Introduction
Squamous cell carcinoma (SCC) is the most frequent
type of cancer in the oral and maxillofacial region, and its
metastatic and invasive abilities result in a poor prognosis
(1,2). Standard care for oral cancer includes
a combination of surgery, radiation and chemotherapy. Although
cancer treatment is progressing substantially, the survival rate of
patients with oral cancer has not changed over the past 30 years
(3). To develop a novel effective
therapy for oral cancer, a further understanding of the processes
and molecules that lead to the initiation and progression of oral
cancer is required. Cancer proteins not only cause and generate
cancer, but also contribute to continuous proliferation and cancer
survival; therefore, the proteins are considered useful therapeutic
targets.
Cyclin D1 has been identified as a human oncogene
(4). Rearrangement of the cyclin D1
gene locus, resulting in protein overexpression, has been
associated with prognosis in a variety of malignant tumours,
including oral SCC (5–9). Cyclin D1 is a protein with various
functions, including cell cycle induction, transcription regulation
and DNA damage-induced apoptosis (10,11).
In oral SCC, there is a possibility that the expression of cyclin
D1 may be associated with proliferation of a cancer cell, based on
an association with Ki-67 expression (12). However, little evidence has been
reported with regard to other possibilities or effects of cyclin
D1.
The present study analysed the expression of cyclin
D1 and Ki-67 using the immunohistochemical analysis of serial
tissue sections and the double-staining method for samples of oral
SCC.
Materials and methods
Patients
Between the years 2001 and 2011, 35 patients with
operable oral cancer underwent surgery at the Department of Oral
and Maxillofacial Surgery, Osaka Dental University Hospital (Osaka,
Japan; Table I). The present study
followed the tenets of the Declaration of Helsinki and was approved
by the ethics committee of Osaka Dental University. Informed
consent was obtained from the patients. None of the primary foci
received pre-operative adjuvant therapy, and among 16 metastatic
samples, 6 received pre-operative adjuvant therapy. The specific
parameters of adjuvant therapy are shown in Table II. The histological classification
of tumours was evaluated based on the Union for International
Cancer Control (UICC) classification (13).
 | Table IClinicopathological factors in 35
patients with OSCC. |
Table I
Clinicopathological factors in 35
patients with OSCC.
| Variable |
Well-differentiated |
Poorly-differentiated |
|---|
| Gender, n | | |
| Male | 10 | 10 |
| Female | 13 | 2 |
| Age, years | | |
| Mean | 66.2 | 63.7 |
| Range | 39–82 | 47–77 |
| Region, n | | |
| Tongue | 15 | 3 |
| Gingiva | 4 | 8 |
| Oral cavity
floor | 0 | 1 |
| Buccal mucosa | 3 | 0 |
| Palate | 1 | 0 |
| T status, n | | |
| T1 | 8 | 1 |
| T2 | 12 | 6 |
| T3 | 3 | 2 |
| T4 | 0 | 3 |
| N status, n | | |
| N0 | 13 | 6 |
| N1 | 4 | 1 |
| N2a | 0 | 0 |
| N2b | 6 | 5 |
| Pre-operative
adjuvant therapy, n | | |
| Yes | 1 | 5 |
| No | 9 | 1 |
 | Table IIPre-operative adjuvant therapy
regimen. |
Table II
Pre-operative adjuvant therapy
regimen.
| Patient no. | Differentiation
level | Regimen |
|---|
| 1 |
Well-differentiated |
PEP+CDDP+TS-1®+RT |
| 2 |
Poorly-differentiated | PEP+RT |
| 3 |
Poorly-differentiated | CDDP+5-FU |
| 4 |
Poorly-differentiated |
TS-1®+RT |
| 5 |
Poorly-differentiated | PEP+RT |
| 6 |
Poorly-differentiated | CDDP+5-FU+RT |
Immunohistochemistry
Tissue samples obtained from patients with different
stages of oral cancer were immediately fixed in 10% neutral
buffered formalin solution (Sumitani Ind Ltd, Tottori, Japan)
subsequent to resection and then embedded in paraffin (Thermo
Fisher Scientific, Waltham, MA, USA). Sections (4-μm thick) were
cut and mounted onto silane-coated glass slides (Matsunami Glass
Ind Ltd., Osaka, Japan). Sections were deparaffinised in L-limonene
(Falma Co., Ltd., Tokyo, Japan) and dehydrated through a graded
ethanol series. Antigen retrieval was performed by autoclaving at
121°C for 15 min in Tris-EDTA buffer (pH 7.0). Endogenous
peroxidase activity was blocked with 3% H2O2
for 10 min, and non-specific reactions were blocked by incubation
with blocking solution (Nacalai Tesque, Kyoto, Japan) for 10 min.
The tissue sections were incubated with a rabbit anti-cyclin D1
monoclonal antibody (1:500; Dako, Tokyo, Japan) or mouse anti-Ki-67
monoclonal antibody (1:100; Dako) at room temperature for 1 h. The
tissue slides were then incubated with peroxidase
micropolymer-conjugated secondary antibodies (Vector Laboratories,
Burlingame, CA, USA) at room temperature for 30 min and visualised
by incubation with a 3,3′-diaminobenzidine tetrahydrochroride
liquid system (Dako) at room temperature for 5 min. The sections
were then counterstained with hematoxylin (Merck KGaA, Daarmstadt,
Germany) and observed by light microscopy (BX50, Olympus
Corporation, Tokyo, Japan).
For double-immunostaining, the tissue sections were
incubated with rabbit anti-cyclin D1 monoclonal antibody (1:100;
Dako) overnight at 4°C. The tissue slides were then incubated with
alkaline phosphatase-conjugated anti-rabbit immunoglobulin G (IgG)
(Vector Laboratories) at room temperature for 30 min and visualised
with PermaRed (Diagnostic Biosystems, Pleasanton, CA, USA). Antigen
inactivation was performed by incubation at 98°C for 20 min in
citrate buffer (pH 6.0). The tissue sections were incubated with
mouse anti-Ki-67 monoclonal antibody (1:100; Dako) overnight at
4°C, and then the tissue slides were incubated with alkaline
phosphatase-conjugated anti-mouse IgG (Vector Laboratories) at room
temperature for 30 min and visualised with PermaBlue (Diagnostic
Biosystems). The sections were then observed by light microscopy
(Olympus Corporation).
Evaluation of slides
The immunoreactivity of the cyclin D1 and Ki-67
proteins was evaluated by two independent pathologists with no
knowledge of the patients’ clinicopathological factors and
outcomes. The nuclear expression of the cyclin D1 and Ki-67
proteins was scored semi-quantitatively by combination of the
staining intensity (scored as: 1, weak staining; 2, moderate
staining; and 3, strong staining) and the proportion of
positively-stained tumour cells in 1,000 tumour cells per
high-power field (scored as: 0, <20%; 1, 20–40%; 2, 41–60%; 3,
61–80%; and 4, >80%). The sum of the staining intensity scores
and the percentage of positive tumour cell scores were graded as
follows: +, 1–3; ++, 4–5; and +++, 6–7. There was no discrepancy in
the overall interpretation of the immunohistochemistry results
between the two independent pathologists.
Statistical analysis
A Mann-Whitney U test was performed using the SPSS
software package (version 13.0, SPSS, Inc., Chicago, IL, USA) to
assess statistically significant differences between samples. Data
are presented as the means ± SD. P<0.05 was considered to
indicate a statistically significant difference.
Results
Immunohistochemical staining was performed to
investigate the expression of cyclin D1 and Ki-67 proteins in oral
SCC clinical samples. Cyclin D1 and Ki-67 proteins were detected in
the nuclei of the cells at various levels in all 35 samples
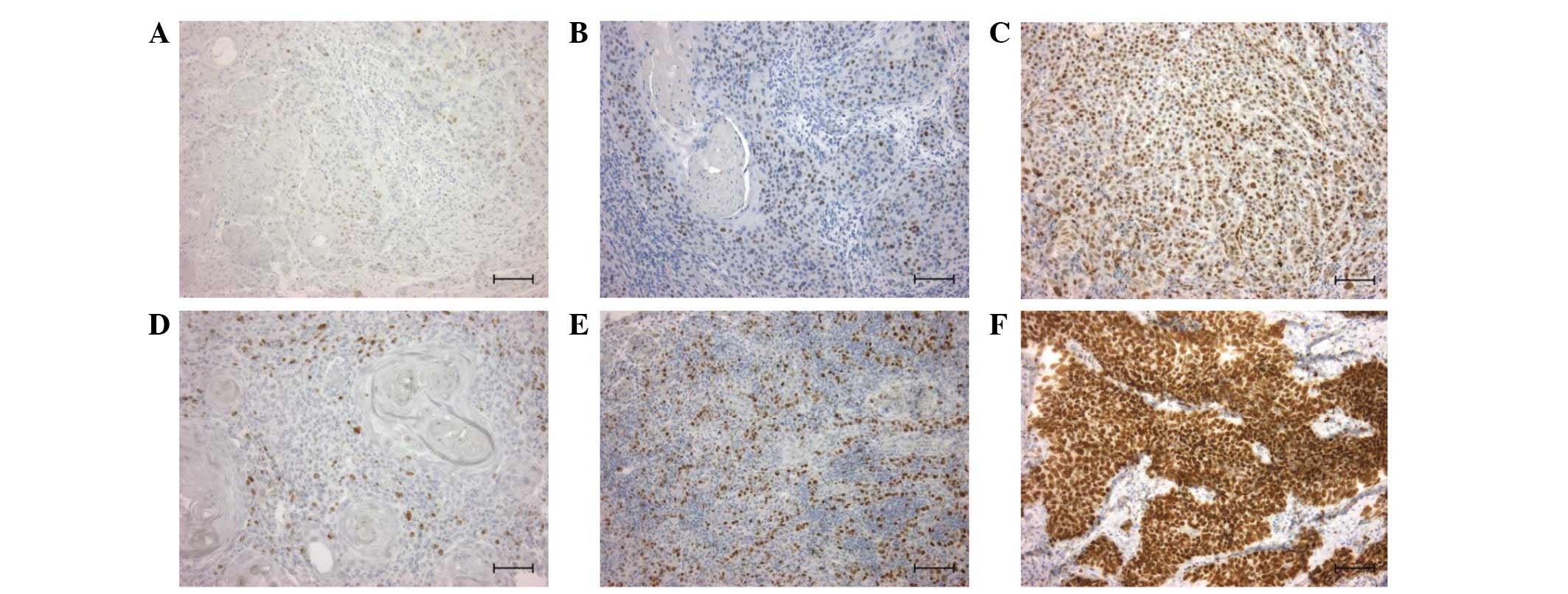
examined. Cyclin D1 expression was observed in 4 cases with weak
expression (+; Fig. 1A), 16 cases
with moderate expression (++; Fig.
1B) and 15 cases with strong expression (+++; Fig. 1C) in primary foci (Table III). Ki-67 was observed in 2 cases
with weak expression (+; Fig. 1D),
17 cases with moderate expression (++; Fig. 1E) and 16 cases with strong
expression (+++; Fig. 1F) in
primary foci (Table III). No
correlation was found between the overexpression of cyclin D1 and
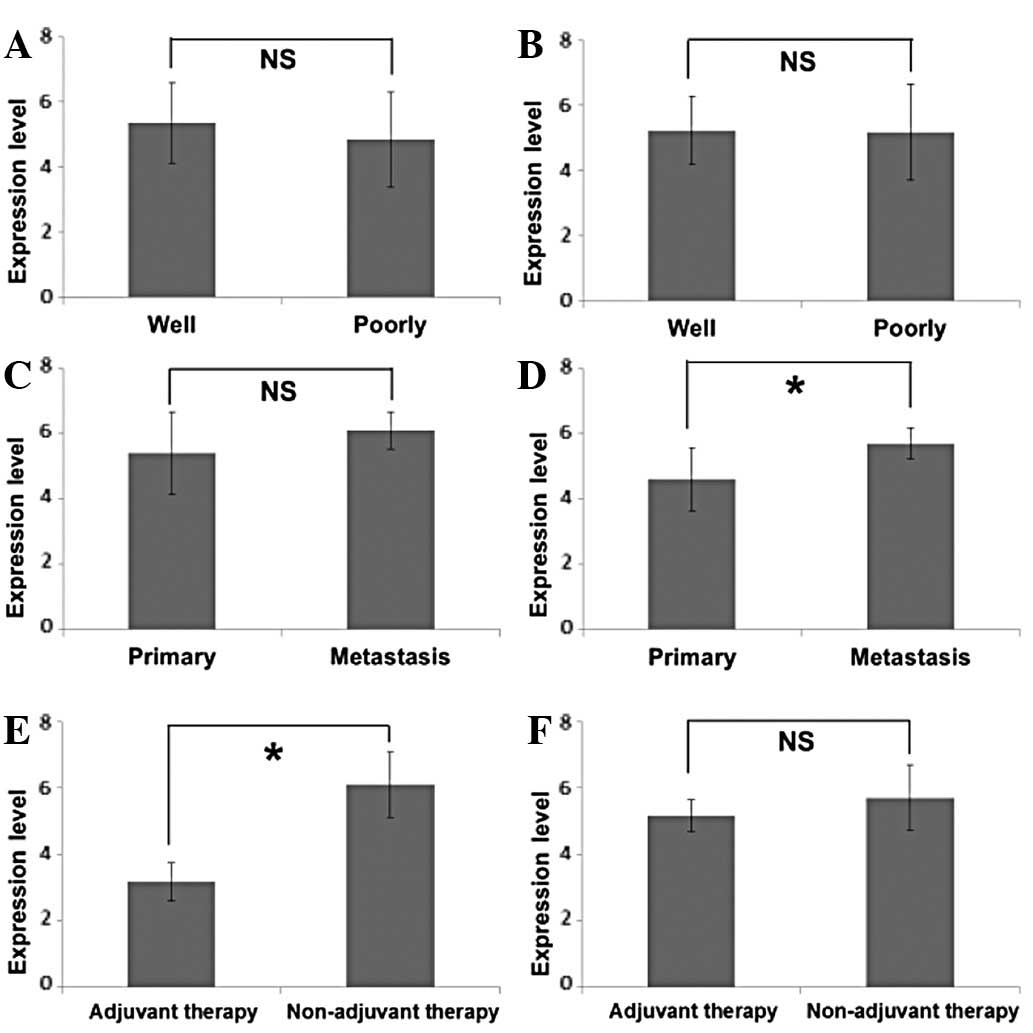
Ki-67, and gender, region, tumour status or nodal status (Table III). No difference was observed in
the expression levels of cyclin D1 and Ki-67 between poorly- and
well-differentiated SCC (Figs. 2A and
B). A statistical difference in cyclin D1 expression was not
identified between primary foci and metastatic foci (Fig. 2C), but it should be noted that 90%
of metastatic foci (9/10) showed strong cyclin D1 expression, while
43% (15/35) of primary foci demonstrated strong cyclin D1
expression (Table III). Ki-67
expression was significantly higher in metastatic foci than in
primary foci (Fig. 2D). These
results indicate a correlation between high levels of cyclin D1
expression and active cell proliferation of metastatic foci.
 | Table IIICorrelation of Cyclin D1 and Ki-67
expression with clinicopathological factors in 35 patients with
OSCC. |
Table III
Correlation of Cyclin D1 and Ki-67
expression with clinicopathological factors in 35 patients with
OSCC.
| Expression of Cyclin
D1 | | Expression of
Ki-67 | |
|---|
|
| |
| |
|---|
| Variable | + | ++ | +++ | P-value | + | ++ | +++ | P-value |
|---|
| Gender, n |
| Male | 2 | 8 | 10 | NS | 0 | 12 | 8 | NS |
| Female | 2 | 8 | 5 | | 2 | 5 | 8 | |
| Region, n |
| Tongue | 2 | 5 | 11 | NS | 0 | 9 | 9 | NS |
| Gingiva | 2 | 7 | 3 | | 2 | 5 | 5 | |
| Oral cavity
floor | 0 | 1 | 0 | | 0 | 0 | 1 | |
| Buccal mucosa | 0 | 2 | 1 | | 0 | 2 | 1 | |
| Palate | 0 | 1 | 0 | | 0 | 1 | 0 | |
| T status, n |
| T1 | 0 | 5 | 4 | NS | 0 | 6 | 3 | NS |
| T2 | 3 | 8 | 7 | | 2 | 9 | 7 | |
| T3 | 1 | 1 | 3 | | 0 | 1 | 4 | |
| T4 | 0 | 2 | 1 | | 0 | 1 | 2 | |
| N status, n |
| N1 | 0 | 1 | 3 | NS | 0 | 1 | 3 | NS |
| N2a | 0 | 0 | 0 | | 0 | 0 | 0 | |
| N2b | 0 | 0 | 6 | | 0 | 2 | 4 | |
| N3 | 0 | 0 | 0 | | 0 | 0 | 0 | |
| Primary foci,
n |
|
Well-differentiated | 2 | 11 | 10 | NS | 1 | 12 | 10 | NS |
|
Poorly-differentiated | 2 | 5 | 5 | | 1 | 5 | 6 | |
| Metastasic foci,
n |
|
Well-differentiated | 0 | 1 | 8 | NS | 0 | 3 | 6 | NS |
|
Poorly-differentiated | 0 | 0 | 1 | | 0 | 0 | 1 | |
| Adjuvant therapy
(metastasis), n |
| Yes | 3 | 3 | 0 | <0.01 | 0 | 3 | 3 | NS |
| No | 0 | 1 | 9 | | 0 | 3 | 7 | |
Furthermore, although lower levels of cyclin D1
expression were detected in metastatic foci with pre-operative
adjuvant therapy (Fig. 2E), no
effect was observed in Ki-67 (Fig.
2F). To date, the high expression of cyclin D1 in cancer tissue
has been believed to play a role in cell proliferation due to its
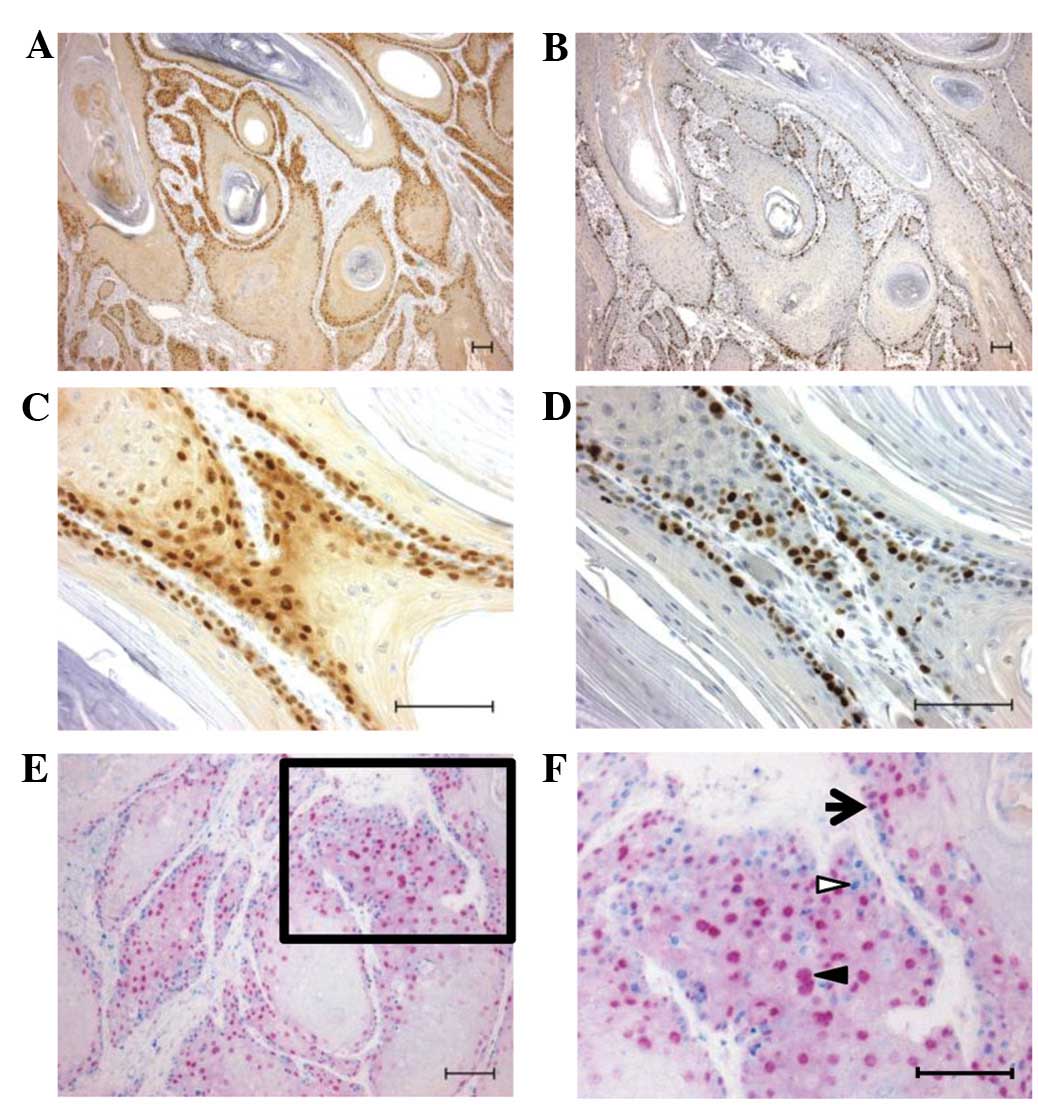
correlation with Ki-67 expression (12). To confirm the simultaneous
occurrence of cyclin D1 and Ki-67 expression in the present study,
immunohistochemistry was applied to serial tissue sections, and it
was identified that cyclin D1 (Fig. 3A
and C) and Ki-67 (Fig. 3B and
D) were expressed in the basal to suprabasal cells.
In addition, the expression of cyclin D1 and Ki-67
was analysed using a double-immunostaining method in the same
tissue sections. Cells expressing cyclin D1, but not Ki-67, were
found to be located away from the basal cell layer (Fig. 3E and F).
Discussion
Immunohistochemical staining was performed to
investigate the expression of cyclin D1 in oral SCC. Cyclin D1
protein was detected in the nuclei of cells in all 35 samples
examined. The expression level did not correlate with tumour
differentiation, but it was higher in the metastatic foci than in
the primary foci. Furthermore, there was a low level of expression
in the metastatic foci with pre-operative adjuvant therapy. Ki-67
protein was also detected in all the samples investigated, with
specific differences in expression levels. These results are
fundamentally consistent with previous studies (12,14),
in which cyclin D1 and Ki-67 expression was detected in the same
region of oral SCC. Other studies have reported contradictory
reports with regard to cyclin D1 expression, with certain studies
indicating high expression of cyclin D1 in poorly-differentiated
SCC (15,16), and others reporting the opposite,
namely, high expression in well-differentiated SCC (17). However, these studies also reported
rather low rates of cyclin D1-positive tumours compared with the
data of the present study; potential bias caused by the low
positive rate could partially explain these discrepancies.
In the present study, the expression of cyclin D1
and Ki-67 was examined in detail using the double-immunostaining
method. Co-expression of cyclin D1 and Ki-67 was observed in the
basal to suprabasal cells, and numerous cyclin D1-positive and
Ki-67-negative cells existed toward the central section of the
tumour. In well-differentiated oral SCC, poorly-differentiated and
highly-proliferative cells that express Ki-67 are located in the
basal layer, and proliferation slows down as it moves from the
periphery to the centre of a tumour tissue and is occupied by
differentiated cells that express keratin 17 (18). Therefore, the results of the present
study indicate that the high expression of cyclin D1 observed in
the cells was involved in the process of differentiation. Cyclin D1
in the nucleus is believed to promote the cell cycle by regulating
the G1/S transition through an interaction with cyclin
dependent kinase (CDK)2/4 (19). If
cyclin D1 is highly expressed and the complex level of CDK2/4
increases, it not only promotes proliferation, but also reduces
cell differentiation without entering into the G0 phase
(20). Additionally, when cyclin D1
works with proteins other than CDKs, it may control apoptosis,
aging, invasion and other processes through transcription or the
DNA damage response (11,21–25).
Therefore, in oral SCC, it is possible that cyclin D1 is involved
in cell differentiation and the prevention of cell death, in
addition to the cell proliferation that has been observed when
working with proteins other than CDKs.
Lower levels of cyclin D1 expression were also found
in the present study in the metastatic foci of patients with
pre-operative adjuvant therapy compared with patients who did not
receive pre-operative adjuvant therapy. By contrast, Ki-67 levels
did not differ between the two groups of patients. The high
expression of cyclin D1 may contribute to drug resistance in cancer
cells, not only by increasing cell proliferation, but also by
suppressing cancer cell apoptosis (26). The results of the present study
indicate that cancer cells with a high expression of cyclin D1 and
with drug resistance may survive, even if certain tumour cells of
primary foci die and local control occurs as the result of
pre-operative adjuvant therapy. Therefore, it is believed to be
necessary to use certain methods to aid in the reduction of cyclin
D1 levels, with respect to conventional anticancer drug
medication.
References
|
1
|
Chen YJ, Lin SC, Kao T, et al: Genome-wide
profiling of oral squamous cell carcinoma. J Pathol. 204:326–332.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pentenero M, Gandolfo S and Carrozzo M:
Importance of tumor thickness and depth of invasion in nodal
involvement and prognosis of oral squamous cell carcinoma: a review
of the literature. Head Neck. 27:1080–1091. 2005. View Article : Google Scholar
|
|
3
|
Myers JN, Elkins T, Roberts D and Byers
RM: Squamous cell carcinoma of the tongue in young adults:
increasing incidence and factors that predict treatment outcomes.
Otolaryngol Head Neck Surg. 122:44–51. 2000. View Article : Google Scholar
|
|
4
|
Motokura T, Bloom T, Kim HG, Jüppner H,
Ruderman JV, Kronenberg HM and Arnold A: A novel cyclin encoded by
a bcl1-linked candidate oncogene. Nature. 350:512–515. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bellacosa A, Almadori G, Cavallos S, et
al: Cyclin D1 gene amplification in human laryngeal squamous cell
carcinomas: prognostic significance and clinical implications. Clin
Cancer Res. 2:175–180. 1996.
|
|
6
|
Michalides R, van Veelen N, Hart A, Loftus
B, Wientjens E and Balm A: Overexpression of cyclin D1 correlates
with recurrence in a group of forty-seven operable squamous cell
carcinomas of the head and neck. Cancer Res. 55:975–978.
1995.PubMed/NCBI
|
|
7
|
Vora HH, Shah NG, Trivedi TT, et al:
Cyclin D1 expression in prediction of survival in carcinoma of the
tongue. GCRI Bulletin. 7:130–135. 1997.
|
|
8
|
Mishra R and Das BR: Cyclin D1 expression
and its possible regulation in chewing tobacco mediated oral
squamous cell carcinoma progression. Arch Oral Biol. 54:917–923.
2009. View Article : Google Scholar
|
|
9
|
Santarius T, Shipley J, Brewer D, Stratton
MR and Cooper CS: A census of amplified and overexpressed human
cancer genes. Nat Rev Cancer. 10:59–64. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Malumbres M and Barbacid M: Cell cycle,
CDKs and cancer: a changing paradigm. Nature Rev Cancer. 9:153–166.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bienvenu F, Jirawatnotai S, Elias JE, et
al: Transcriptional role of cyclin D1 in development revealed by a
genetic-proteomic screen. Nature. 463:374–378. 2010. View Article : Google Scholar
|
|
12
|
Carlos de Vicente J, Herrero-Zapatero A,
Fresno MF and López-Arranz JS: Expression of cyclin D1 and Ki-67 in
squamous cell carcinoma of the oral cavity: clinicopathological and
prognostic significance. Oral Oncol. 38:301–308. 2002.PubMed/NCBI
|
|
13
|
Sobin LH and Wittekind C: International
Union Against Cancer: TNM Classification of Malignant Tumors. 5th
edition. Wiley-Liss Publications; New York, NY: 1997
|
|
14
|
Wang L, Liu T, Nishioka M, Aguirre RL, Win
SS and Okada N: Activation of ERK1/2 and cyclin D1 expression in
oral tongue squamous cell carcinomas: relationship between
clinicopathological appearances and cell proliferation. Oral Oncol.
42:625–631. 2006. View Article : Google Scholar
|
|
15
|
Lam KY, Ng IO, Yuen AP, Kwong DL and Wei
W: Cyclin D1 expression in oral squamous cell carcinomas:
clinicopathological relevance and correlation with p53 expression.
J Oral Pathol Med. 29:167–172. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Angadi PV and Krishnapillai R: Cyclin D1
expression in oral squamous cell carcinoma and verrucous carcinoma:
correlation with histological differentiation. Oral Surg Oral Med
Oral Pathol Oral Radiol Endod. 103:e30–e35. 2007. View Article : Google Scholar
|
|
17
|
Bartkova J, Lukas J, Müller H, Strauss M,
Gusterson B and Bartek J: Abnormal patterns of D-type cyclin
expression and G1 regulation in human head and neck cancer. Cancer
Res. 55:949–956. 1995.PubMed/NCBI
|
|
18
|
Mikami T, Cheng J, Maruyama S, et al:
Emergence of keratin 17 vs. loss of keratin 13: their reciprocal
immunohistochemical profiles in oral carcinoma in situ. Oral Oncol.
47:497–503. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Musgrove EA, Lee CS, Buckley MF and
Sutherland RL: Cyclin D1 induction in breast cancer cells shortens
G1 and is sufficient for cells arrested in G1 to complete the cell
cycle. Proc Natl Acad Sci USA. 91:8022–8026. 1994. View Article : Google Scholar
|
|
20
|
Skapek SX, Rhee J, Spicer DB and Lasser
AB: Inhibition of myogenic differentiation in proliferating
myoblasts by cyclin D1-dependent kinase. Science. 267:1022–1024.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kotelnikov VM, Coon JS IV, Mundle S, et
al: Cyclin D1 expression in squamous cell carcinomas of the head
and neck and in oral mucosa in relation to proliferation and
apoptosis. Clin Cancer Res. 3:95–101. 1997.PubMed/NCBI
|
|
22
|
Lucibello FC, Sewing A, Brüsselbach S,
Bürger C and Müller R: Deregulation of cyclins D1 and E and
suppression of cdk2 and cdk4 in senescent human fibroblasts. J Cell
Sci. 105:123–133. 1993.PubMed/NCBI
|
|
23
|
Sauter ER, Nesbit M, Litwin S,
Klein-Szanto AJ, Cheffetz S and Herlyn M: Antisense cyclin D1
induces apoptosis and tumor shrinkage in human squamous carcinomas.
Cancer Res. 59:4876–4881. 1999.PubMed/NCBI
|
|
24
|
Li Z, Jiao X, Wang C, et al: Alternative
cyclin D1 splice forms differentially regulate the DNA damage
response. Cancer Res. 70:8802–8811. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang J, Wang Q, Cui Y, et al: Knockdown of
cyclin D1 inhibits proliferation, induces apoptosis, and attenuates
the invasive capacity of human glioblastoma cells. J Neurooncol.
106:473–484. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Biliran H Jr, Wang Y, Banerjee S, et al:
Overexpression of cyclin D1 promotes tumor cell growth and confers
resistance to cisplatin-mediated apoptosis in an elastase-myc
transgene-expressing pancreatic tumor cell line. Clin Cancer Res.
11:6075–6086. 2005. View Article : Google Scholar
|