Introduction
Epithelial ovarian carcinoma (EOC) is a predominant,
lethal gynecologic malignancy, with a five-year survival rate of
<25% for patients that are diagnosed with stage III–IV disease
(1,2). The treatment for EOC remains
challenging, regardless of advances in surgical procedures and
chemotherapy. The current ovarian cancer classification scheme
distinguishes tumors based on the histopathological subtype, grade
and surgical stage. Although established differences are observed
in the clinical behavior, all of the ovarian neoplasia are subject
to the same treatment paradigm. Therefore, the development of
predictive markers that will direct the treatment selection, in
addition to novel targeted therapies, are required for the
treatment of EOC.
Aberrant DNA methylation is currently recognized as
a common molecular abnormality in cancer. The abnormal promoter
methylation may be a potential molecular marker for cancer
diagnosis, prognosis and treatment (3–5).
Recent studies have focused on the identification of methylated
genes and the evaluation of their potential application as
biomarkers for the prognosis and diagnosis of EOC (6,7).
However, it is particularly unclear which genes should be used to
identify a methylator phenotype that may become instrumental in the
clinical diagnosis, prognosis and treatment of cancer.
The breast cancer susceptibility gene 1
(BRCA1) is a significant breast and ovarian cancer
susceptibility gene; it has been mapped to chromosome 17q21 and
encodes a nuclear protein, which is comprised of 1863 amino acids
(8). BRCA1 contributes to
the regulation of transcriptional activation, DNA repair,
apoptosis, cell-cycle checkpoint control and chromosomal remodeling
(9,10). BRCA1 dysfunction, frequently
observed in high-grade serous ovarian carcinomas, commonly results
from germline and somatic mutations, as well as promoter
methylation. Therefore, the identification of tumors exhibiting
BRCA1 defects has therapeutic and prognostic implications (11–16).
The hypermethylation of the BRCA1 promoter is important in
silencing BRCA1 in sporadic EOCs, and previous studies have
demonstrated that methylation of BRCA1 is associated with
chemotherapy sensitivity and patient survival (17–20).
BRCA1 CpG island hypermethylation predits sensitivity to
poly (ADP-ribose) polymerase-1 inhibitors (21), thus indicating a potential role for
BRCA1 methylation as a biological marker in the clinical
treatment of EOC.
DNA methyltransferases (DNMTs) are a family of
enzymes responsible for the transfer of methyl groups to cytosine
(22,23) and the DNMT family includes DNMT1, 2,
3a, 3b and 3L (24,25). DNMT2 (whose substrate and DNA
methylation activity is unclear) (26), was shown to methylate transfer RNA
(27,28) and DNMT3L (which is essential for
establishing maternal genomic imprints, however, lacks key
methyltransferase motifs) is potentially a regulator of methylation
rather than an enzyme that methylates DNA (29). It has been suggested that that
global genomic DNA methylation patterns are established and
predominantly maintained by the combined action of three
enzymatically active DNMTs, 1, 3a and 3b. DNMT1 is traditionally
referred to as a maintenance enzyme as it copies methylation
following replication, whereas DNMT3a and DNMT3b are de novo
enzymes, which establish novel patterns of methylation during
differentiation (30,31). Aberrant expression of DNMTs and
disruption of DNA methylation patterns are closely associated with
numerous types of cancer. Our previous study demonstrated that
DNMT3a expression was higher in EOC (32), indicating an important role for the
differential expression of DNMTs in aberrant DNA methylation
patterns of EOC. However, there is currently no data concerning the
association between DNMT expression and DNA methylation in EOC.
In the present study, the expression and methylation
of BRCA1 was investigated in the same cohort of ovarian
tumor patients as our previous study (32) and the association between BRCA1
expression and methylation was analyzed. The clinicopathological
and prognostic differences, based on BRCA1 promoter
methylation in EOC patients, were analyzed to determine the role of
BRCA1 methylation in EOC and to understand the clinical
significance. Furthermore, the association between BRCA1
methylation and DNMT protein expression was analyzed to investigate
the role of DNMTs in BRCA1 methylation.
Patients and methods
Patients and tissue samples
The present study included 142 EOC cases and 32
ovarian benign tumor cases, which were selected from the Department
of Surgical Oncology and General Surgery at the China Medical
University-Affiliated First and Second Hospitals (Liaoning, China).
All of the cases included in the present study were clinical cases
that were routinely examined and diagnosed between 2002 and 2010.
None of the patients had a family history of cancer and they were
surgically staged according to the current International Federation
of Gynecologists and Obstetricians (FIGO) classification system.
The histological diagnosis was determined based on criteria set out
by the World Health Organization (33). Approval for the present study was
obtained from the Institute Research Medical Ethics Committee of
the China Medical University (Liaoning, China). Consent was
obtained from the families of all patients.
Immunohistochemical analysis
Formalin-fixed and paraffin-embedded tissue samples
were cut into 4-μm sections and mounted onto poly-L-lysine-coated
glass slides. For immunohistochemical staining, the sections were
deparaffinized in xylene, rehydrated in a series of alcohol and
washed using tap water. The sections were autoclaved in 10 mM
sodium citrate buffer (pH 6.0; China National Pharmaceutical Group
Corporation, Beijing, China) for 10 min for antigen retrieval.
Endogenous peroxidase activity was blocked by incubating the
sections in 3% H2O2 (China National
Pharmaceutical Group Corporation) at 37°C for 20 min. The sections
were blocked for non-specific binding by 10% normal goat serum at
37°C for 30 min and were incubated at 4°C overnight with mouse
monoclonal anti-BRCA1 antibodies (1:100; MS110, Calbiochem,
Darmstadt, Germany). The following day, the sections were washed
three times with 0.01 mol/l phosphate-buffered saline (PBS; pH 7.4)
for 15 min and incubated with a goat anti-mouse IgG secondary
antibody (catalogue no. sc-2039; Santa Cruz Biotechnology Inc.,
Santa Cruz, CA, USA) for 30 min at 37°C. Subsequently, the sections
were incubated with a streptavidin horseradish peroxidase solution
(LSABTM kit, Dako, Glostrup, Denmark) for 30 min, washed
with PBS and stained with 3,3′-diaminobenzidine. The sections were
then counterstained with Mayer’s hematoxylin (Sigma-Aldrich, St.
Louis, MO, USA), dehydrated and mounted. The negative controls were
generated using PBS as a replacement for the anti-BRCA1
antibodies.
Evaluation of immunohistochemistry
The immunostained sections were independently
reviewed and scored by two investigators who were blinded to the
clinicopathological characteristics of the patients; a positive
correlation was observed between them. The nuclear positivity of
the BRCA1 proteins was evaluated using semi-quantitative scoring
criteria according to the staining intensity (0, negative; 1, weak;
2, moderate; and 3, strong) and the proportion of positive cells
(0, negative; 1, positive in ≤10%; 2, positive in >10% and ≤50%;
3, positive in >50% and ≤80%; 4, positive in >80% of tumor
cells). The two scores were multiplied together for each case and
the expression was graded as: 0, negative score; 1–4, weak
expression score; 5–8, moderate expression score; and 9–12, strong
expression score.
Methylation-specific polymerase chain
reaction (MSP)
The promoter methylation status of the BRCA1
gene was analyzed using MSP. The bisulfite modification, primer
sequences and PCR conditions were performed as previously described
(29). Lymphocyte DNA, which was
treated with SssI bacterial methylase (New England BioLabs,
Hithcin, UK) served as the positive control for the methylated
alleles and the DNA from normal lymphocytes served as the control
for the unmethylated alleles; the negative controls, without DNA,
were included in each experiment. All PCR reactions were conducted
in duplicate and a methylated band, which was detected in either or
both duplicates, was recorded as positive for promoter
methylation.
Statistical analysis
Pairwise correlations between the categorical
variables were investigated using the χ2 test or
Fisher’s exact test where appropriate. Overall survival (OS) and
disease-free survival (DFS) were estimated using the Kaplan-Meier
method, and the log-rank test was used to compare the patient
survival time between or among the groups. The Cox proportional
hazards model (Cox regression) was used with backward elimination
to identify the significant, independent, prognostic factors. OS
was defined as the time interval from initial surgery to mortality
or, for surviving patients, the time interval between the initial
surgery and the final follow-up. DFS was defined as the time
interval between the initial surgery and the date of disease
progression or recurrence, or the final follow-up. The statistical
tests were two-sided and P<0.05 was considered to indicate a
statistically significant difference. All statistical analyses were
performed using the SPSS statistical software program (SPSS,
Chicago, IL, USA).
Results
Patient characteristics
In the present study, the tissue sections were
obtained from 174 ovarian tumor samples for the evaluation of BRCA1
protein expression and promoter methylation. The
clinicopathological data from the patients are shown in Table I. Briefly, the mean age of the
patients at the time of surgery was 53 years (range, 20–74 years).
Twenty-seven (23.3%) patients exhibited lymph node-metastasized
disease at the time of surgery and 110 (80.9%) patients exhibited
serous carcinoma as the predominant histological diagnosis,
followed by mucinous carcinoma (8.5%), clear cell carcinoma (5.6%)
and undifferentiated carcinoma (5.6%). The preoperative serum
levels of carbohydrate antigen (CA)-125 and CA19-9 were elevated
prior to surgery (higher than in the levels of healthy individuals)
in the majority of patients but none of the patients received any
neo-adjuvant chemotherapy. Follow-up data were available for 85
patients. The mean and median OS times were 56.1 and 41.0 months
with 95% confidence intervals (CIs) of 45.3–66.9 and 33.2–48.8
months, respectively. The mean and median DFS times were 46.6 and
26.0 months with 95% CIs of 36.4–56.9 and 14.6–37.4 months,
respectively. The 32 benign tumors comprised of 27 that were serous
and five that were mucinous.
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Characteristic | n | % |
|---|
| Age (years) |
| ≤53 | 79 | 57.2 |
| >53 | 59 | 42.8 |
| Unknown | 4 | - |
| Menopause
state |
| Pre-menopause | 45 | 34.6 |
|
Post-menopause | 85 | 65.4 |
| Unknown | 12 | - |
| Histological
type |
| Serous | 110 | 77.5 |
| Mucinous | 12 | 8.5 |
| Clear cell | 8 | 5.6 |
| Transitional | 2 | 1.4 |
| Endometrioid | 2 | 1.4 |
|
Undifferentiated | 8 | 5.6 |
| Tumor size
(cm) |
| ≤5 | 15 | 12.5 |
| 5–10 | 53 | 44.2 |
| >10 | 51 | 43.3 |
| Unknown | 23 | - |
| FIGO stage |
| I–II | 31 | 24.4 |
| III–IV | 96 | 75.6 |
| Unknown | 15 | - |
| Node
metastasis |
| No | 89 | 76.7 |
| Yes | 27 | 23.3 |
| Unknown | 26 | - |
| Location of
tumor |
| Single side | 56 | 43.4 |
| Both sides | 73 | 56.6 |
| Unknown | 13 | - |
| CA-125 (U/ml) |
| 0–35 | 10 | 10.1 |
| 35–500 | 40 | 40.4 |
| 500–1000 | 32 | 32.3 |
| >1000 | 17 | 17.2 |
| Unknown | 43 | - |
| CA19–9 (U/ml) |
| 0–37 | 67 | 76.1 |
| 37–100 | 10 | 11.4 |
| >100 | 11 | 12.5 |
| Unknown | 54 | - |
| CEA (ng/ml) |
| 0–5 | 72 | 91.1 |
| >5 | 7 | 8.9 |
| Unknown | 63 | - |
| Chemotherapy |
|
Platinum-based | 111 | 94.9 |
| Non-platinum | 3 | 2.6 |
| No
chemotherapy | 3 | 2.6 |
| Unknown | 25 | - |
BRCA1 promoter methylation is associated
with low BRCA1 expression in EOC
The methylation of BRCA1 was detected in 142
cases of malignant tumors and 32 cases of benign, ovarian tumors.
BRCA1 methylation was detected in 50 (35.2%) of the 142
carcinomas, however, no methylation was detected in the benign
tumors. The frequency of BRCA1 methylation was significantly
higher in the EOC samples than that observed in the benign tumor
samples (P<0.001, Pearson’s χ2 test).
The BRCA1 expression in the 142 carcinoma samples
was as follows, 67 (47.2%) were negative and 62 (43.7%) were weakly
positive, nine (6.3%) were moderately positive and four (2.8%) were
strongly positive for BRCA1. Among the 32 cases of benign tumors;
nine (28.1%) were negative and 15 (46.9%) were weakly positive,
four (12.5%) were moderately positive and four (12.5%) were
strongly positive for nuclear BRCA1. The expression of BRCA1 in the
carcinoma samples was significantly reduced compared with that
observed in the benign tumors (P=0.031, Pearson’s χ2
test).
In the EOC samples, a reduction of the BRCA1 protein
was significantly associated with BRCA1 methylation
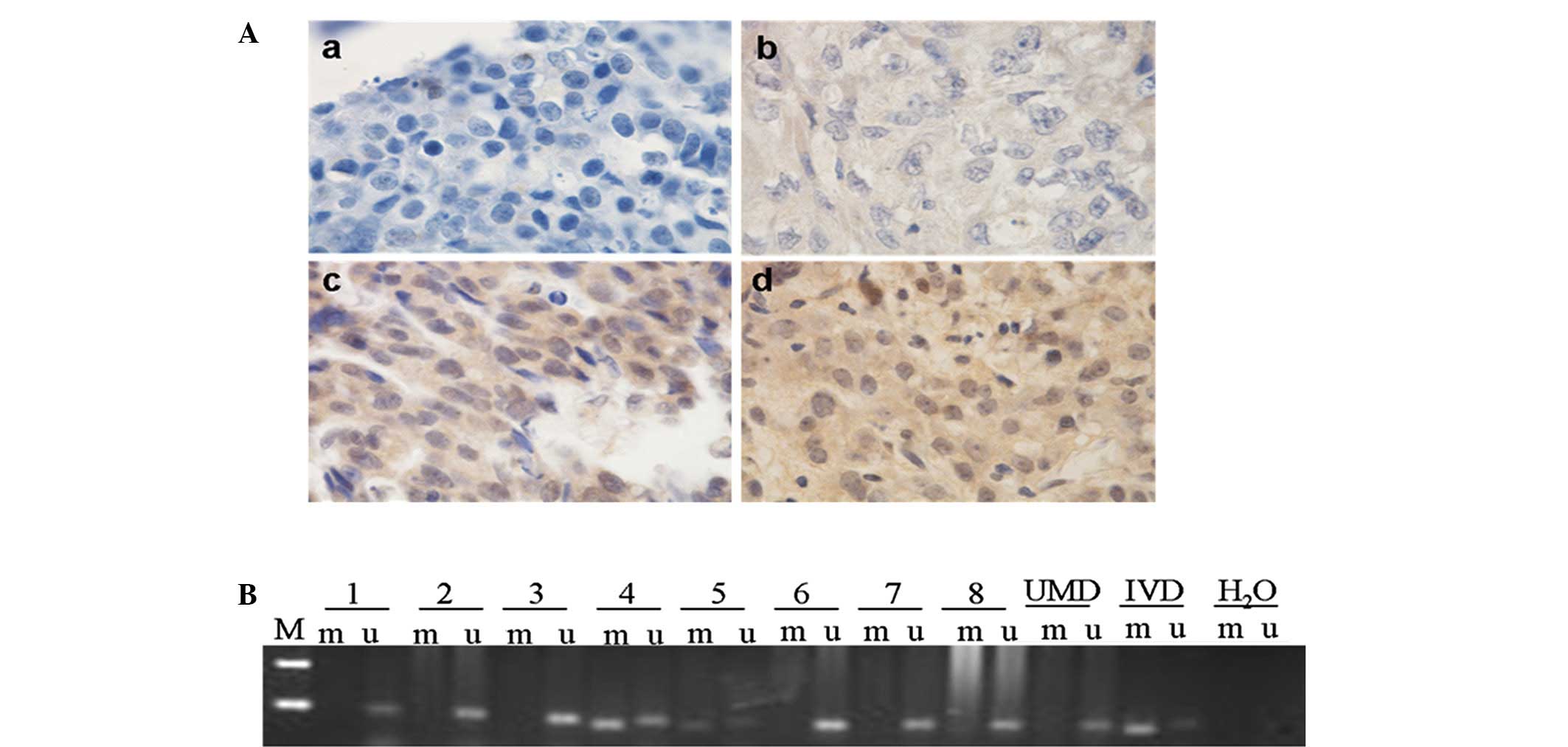
(P<0.001, Pearson’s χ2 test; Table II). Representative results of the
immunohistochemical staining and BRCA1 promoter methylation
in EOC tissues are shown in Fig.
1.
 | Table IICorrelation between BRCA1
expression and BRCA1 methylation in 142 sporadic EOC
cases. |
Table II
Correlation between BRCA1
expression and BRCA1 methylation in 142 sporadic EOC
cases.
| | BRCA1
methylation, n (%) | |
|---|
| |
| |
|---|
| BRCA1
expression | n | Positive | Negative | P-value |
|---|
| Malignant | 142 | 50 (35.2) | 92 (64.8) | <0.001 |
| Negative | 67 | 35 (52.2) | 32 (48.7) | |
| Positive | 75 | 15 (20.0) | 60 (80.0) | |
BRCA1 promoter methylation is associated
with clinicopathological parameters in EOC
The association between BRCA1 promoter
methylation and clinicopathological parameters is shown in Table III. The data indicated that
BRCA1 methylation was significantly associated with tumor
localization. In addition, the frequency of BRCA1
methylation was observed to be higher in the patients whose tumor
was bilateral than that of patients whose tumor unilateral
(P=0.015, Pearson’s χ2 test). Moreover, the methylation
of the BRCA1 gene was marginally associated with the FIGO
clinical stage (P=0.085, Pearson’s χ2 test).
 | Table IIICorrelation between BRCA1
promoter methylation and clinicopathological features of sporadic
EOC patients. |
Table III
Correlation between BRCA1
promoter methylation and clinicopathological features of sporadic
EOC patients.
| | BRCA1
methylation, n (%) | |
|---|
| |
| |
|---|
| Clinicopathological
features | n | Positive [50
(35.2)] | Negative [92
(64.8)] | P-valuea |
|---|
| Age at diagnosis
(years) | 138 | | | |
| ≤53 | 79 | 26 (32.9) | 53 (67.1) | 0.742 |
| >53 | 59 | 21 (35.6) | 38 (64.4) | |
| Menopause
state | 130 | | | |
| Pre-menopause | 45 | 13 (28.9) | 32 (71.1) | 0.318 |
|
Post-menopause | 85 | 32 (37.6) | 53 (62.4) | |
| Tumor size
(cm) | 119 | | | |
| ≤5.0 | 15 | 5 (33.3) | 10 (66.7) | 0.282 |
| 5–10 | 53 | 15 (28.3) | 38 (71.7) | |
| >10 | 51 | 22 (43.1) | 29 (56.9) | |
| Node
metastasis | 116 | | | |
| No | 89 | 31 (34.8) | 58 (65.2) | 0.834 |
| Yes | 27 | 10 (37.0) | 17 (63.0) | |
| FIGO stage | 127 | | | |
| I–II | 31 | 7 (22.6) | 24 (77.4) | 0.085 |
| III–IV | 96 | 38 (39.6) | 58 (60.4) | |
| Histological
type | 130 | | | |
| Serous | 110 | 38 (34.5) | 72 (65.5) | 0.506 |
| Mucinous | 12 | 2 (16.7) | 10 (83.3) | |
| Clear cell | 8 | 3 (37.5) | 5 (62.5) | |
| Tumor location | 129 | | | |
| Single side | 56 | 11 (19.6) | 45 (80.4) | 0.015 |
| Both sides | 73 | 29 (39.7) | 44 (60.3) | |
| CA-125 (U/ml) | 89 | | | |
| 35–500 | 40 | 9 (22.5) | 31 (77.5) | 0.013 |
| 500–1000 | 32 | 18 (56.2) | 14 (43.8) | |
| >1000 | 17 | 7 (41.2) | 10 (58.8) | |
| CA19-9 (U/ml) | 88 | | | |
| 0–37 | 67 | 24 (35.8) | 43 (64.2) | 0.083 |
| >37 | 21 | 12 (57.1) | 9 (42.9) | |
| CEA (U/ml) | 79 | | | |
| 0–5 | 72 | 20 (27.8) | 52 (72.2) | 0.191 |
| >5 | 7 | 4 (57.1) | 3 (42.9) | |
CA-125, CA19-9 and carcinoembryonic antigen (CEA)
are the predominant biomarkers for confirming the diagnosis and
management of EOC. Although they commonly aid with the diagnosis of
ovarian malignancies, there are significant limitations relating to
their sensitivity and specificity. The association between
BRCA1 gene promoter methylation and preoperative serum
levels of CA-125, CA19-9 and CEA were analyzed in the present study
and BRCA1 methylation was observed to be significantly
associated with the CA-125 level. The frequency of BRCA1
methylation was greater in patients exhibiting higher preoperative
CA-125 levels than that observed in patients exhibiting lower
CA-125 levels (P=0.013, Pearson’s χ2 test).
BRCA1 promoter methylation is associated
with an improved survival rate
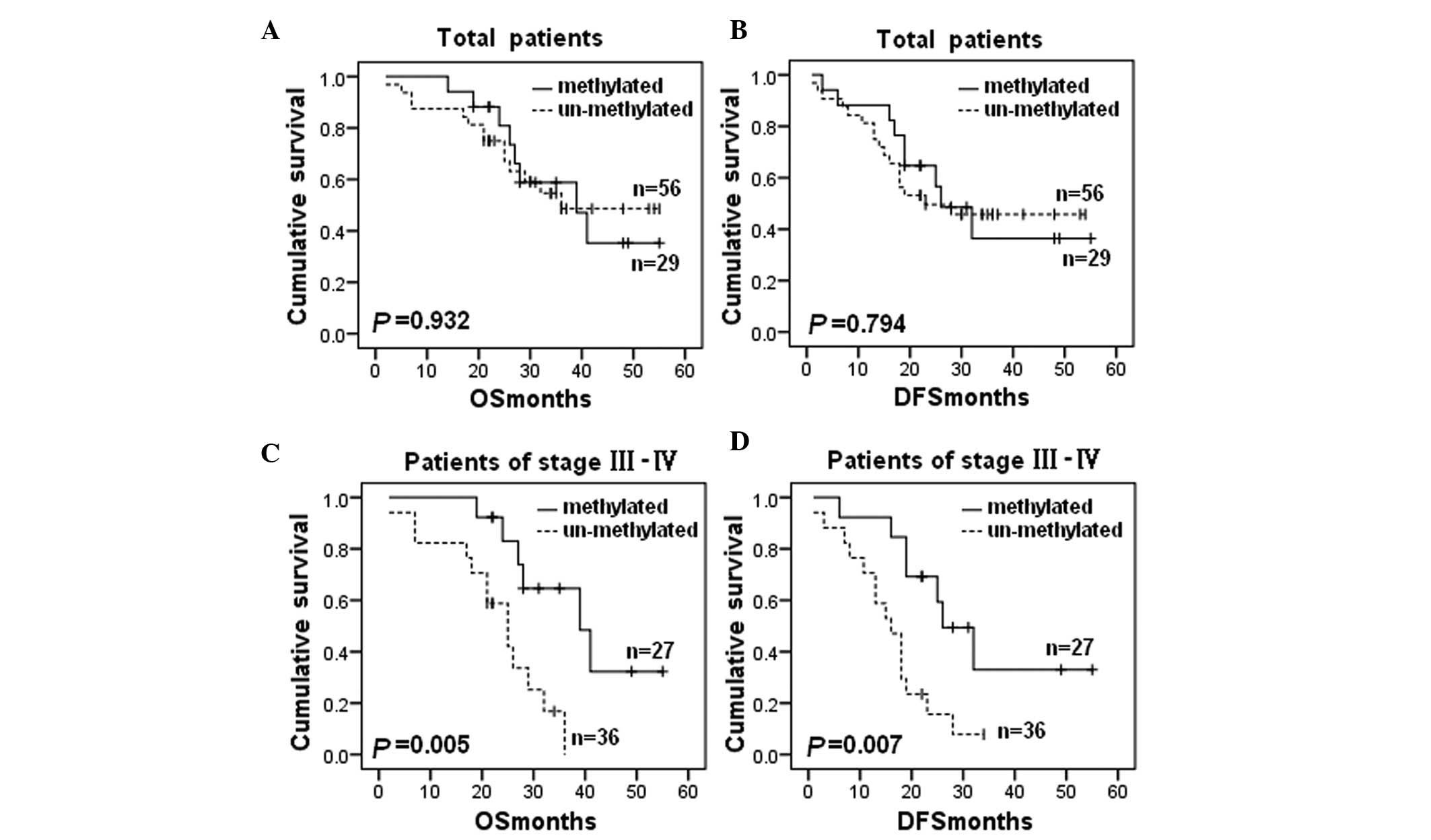
In the present study BRCA1 methylation did
not correlate with the patient survival rate in all of the patients
(OS: P=0.932; Fig. 2A and DFS:
P=0.794; Fig. 2B). However, in the
patients exhibiting advanced stage (III–IV) cancer, BRCA1
methylation was significantly associated with improved OS (P=0.005;
Fig. 2C) and DFS (P=0.007; Fig. 2D).
Cox regression univariate analysis of the potential
prognostic impact of the clinical and histopathological parameters
enabled identification of the FIGO clinical stage (OS: P=0.003;
RR=8.750; 95% CI, 2.072–36.951; and DFS: P=0.001; RR=7.446; 95% CI,
2.279–24.331), the location of the tumor (OS: P=0.075; RR=2.019;
95% CI, 0.931–4.376; and DFS: P=0.024; RR=2.227; 95% CI,
1.112–4.462) and the serum CA-125 level (OS: P=0.113; RR=1.688; 95%
CI, 0.884–3.224; and DFS, P=0.007; RR=2.067; 95% CI, 1.218–3.508)
are significantly associated with a reduced OS and DFS (Table IV). Subsequently, multivariate Cox
regression models using the clinical stage, tumor size, location of
neoplasia and BRCA1 methylation revealed that the clinical
stage alone provided the independent prognostic factor (OS:
P=0.012; RR=7.315; 95% CI, 1.552–34.481; and DFS: P=0.008;
RR=5.535; 95% CI, 1.552–19.739) (Table
V).
 | Table IVUnivariate Cox regression analysis of
OS and DFS in EOC. |
Table IV
Univariate Cox regression analysis of
OS and DFS in EOC.
| | OS | DFS |
|---|
| |
|
|
|---|
| Factor | n | RR | 95% CI | P-value | RR | 95% CI | P-value |
|---|
| Age (years) |
| >53/≤53 | 85 | 1.283 | 0.638–2.583 | 0.485 | 1.330 | 0.724–2.442 | 0.357 |
| Menopause
state |
| Post/pre- | 83 | 1.187 | 0.532–2.649 | 0.676 | 1.335 | 0.668–2.667 | 0.413 |
| Tumor size
(cm) |
| >5/≤5 | 70 | 1.599 | 0.371–6.889 | 0.529 | 1.759 | 0.418–7.399 | 0.441 |
| Node
metastasis |
| Yes/no | 68 | 1.695 | 0.708–4.055 | 0.236 | 1.114 | 0.483–2.571 | 0.799 |
| Histological
type |
|
Serous/non-serous | 84 | 0.950 | 0.411–2.197 | 0.904 | 0.886 | 0.423–1.853 | 0.748 |
| FIGO stage |
| III–IV/I–II | 79 | 8.750 | 2.072–36.951 | 0.003 | 7.446 | 2.279–24.331 | 0.001 |
| Location of
neoplasia |
|
Bilateral/unilateral | 78 | 2.019 | 0.931–4.376 | 0.075 | 2.227 | 1.112–4.462 | 0.024 |
| CA-125 (U/ml) |
|
>1000/500-1000/35-500 | 56 | 1.688 | 0.884–3.224 | 0.113 | 2.067 | 1.218–3.508 | 0.007 |
| CA19-9 (U/ml) |
| >37/0–37 | 53 | 0.366 | 0.085–1.579 | 0.178 | 0.399 | 0.119–1.342 | 0.138 |
 | Table VMultivariate Cox regression analysis
of OS and DFS in EOC. |
Table V
Multivariate Cox regression analysis
of OS and DFS in EOC.
| OS | DFS |
|---|
|
|
|
|---|
| Factor | RR (95% CI) | P-value | RR (95% CI) | P-value |
|---|
| Clinical stage |
| III–IV/I–II | 7.315
(1.552–34.481) | 0.012 | 5.535
(1.552–19.739) | 0.008 |
| Tumor size
(cm) |
| >5/≤5 | 2.383
(0.534–10.636) | 0.255 | 2.435
(0.567–10.458) | 0.231 |
| Location of
neoplasia |
|
Bilateral/unilateral | 1.210
(0.425–3.445) | 0.721 | 1.429
(0.563–3.625) | 0.453 |
| BRCA1
methylation |
|
Positive/negative | 0.448
(0.157–1.281) | 0.134 | 0.584
(0.256–1.332) | 0.201 |
DNMT co-expression is associated with
BRCA1 methylation in EOC
In the present study the correlation between DNMT
expression and BRCA1 methylation was analyzed (Table VI). No significant correlation
between the methylation status of BRCA1 and DNMT1, 3a, or 3b
protein expression was observed (P=0.135, P=0.824 and P=0.260,
respectively). However, a significant correlation was observed
between the methylation status of BRCA1 and the
co-expression of any two of the DNMTs or all three of the DNMTs.
The methylation rates of BRCA1 in the samples exhibiting
co-expression of DNMT1 and 3a, DNMT1 and 3b, or DNMT3a and 3b, were
greater than the rates observed in the non-co-expression samples
(P=0.030, P=0.034 and P=0.046, respectively). Additionally, when
all three of the DNMTs were positive, the highest BRCA1
methylation rate was observed (59.0%).
 | Table VICorrelation between DNMT expression
and BRCA1 methylation in 142 sporadic EOC cases. |
Table VI
Correlation between DNMT expression
and BRCA1 methylation in 142 sporadic EOC cases.
| | BRCA1
methylation, n (%) | |
|---|
| |
| |
|---|
| Features | n | Positive [92
(64.8)] | Negative [50
(35.2)] | P-valuea |
|---|
| DNMT3a status |
| Negative | 50 (35.2) | 33 (66.0) | 17 (34.0) | 0.824 |
| Positive | 92 (64.8) | 59 (64.1) | 33 (35.9) | |
| DNMT3b status |
| Negative | 63 (44.4) | 44 (69.8) | 19 (30.2) | 0.260 |
| Positive | 79 (55.6) | 48 (60.8) | 31 (39.2) | |
| DNMT1 status |
| Negative | 66 (46.5) | 47 (71.2) | 19 (28.8) | 0.135 |
| Positive | 76 (53.5) | 45 (59.2) | 31 (40.8) | |
| DNMT1+DNMT3a |
| No
co-expression | 88 (62.0) | 63 (71.6) | 25 (28.4) | 0.030 |
| Co-expression | 54 (38.0) | 29 (53.7) | 25 (46.3) | |
| DNMT1+DNMT3b |
| No
co-expression | 93 (65.5) | 66 (71.0) | 27 (29.0) | 0.034 |
| Co-expression | 49 (34.5) | 26 (53.1) | 23 (46.9) | |
| DNMT3a+DNMT3b |
| No
co-expression | 84 (59.2) | 60 (71.4) | 24 (28.6) | 0.046 |
| Co-expression | 58 (40.8) | 32 (55.2) | 26 (44.8) | |
|
DNMT1+DNMT3a+DNMT3b |
| No
co-expression | 103 (72.5) | 76 (73.8) | 27 (26.2) | <0.001 |
| Co-expression | 39 (27.5) | 16 (41.0) | 23 (59.0) | |
Discussion
The loss of expression of tumor suppressor genes is
known to occur via biallelic inactivation (35). The current consensus is that the
somatic mutation of BRCA1 is rare in sporadic EOC, thus, the
loss of BRCA1 expression is considered to be due to a combination
of allelic loss and methylation or biallelic methylation (36,37).
Promoter methylation is significant in silencing BRCA1. The
varying experimental methods and, in particular, the region of the
BRCA1 promoter that has been investigated across studies
have identified a range of frequencies of BRCA1 promoter
methylation, from 5 to 40% of clinical specimens (38). In the present study, the methylation
rate of BRCA1 in EOC samples is consistent with that
observed in previous studies and BRCA1 promoter methylation
was significantly associated with a decreased expression of
BRCA1. These results corroborated the hypothesis that
BRCA1 hypermethylation was a predominant cause of BRCA1 loss
of expression.
Previous studies regarding the association between
BRCA1 gene promoter methylation and patient survival remain
controversial. Montavon et al (6) and Yang et al (39) indicated that BRCA1
methylation was not associated with patient survival, however,
another study demonstrated a survival disadvantage in patients
whose neoplasms were methylated at BRCA1 (17). In the present study, a significant
association was observed between BRCA1 methylation and an
improved survival rate in patients with an advanced FIGO stage
(III–IV). The inconsistency of these results may be due to the
varying regions of the BRCA1 promoter that were investigated
in addition to the different populations that were involved in the
studies. The data from the present study resulted in the hypothesis
that the cells from the patients with methylation of the
BRCA1 promoter may exhibit an impaired ability to repair DNA
damage via downregulation of BRCA1 expression. The patients
may experience an increased sensitivity to platinum chemotherapy,
resulting in an improved survival outcome. These results were
consistent with previous studies, which demonstrated that
BRCA1 gene promoter methylation may be an effective
indicator of the patient response to chemotherapy (20,21).
As there were limitations in the present study due to the small
number of cases, further investigation is required to clarify the
role of BRCA1 gene methylation in chemoresponsiveness and
patient survival in EOC cases.
The present study indicated that BRCA1
methylation was significantly associated with the tumor location.
Furthermore, the frequency of BRCA1 methylation was observed
to be greater in patients with bilateral ovarian cancer than in
unilateral cancer patients, which may indicate a contrast between
the unilateral and bilateral ovarian cancers regarding their
biological characteristics, genetics and mechanisms of
carcinogenesis. Moreover, these data indicated that BRCA1
methylation was significantly associated with the preoperative
serum CA-125 levels. CA-125 was established as a prognostic marker
in cancer, specifically in ovarian carcinoma (40) and the present results indicated that
BRCA1 methylation and CA-125 levels, in combination, may be
used as biomarkers for the diagnosis and prognosis of EOC.
The causes of DNA methylation at specific CpG
islands in cancer are ambiguous and potentially multifactorial.
DNMTs were a predominant cause of DNA methylation and the
differential expression of DNMTs has been identified in numerous
types of cancer (41–43), including EOC, which we previously
reported (32). It was hypothesized
in the present study that the over-expression of DNMT proteins may
contribute to BRCA1 methylation; therefore, the association
between BRCA1 methylation and DNMT protein expression was
analyzed. The results indicated that there was no significant
correlation identified between the methylation status of
BRCA1 and the individual protein expression of DNMT1, 3a or
3b. However, the co-expression of DNMTs was identified to be
significantly associated with BRCA1 methylation. The results
of the present study supported the hypothesis that genomic
methylation patterns may be established depending on the
interaction of these three enzymes. Further studies are required to
clarify how these DNMTs interact with each other.
In conclusion, BRCA1 gene promoter
methylation was identified to be significant in reducing protein
expression in a Chinese population and the co-expression of DNMTs
may have contributed to BRCA1 gene promoter
hypermethylation. Moreover, BRCA1 gene promoter
hypermethylation was observed to be significantly associated with a
favorable survival rate, thus supporting the hypothesis that
BRCA1 methylation may be an important prognostic marker for
EOC.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 81173092
and 81102472), and was partly supported by the Doctoral Scientific
Research Foundation of Liaoning Province of China (grant no.
20092104110020) and the Liaoning Provincial Science and Technology
Program (grant no. 2011415052).
References
|
1
|
Bukowski RM, Ozols RF and Markman M: The
management of recurrent ovarian cancer. Semin Oncol. 34(2 Suppl 2):
S1–S15. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yap TA, Carden CP and Kaye SB: Beyond
chemotherapy: targeted therapies in ovarian cancer. Nat Rev Cancer.
9:167–181. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Laird PW: The power and the promise of DNA
methylation markers. Nat Rev Cancer. 3:253–266. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ozdemir F, Altinisik J, Karateke A,
Coksuer H and Buyru N: Methylation of tumor suppressor genes in
ovarian cancer. Exp Ther Med. 4:1092–1096. 2012.PubMed/NCBI
|
|
5
|
Tahara T and Arisawa T: Potential
usefulness of DNA methylation as a risk marker for digestive cancer
associated with inflammation. Expert Rev Mol Diagn. 12:489–497.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Montavon C, Gloss BS, Warton K, et al:
Prognostic and diagnostic significance of DNA methylation patterns
in high grade serous ovarian cancer. Gynecol Oncol. 124:582–588.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kashuba V, Dmitriev AA, Krasnov GS, et al:
NotI microarrays: Novel epigenetic markers for early detection and
prognosis of high grade serous ovarian cancer. Int J Mol Sci.
13:13352–13377. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Miki Y, Swensen J, Shattuck-Eidens D, et
al: A strong candidate for the breast and ovarian cancer
susceptibility gene BRCA1. Science. 266:66–71. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yoshida K and Miki Y: Role of BRCA1 and
BRCA2 as regulators of DNA repair, transcription, and cell cycle in
response to DNA damage. Cancer Sci. 95:866–871. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu J, Lu LY and Yu X: The role of BRCA1 in
DNA damage response. Protein Cell. 1:117–123. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Carser JE, Quinn JE, Michie CO, et al:
BRCA1 is both a prognostic and predictive biomarker of response to
chemotherapy in sporadic epithelial ovarian cancer. Gynecol Oncol.
123:492–498. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Weberpals JI, Tu D, Squire JA, et al:
Breast cancer 1 (BRCA1) protein expression as a prognostic marker
in sporadic epithelial ovarian carcinoma: an NCIC CTG OV.16
correlative study. Ann Oncol. 22:2403–2410. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Weberpals J, Garbuio K, O’Brien A, et al:
The DNA repair proteins BRCA1 and ERCC1 as predictive markers in
sporadic ovarian cancer. Int J Cancer. 124:806–815. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Quinn JE, James CR, Stewart GE, et al:
BRCA1 mRNA expression levels predict for overall survival in
ovarian cancer after chemotherapy. Clin Cancer Res. 13:7413–7420.
2007. View Article : Google Scholar
|
|
15
|
Bolton KL, Chenevix-Trench G, Goh C, et
al; EMBRACE; kConFab Investigators; Cancer Genome Atlas Research
Network. Association between BRCA1 and BRCA2 mutations and survival
in women with invasive epithelial ovarian cancer. JAMA.
307:382–390. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vencken PM, Kriege M, Hoogwerf D, et al:
Chemosensitivity and outcome of BRCA1- and BRCA2-associated ovarian
cancer patients after first-line chemotherapy compared with
sporadic ovarian cancer patients. Ann Oncol. 22:1346–1352. 2011.
View Article : Google Scholar
|
|
17
|
Chiang JW, Karlan BY, Cass L and Baldwin
RL: BRCA1 promoter methylation predicts adverse ovarian cancer
prognosis. Gynecol Oncol. 101:403–410. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Drew Y, Mulligan EA, Vong WT, et al:
Therapeutic potential of poly(ADP-ribose) polymerase inhibitor
AG014699 in human cancers with mutated or methylated BRCA1 or
BRCA2. J Natl Cancer Inst. 103:334–346. 2011. View Article : Google Scholar
|
|
19
|
Wang YQ, Zhang JR, Li SD, et al: Aberrant
methylation of breast and ovarian cancer susceptibility gene 1 in
chemosensitive human ovarian cancer cells does not involve the
phosphatidylinositol 3′-kinase-Akt pathway. Cancer Sci.
101:1618–1623. 2010.PubMed/NCBI
|
|
20
|
Chaudhry P, Srinivasan R and Patel FD:
Utility of gene promoter methylation in prediction of response to
platinum-based chemotherapy in epithelial ovarian cancer (EOC).
Cancer Invest. 27:877–884. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Veeck J, Ropero S, Setien F, et al: BRCA1
CpG island hypermethylation predicts sensitivity to poly(adenosine
diphosphate)-ribose polymerase inhibitors. J Clin Oncol.
28:e563–e566. 2010. View Article : Google Scholar
|
|
22
|
Jones PA and Baylin SB: The fundamental
role of epigenetic events in cancer. Nat Rev Genet. 3:415–428.
2002.PubMed/NCBI
|
|
23
|
Laird PW: Cancer epigenetics. Hum Mol
Genet. 14:R65–R76. 2005. View Article : Google Scholar
|
|
24
|
Cheng X and Blumenthal RM: Mammalian DNA
methyltransferases: a structural perspective. Structure.
16:341–350. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bestor TH: The DNA methyltransferases of
mammals. Hum Mol Genet. 9:2395–2402. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vilain A, Apiou F, Dutrillaux B and Malfoy
B: Assignment of candidate DNA methyltransferase gene (DNMT2) to
human chromosome band 10p15.1 by in situ hybridization. Cytogenet
Cell Genet. 82:1201998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rai K, Chidester S, Zavala CV, et al:
Dnmt2 functions in the cytoplasm to promote liver, brain, and
retina development in zebrafish. Genes Dev. 21:261–266. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Goll MG, Kirpekar F, Maggert KA, et al:
Methylation of tRNAAsp by the DNA methyltransferase homolog Dnmt2.
Science. 311:395–398. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hermann A, Gowher H and Jeltsch A:
Biochemistry and biology of mammalian DNA methyltransferases. Cell
Mol Life Sci. 61:2571–2587. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Goll MG and Bestor TH: Eukaryotic cytosine
methyltransferases. Annu Rev Biochem. 74:481–514. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Baylin SB and Jones PA: A decade of
exploring the cancer epigenome - biological and translational
implications. Nat Rev Cancer. 11:726–734. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bai X, Song Z, Fu Y, et al:
Clinicopathological significance and prognostic value of DNA
methyltransferase 1, 3a, and 3b expressions in sporadic epithelial
ovarian cancer. PLoS One. 7:e400242012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kaku T, Ogawa S, Kawano Y, et al:
Histological classification of ovarian cancer. Med Electron
Microsc. 36:9–17. 2003. View Article : Google Scholar
|
|
34
|
Matros E, Wang ZC, Lodeiro G, Miron A,
Iglehart JD and Richardson AL: BRCA1 promoter methylation in
sporadic breast tumors: relationship to gene expression profiles.
Breast Cancer Res Treat. 91:179–186. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Knudson AG Jr: Hereditary cancer,
oncogenes, and antioncogenes. Cancer Res. 45:1437–1443.
1985.PubMed/NCBI
|
|
36
|
Esteller M, Silva JM, Dominguez G, et al:
Promoter hypermethylation and BRCA1 inactivation in sporadic breast
and ovarian tumors. J Natl Cancer Inst. 92:564–569. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Geisler JP, Hatterman-Zogg MA, Rathe JA
and Buller RE: Frequency of BRCA1 dysfunction in ovarian cancer. J
Natl Cancer Inst. 94:61–67. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Senturk E, Cohen S, Dottino PR and
Martignetti JA: A critical re-appraisal of BRCA1 methylation
studies in ovarian cancer. Gynecol Oncol. 119:376–383. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yang D, Khan S, Sun Y, et al: Association
of BRCA1 and BRCA2 mutations with survival, chemotherapy
sensitivity, and gene mutator phenotype in patients with ovarian
cancer. JAMA. 306:1557–1565. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bast RC Jr, Badgwell D, Lu Z, et al: New
tumor markers: CA125 and beyond. Int J Gynecol Cancer. 15(Suppl 3):
274–281. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yang J, Wei X, Wu Q, et al: Clinical
significance of the expression of DNA methyltransferase proteins in
gastric cancer. Mol Med Rep. 4:1139–1143. 2011.PubMed/NCBI
|
|
42
|
Qu Y, Mu G, Wu Y, et al: Overexpression of
DNA methyltransferases 1, 3a, and 3b significantly correlates with
retinoblastoma tumorigenesis. Am J Clin Pathol. 134:826–834. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Amara K, Ziadi S, Hachana M, Soltani N,
Korbi S and Trimeche M: DNA methyltransferase DNMT3b protein
overexpression as a prognostic factor in patients with diffuse
large B-cell lymphomas. Cancer Sci. 101:1722–1730. 2010. View Article : Google Scholar
|