Introduction
As a tumor suppressor gene regulating the cell
cycle, cyclin-dependent kinase inhibitor 2A (CDKN2A/p16), is often
found to be mutational in various types of human cancer (1). Loss of CDKN2A/p16 function arises by
two main mechanisms: Homozygous deletion and 5′ CpG island
hypermethylation with low transcription (2). Mutations in primary ovarian cancer and
metastatic ovarian cancer do not occur at high frequency (3). However, the present study identified a
single-base substitution from G to A in codon 148 of CDKN2A/p16 by
sequencing human ovarian cancer cell line UACC-1598. The mutation
results in the amino acid alanine (A) being substituted with
threonine (T). It is known that the abnormal expression of cell
cycle-regulating genes plays important roles in cancer cells
(4), prompting the use of
recombinant expression systems to investigate their function
(5,6). The pTUNE vector establishes a gene
expression system induced by isopropyl-β-D-thiogalactoside (IPTG)
which utilizes repressor proteins and RNA interference to switch
genes on or off while preserving gene function (7). Therefore, the present study used a
pTUNE vector to construct human CDKN2A/p16-A148T mutant and
CDKN2A/p16-wild-type gene expression systems in order to
investigate the effect of CDKN2A/p16-A148T on the cell cycle.
Materials and methods
Cell culture and transfection
Human ovarian cancer cell line UACC-1598 was a gift
from Dr Xin-Yuan Guan (University of Hong Kong, Hong Kong SAR,
China). Human ovarian cancer cell line SKOV3 was purchased from
American Type Culture Collection (Manassas, VA, USA). These cells
were cultured in RPMI-1640 (Gibco, Carlsbad, CA, USA) containing
10% fetal bovine serum (PAA Laboraties GmbH, Pasching, Austria) at
37°C in a 5% CO2 humidified atmosphere.
Cells were seeded in six-well plates at a density of
5×106 cells/well, and the constructed expression vectors
were transfected into cells using Lipofectamine 2000 (Invitrogen
Life Technologies, Carlsbad, CA, USA).
Vector construction
The pTUNE inducible vector was obtained from OriGene
Technologies Inc. (Rockville, MD, USA) as control vector. The
primers specific for amplifying CDKN2A/p16 open reading frame (ORF)
were synthesized by Invitrogen Life Technologies as follows: Primer
1 (hCDKN2A-SgfI), 5′-GAGGCGATGAGGCGATC GCATGGAGCCGGCGGCG-3′; primer
2 (hCDKN2A-XhoI), 5′-GCGCTCGAGATCGGGGATGTCTGAGGGACCTTC-3′; and
primer 3 (hCDKN2A-XhoI), 5′-GCGCTCGAGAT
CGGGGATGTCTGAGGGACCTTCCGCGGCATCTAG-3′.
The CDKN2A/p16-A148T ORF fragment was cloned from
the cDNA template of UACC-1598 cells by polymerase chain reaction
(PCR) with primers 1 and 2 specific to CDKN2A/p16. The PCR product
was electrophoresed on a 2% agarose gel (Gene Tech Company Limited,
Shanghai, China)and the predicted 490 bp fragment was extracted.
The amplified ORF and the pTUNE inducible vector (OriGene
Technologies, Inc.) were digested with endonuclease XhoI
(New England Biolabs, Inc., Beijing, China) and SgfI
(Promega Corporation, Madison, WI, USA). A ligation reaction was
set up with linearized pTUNE vector and CDKN2A/p16 ORF using T4 DNA
ligase (TransGen Biotech Co., Ltd., Beijing, China). The positive
clone was picked up and the authenticity of the plasmid was
confirmed by sequencing.
The CDKN2A/p16-wild-type ORF fragment was amplifed
with primers 1 and 3 using the pTUNE-CDKN2A/p16-A148T vector
template, which covers the mutation site and contains the wild-type
base G substitution for correcting the sequence. The PCR product
was separated by electrophoresis and the 490 bp fragment was
extracted. The CDKN2A/p16-wild-type ORF was subsequently digested
by the restriction enzymes XhoI and SgfI to construct
the pTUNE-CDKN2A/p16-wild-type inducible vector. The positive clone
was also sequenced for verification.
Flow cytometric analysis of the cell
cycle
Cells were harvested by trypsinization and fixed
with 70% ethanol at 4°C for 24 h. The cells were then centrifuged
at 500 × g for 5 min, washed twice with phosphate-buffered saline
and stained with buffers provided in the Cycletest™ Plus DNA
Reagent kit (BD Biosciences, Franklin Lakes, NJ, USA) for analysis
by flow cytometry.
Results
Verification of the transfection
efficiency of pTUNE-CDKN2A/p16-wild-type and pTUNE-CDKN2A/p16-A148T
mutant vectors
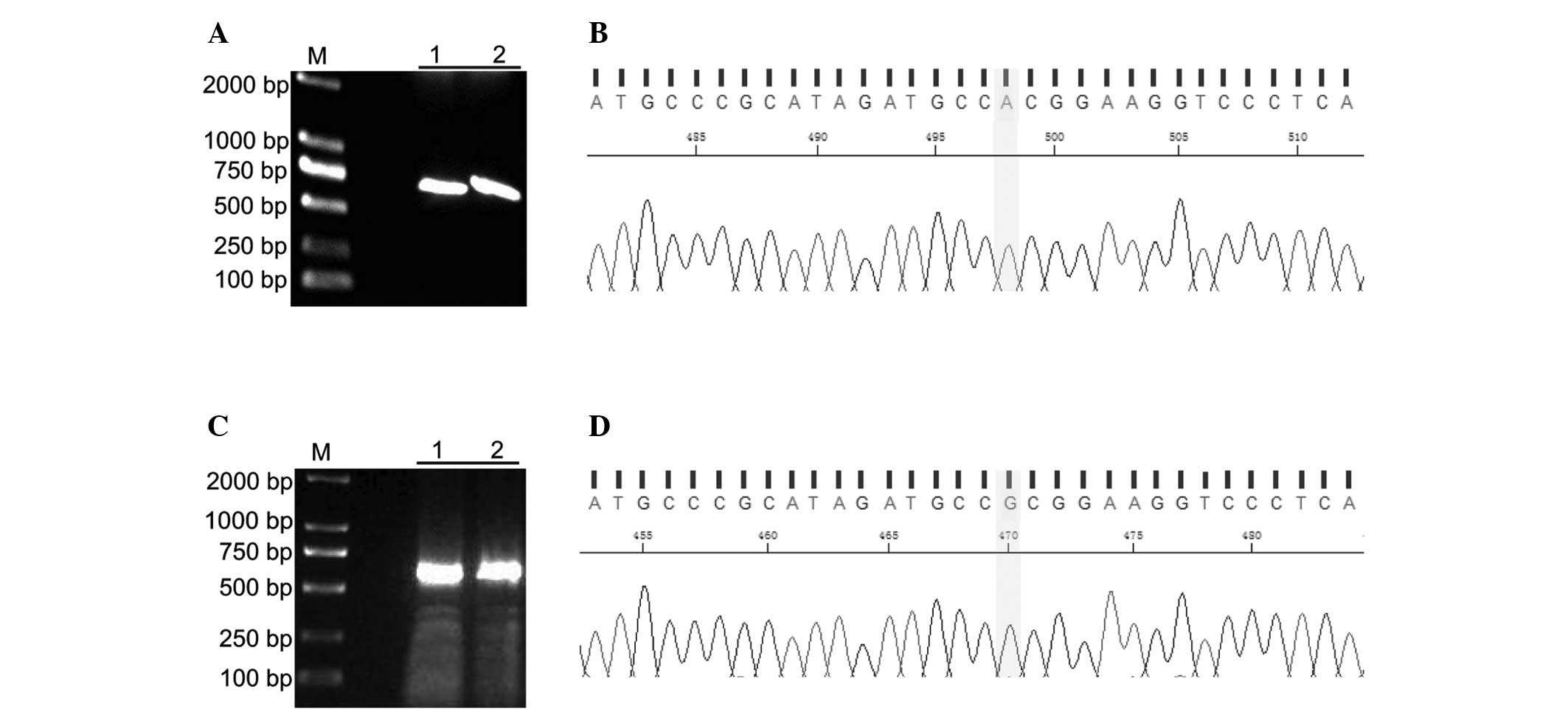
The present study identified a single-base
substitution from G to A in codon 148 of CDKN2A/p16 by sequencing
the human ovarian cancer cell line UACC-1598. This mutation
resulted in the amino acid substitution of alanine (A) with
threonine (T). pTUNE-CDKN2A/p16-A148T and
pTUNE-CDKN2A/p16-wild-type expression vectors were successfully
constructed (Figs. 1).
To verify the effect of the pTUNE-CDKN2A/p16-A148T
and pTUNE-CDKN2A/p16-wild-type vectors, a CDKN2A homozygous
deletion human ovarian cancer cell line, SKOV3, was selected
(8). The constructed expression
vectors pTUNE-CDKN2A/p16-A148T and pTUNE-CDKN2A/p16-wild-type were
transfected into SKOV3 cells using Lipofectamine 2000 when the
cells were ~80% confluent. After 24 h had elapsed, the cells were
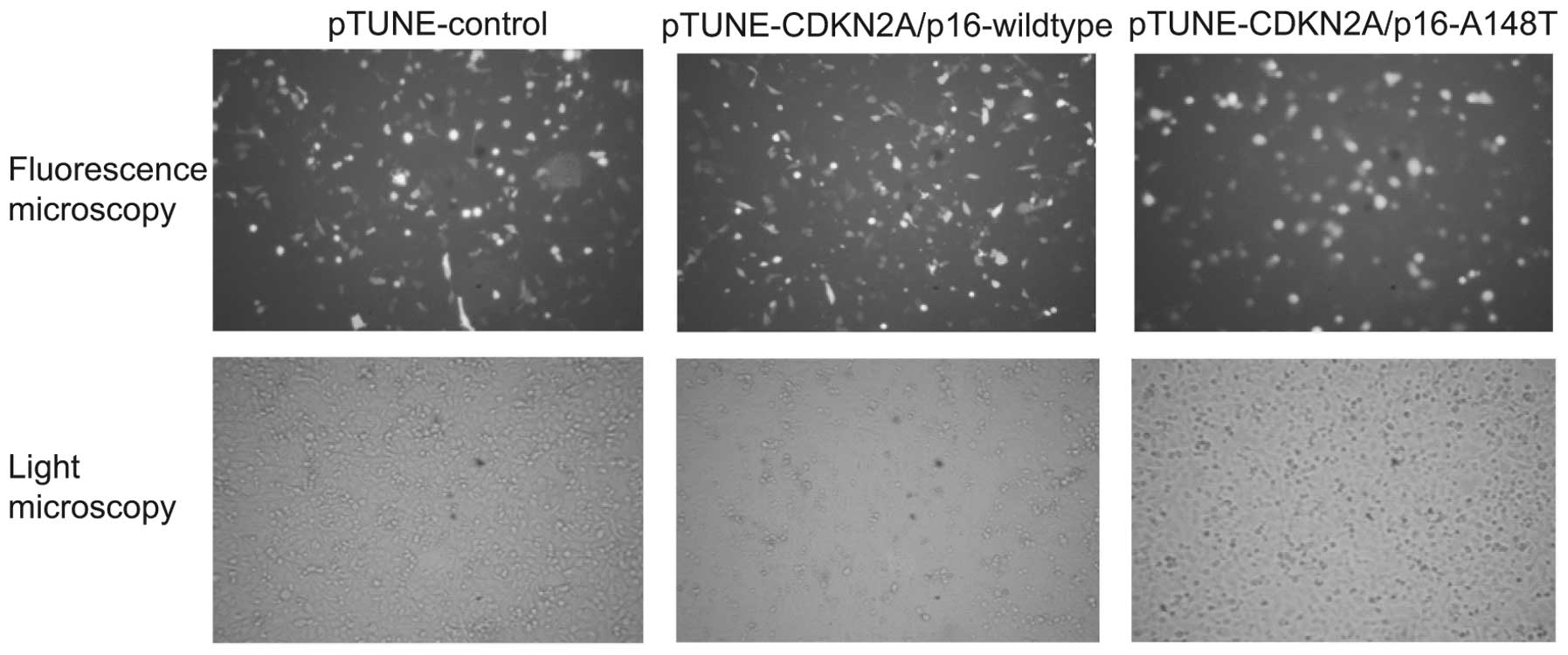
induced with 500 mM IPTG for an additional 36 h. As pTUNE-vectors
contain green fluorescent protein (GFP) fragments, the efficiency
of transfection was detected by fluorescence microscopy. The
expression of wild-type and mutant CDKN2A/p16-GFP fusion protein
increased markedly in the transfected SKOV3 cells following IPTG
induction (Fig. 2). These results
indicate that the vectors pTUNE-CDKN2A/p16-A148T and
pTUNE-CDKN2A/p16-wild-type are effectively induced by IPTG and
could be used for further study.
Influence of CDKN2A/p16-A148T on the cell
cycle
CDKN2A/p16 is a cell cycle-regulating gene. In the
present study, the effects of pTUNE-CDKN2A/p16-A148T and
pTUNE-CDKN2A/p16-wild-type vectors on cell cycle regulation were
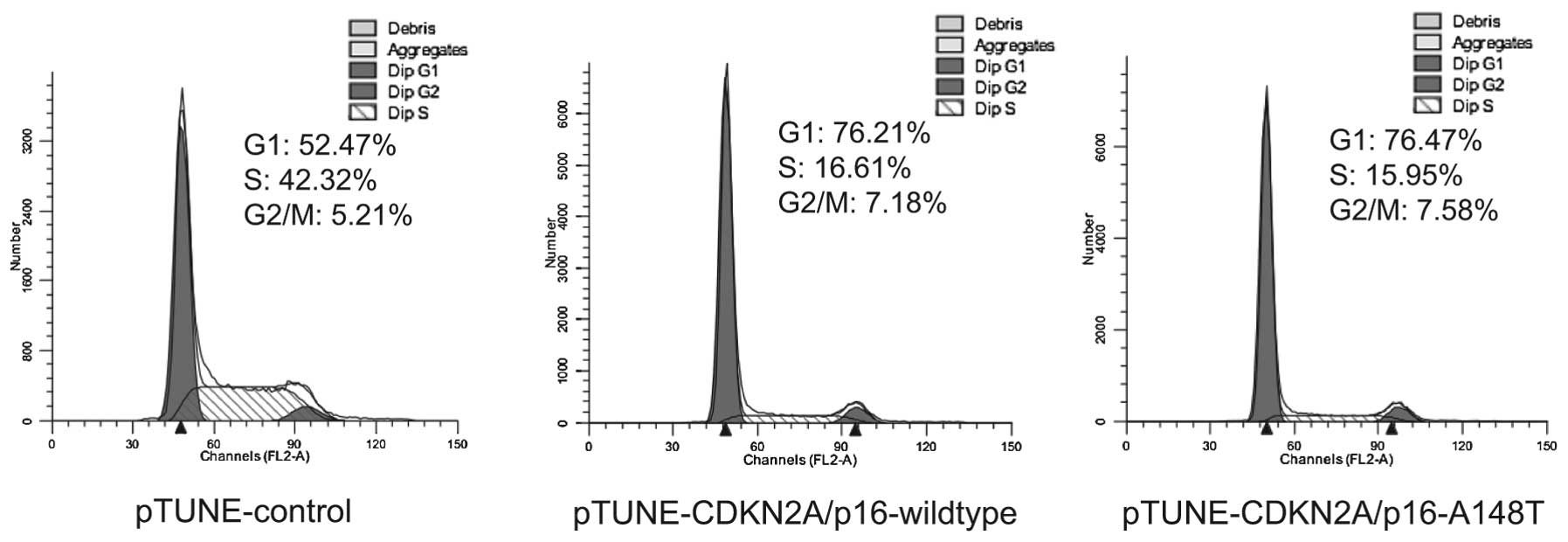
examined. Cell cycle distribution of the transfected and
IPTG-induced SKOV3 cells were determined by flow cytometry in three
independent experiments. The results demonstrated that wild-type
and A148T mutant expression of CDKN2A/p16 can induce cell cycle
arrest in G1 phase (Fig. 3). This
suggests that the amino acid substitution of A with T at codon 148
of CDKN2A/p16 does not influence the function of CDKN2A/p16 on cell
cycle arrest.
Discussion
CDKN2A/p16 is an important factor in cell cycle
regulation (9). As a tumor
suppressor gene, the expression of CDKN2A/p16 is strictly
controlled. When overexpressed, location-specific CDKN2A/p16
expression is lost and it appears in the cytoplasm and the nucleus.
This exerts abnormal influence on cell growth and division,
resulting in cellular senescence and apoptosis (10). In order to control CDKN2A/p16 gene
expression, an inducible pTUNE vector system was selected. This
system is designed for IPTG-inducible expression, compared with a
standard system in which IPTG treatment would induce only modestly
elevated expression of proteins. In the present study, SKOV3 cells
transfected with pTUNE-CDKN2A/p16-wild-type and
pTUNE-CDKN2A/p16-A148T plasmids demonstrated significant G1 arrest
compared with control cells. This illustrates that the amino acid
substitution of A with T at CDKN2A/p16 codon 148 does not interfere
with its role in cell cycle regulation. Wolf et al have
previously demonstrated G1 phase arrest when CDKN2A/p16 was
overexpressed in SKOV3 cells, which caused inhibition of cell
growth (11). It has been confirmed
that the CDKN2A/p16-A148T mutant exists in several types of cancer,
including ovarian cancer, and, with the exception of certain
populations, it is associated with cancer risk (3,12–14).
However, the CDKN2A/p16-A148T mutant in melanoma does not exhibit
impaired CDK4 binding function (15). By sequencing human ovarian cancer
cell line UACC-1598, the present study also identified the
CDKN2A/p16-A148T mutant. Assessment of the effect of the CDKN2A
mutant on the cell cycle yielded results consistent with previous
studies in which the CDKN2A mutant did not decrease the inhibitory
activity of cyclin D1/CDK4 (15,16).
In conclusion, this study identified
CDKN2A/p16-A148T mutation in ovarian cancer cells. The inducible
expression vectors of CDKN2A/p16-wild-type and CDKN2A/p16-A148T
utilized in the study could be useful for further investigation
into whether this somatic mutation can alter the tumor suppression
functions of CDKN2A/p16.
Acknowledgements
This study was supported by the Program for
Changjiang Scholars and Innovative Research Team in University
(IRT1230); the New Century Support Program for the Excellent
Scholar, Ministry of Education of China (NCET-10-0149,
NCET-11-0954); and the National Natural Science Foundation of China
(81272288).
References
|
1
|
Witkiewicz AK, Knudsen KE, Dicker AP and
Knudsen ES: The meaning of p16(ink4a) expression in tumors:
functional significance, clinical associations and future
developments. Cell Cycle. 10:2497–2503. 2011. View Article : Google Scholar
|
|
2
|
Herman JG, Merlo A, Mao L, Lapidus RG,
Issa JP, Davidson NE, Sidransky D and Baylin SB: Inactivation of
the CDKN2/p16/MTS1 gene is frequently associated with aberrant DNA
methylation in all common human cancers. Cancer Res. 55:4525–4530.
1995.PubMed/NCBI
|
|
3
|
Schuyer M, van Staveren IL, Klijn JG, vd
Burg ME, Stoter G, Henzen-Logmans SC, Foekens JA and Berns EM:
Sporadic CDKN2 (MTS1/p16ink4) gene alterations in human ovarian
tumours. Br J Cancer. 74:1069–1073. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rayess H, Wang MB and Srivatsan ES:
Cellular senescence and tumor suppressor gene p16. Int J Cancer.
130:1715–1725. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Outwin E, Carpenter G, Bi W, Withers MA,
Lupski JR and O’Driscoll M: Increased RPA1 gene dosage affects
genomic stability potentially contributing to 17p13.3 duplication
syndrome. PLoS Genet. 7:e10022472011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Walker F, Zhang HH, Odorizzi A and Burgess
AW: LGR5 is a negative regulator of tumourigenicity, antagonizes
Wnt signalling and regulates cell adhesion in colorectal cancer
cell lines. PLoS One. 6:e227332011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Deans TL, Cantor CR and Collins JJ: A
tunable genetic switch based on RNAi and repressor proteins for
regulating gene expression in mammalian cells. Cell. 130:363–372.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schultz DC, Vanderveer L, Buetow KH,
Boente MP, Ozols RF, Hamilton TC and Godwin AK: Characterization of
chromosome 9 in human ovarian neoplasia identifies frequent genetic
imbalance on 9q and rare alterations involving 9p, including CDKN2.
Cancer Res. 55:2150–2157. 1995.
|
|
9
|
Zhang W, Zeng Z, Zhou Y, Xiong W, Fan S,
Xiao L, Huang D, Li Z, Li D, Wu M, Li X, Shen S, Wang R, Cao L,
Tang K and Li G: Identification of aberrant cell cycle regulation
in Epstein-Barr virus-associated nasopharyngeal carcinoma by cDNA
microarray and gene set enrichment analysis. Acta Biochim Biophys
Sin (Shanghai). 41:414–428. 2009. View Article : Google Scholar
|
|
10
|
Romagosa C, Simonetti S, López-Vicente L,
Mazo A, Lleonart ME, Castellvi J and Ramon y Cajal S: p16(Ink4a)
overexpression in cancer: a tumor suppressor gene associated with
senescence and high-grade tumors. Oncogene. 30:2087–2097. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wolf JK, Kim TE, Fightmaster D, Bodurka D,
Gershenson DM, Mills G and Wharton JT: Growth suppression of human
ovarian cancer cell lines by the introduction of a p16 gene via a
recombinant adenovirus. Gynecol Oncol. 73:27–34. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nagore E, Montoro A, García-Casado Z,
Botella-Estrada R, Insa A, Lluch A, López-Guerrero JA and Guillén
C: Germline mutations in CDKN2A are infrequent in female patients
with melanoma and breast cancer. Melanoma Res. 19:211–214. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bakos RM, Besch R, Zoratto GG, Godinho JM,
Mazzotti NG, Ruzicka T, Bakos L, Santos SE, Ashton-Prolla P,
Berking C and Giugliani R: The CDKN2A p.A148T variant is associated
with cutaneous melanoma in Southern Brazil. Exp Dermatol.
20:890–893. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Spica T, Portela M, Gérard B, Formicone F,
Descamps V, Crickx B, Ollivaud L, Archimbaud A, Dupin N,
Wolkenstein P, Vitoux D, Lebbe C, Saiag P, Basset-Seguin N,
Fargnoli MC, Grandchamp B, Peris K and Soufir N: The A148T variant
of the CDKN2A gene is not associated with melanoma risk in the
French and Italian populations. J Invest Dermatol. 126:1657–1660.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ranade K, Hussussian CJ, Sikorski RS,
Varmus HE, Goldstein AM, Tucker MA, Serrano M, Hannon GJ, Beach D
and Dracopoli NC: Mutations associated with familial melanoma
impair p16INK4 function. Nat Genet. 10:114–116. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lilischkis R, Sarcevic B, Kennedy C,
Warlters A and Sutherland RL: Cancer-associated mis-sense and
deletion mutations impair p16INK4 CDK inhibitory activity. Int J
Cancer. 66:249–254. 1996. View Article : Google Scholar
|