovasc Surg.
7:204–209. 2001.
|
|
4
|
Martin J, Ginsberg RJ, Venkatraman ES, et
al: Long-term results of combined-modality therapy in resectable
non-small-cell lung cancer. J Clin Oncol. 20:1989–1995. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Williams BA, Sugimura H, Endo C, et al:
Predicting post-recurrence survival among completely resected
nonsmall-cell lung cancer patients. Ann Thorac Surg. 81:1021–1027.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sugimura H, Nichols FC, Yang P, et al:
Survival after recurrent non-small-cell lung cancer after complete
pulmonary resection. Ann Thorac Surg. 83:409–418. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hung JJ, Hsu WH, Hsieh CC, et al:
Post-recurrence survival in completely resected stage I non-small
cell lung cancer with local recurrence. Thorax. 64:192–196. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shimada Y, Saji H, Yoshida K, et al:
Prognostic factors and the significance of treatment after
recurrence in completely resected stage I non-small cell lung
cancer. Chest. 143:1626–1634. 2013.PubMed/NCBI
|
|
9
|
Maemondo M, Inoue A, Kobayashi K, et al:
Gefitinib or chemotherapy for non-small-cell lung cancer with
mutated EGFR. N Engl J Med. 362:2380–2388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mitsudomi T, Morita S, Yatabe Y, et al:
Gefitinib versus cisplatin plus docetaxel in patients with
non-small-cell lung cancer harbouring mutations of the epidermal
growth factor receptor (WJTOG3405): an open label, randomised phase
3 trial. Lancet Oncol. 11:121–128. 2010. View Article : Google Scholar
|
|
11
|
Mok TS, Wu YL, Thongprasert S, et al:
Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N
Engl J Med. 361:947–957. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Paz-Ares L, de Marinis F, Dediu M, et al:
Maintenance therapy with pemetrexed plus best supportive care
versus placebo plus best supportive care after induction therapy
with pemetrexed plus cisplatin for advanced non-squamous
non-small-cell lung cancer (PARAMOUNT): a double-blind, phase 3,
randomised controlled trial. Lancet Oncol. 13:247–255. 2012.
|
|
13
|
Reck M, von Pawel J, Zatloukal P, et al:
Overall survival with cisplatin-gemcitabine and bevacizumab or
placebo as first-line therapy for nonsquamous non-small-cell lung:
results from a randomised phase III trial (AVAiL). Ann Oncol.
21:1804–1809. 2010. View Article : Google Scholar
|
|
14
|
Sandler A, Gray R, Perry MC, et al:
Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell
lung cancer. N Engl J Med. 355:2542–2550. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Scagliotti GV, Parikh P, von Pawel J, et
al: Phase III study comparing cisplatin plus gemcitabine with
cisplatin plus pemetrexed in chemotherapy-naive patients with
advanced-stage non-small-cell lung cancer. J Clin Oncol.
26:3543–3551. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Paez JG, Jänne PA, Lee JC, et al: EGFR
mutations in lung cancer: correlation with clinical response to
gefitinib therapy. Science. 304:1497–1500. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sasaki H, Endo K, Mizuno K, et al: EGFR
mutation status and prognosis for gefitinib treatment in Japanese
lung cancer. Lung Cancer. 51:135–136. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fukuoka M, Wu YL, Thongprasert S, et al:
Biomarker analyses and first overall survival results from a phase
III, randomized, open-label, first-line study of gefitinib versus
carboplatin/paclitaxel in clinically selected patients with
advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol.
29:2866–2874. 2011. View Article : Google Scholar
|
|
19
|
The Japan Lung Cancer Society. The General
Rule for Clinical and Pathological Record of Lung Cancer. 7th
edition. Kanehara Shuppan; Tokyo, Japan: pp. 1–234. 2010
|
|
20
|
Sasaki H, Endo K, Konishi A, et al: EGFR
mutation status in Japanese lung cancer patients: genotyping
analysis using LightCycler. Clin Cancer Res. 11:2924–2929. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sasaki H, Shimizu S, Endo K, et al: EGFR
and erbB2 mutation status in Japanese lung cancer patients. Int J
Cancer. 118:180–184. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ichinose Y, Yano T, Yokoyama H, et al:
Postrecurrent survival of patients with non-small-cell lung cancer
undergoing a complete resection. J Thorac Cardiovasc Surg.
108:158–161. 1994.PubMed/NCBI
|
|
23
|
Walsh GL, O’Connor M, Willis KM, et al: Is
follow-up of lung cancer patients after resection medically
indicated and cost-effective? Ann Thorac Surg. 60:1563–1572. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ichinose Y, Kato H, Koike T, et al:
Overall survival and local recurrence of 406 completely resected
stage IIIa-N2 non-small cell lung cancer patients: questionnaire
survey of the Japan Clinical Oncology Group to plan for clinical
trials. Lung Cancer. 34:29–36. 2001. View Article : Google Scholar
|
|
25
|
Mitsudomi T, Nishioka K, Maruyama R, et
al: Kinetic analysis of recurrence and survival after potentially
curative resection of nonsmall cell lung cancer. J Surg Oncol.
63:159–165. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yano T, Hara N, Ichinose Y, et al: Local
recurrence after complete resection for non-small-cell carcinoma of
the lung. Significance of local control by radition treatment. J
Thorac Cardiovasc Surg. 107:8–12. 1994.
|
|
27
|
Emami B, Graham MV, Deedy M, Shapiro S and
Kucik N: Radiation therapy for intrathoracic recurrence of
non-small cell lung cancer. Am J Clin Oncol. 20:46–50. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sequist LV, Joshi VA, Jänne PA, et al:
Response to treatment and survival of patients with non-small cell
lung cancer undergoing somatic EGFR mutation testing. Oncologist.
12:90–98. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang CH, Yu CJ, Shih JY, et al: Specific
EGFR mutations predict treatment outcome of stage IIIB/IV patients
with chemotherapy-naive non-small-cell lung cancer receiving
first-line gefitinib monotherapy. J Clin Oncol. 26:2745–2753. 2008.
View Article : Google Scholar
|
|
30
|
Kosaka T, Yatabe Y, Endo H, et al:
Analysis of epidermal growth factor receptor gene mutation in
patients with non-small cell lung cancer and acquired resistance to
gefitinib. Clin Cancer Res. 12:5764–5769. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lynch TJ, Bell DW, Sordella R, et al:
Activating mutations in the epidermal growth factor receptor
underlying responsiveness of non-small-cell lung cancer to
gefitinib. N Engl J Med. 350:2129–2139. 2004. View Article : Google Scholar
|