Introduction
Glycosyl structures, expressed as either
glycoproteins or glycosphingolipids (GSLs), are involved in a
variety of cell functions. Gangliosides (GSLs containing sialic
acid) are abundantly expressed at the membranes of mammalian cells,
particularly neuronal cells. The expression profiles of
gangliosides and other GSLs have been shown to change during cell
differentiation, proliferation and oncogenic transformation
(1). The ganglioside
sialosyllactosylceramide (GM3; NeuAcα3Galβ4Glcβ1Cer) inhibits the
activity of various growth factor receptor (GFR)-associated
tyrosine kinases. For example, the exogenous addition of GM3 has
been shown to inhibit BHK cell growth induced by fibroblast growth
factor (2) and the phosphorylation
of platelet-derived GFR (3) and
epidermal GFR (EGFR) (4).
EGF-induced EGFR activation in human epidermoid carcinoma A431
cells was shown to be strongly inhibited by GM3, but to a much
lesser degree by various other gangliosides and neutral GSLs. The
order of inhibition was GM3>>GM2, GD3, GM4>GM1, GD1a,
GD1b, GT1b, GD2, GQ1b>lactosyl-Cer (5). The inhibition of cell proliferation by
exogenously added GM3 has also been reported (6,7).
In our previous preliminary study, it was found that
fully halogenated GM3, starting from N-glycolyl-GM3, but not
from N-acetyl-GM3, enhanced contact inhibition of tumor cell
growth (8), indicating the
possibility that the inhibitory effect of halogenated GM3
derivatives on EGFR activation is stronger than that of GM3 itself.
The present study describes: (i) The complete chemical synthesis of
monochloro-acetyl-GM3 and dichloro-acetyl-GM3 (referred to
hereafter as monochloro-GM3 and dichloro-GM3, respectively), and
(ii) evidence that these derivatives show stronger inhibitory
effects in comparison with GM3, on the activation of EGFR and of
ΔEGFR, a common mutant detected in cancers (9,10). The
findings of the present study indicate the potential application of
halogenated GM3 derivatives as a novel approach for cancer
therapy.
Materials and methods
Synthesis of GM3 chloro-derivatives
All chemicals were reagent grade and used without
further purification. Solvent ratios are by volume.
Dichloromethane
(CH2Cl2) was freshly distilled from
P2O5
GM3 was synthesized as described previously
(11) at the Institute of Paris
Molecular Chemistry (University Pierre & Marie Curie Paris 6,
Paris, France). Nuclear magnetic resonance (NMR) spectra were
recorded with a Bruker DRX 400 spectrometer (400 MHz for
1H NMR and 100 MHz for 13C NMR; Bruker,
Fällanden, Switzerland). The chemical shifts were referenced to the
solvent peaks; δ=3.31 ppm (1H) and δ=49.00 ppm
(13C) for CD3OD. The coupling constants were
provided in Hz. High-resolution mass spectra (HRMS) were recorded
with a Bruker micrOTOF spectrometer in electrospray ionization
(ESI) mode, using the Tuning-Mix as a reference (Bruker). Reactions
were monitored by thin-layer chromatography on glass plates
precoated with silica gel 60 F254 (Merck, Darmstadt,
Germany) and detected by charring with sulfuric acid. Flash column
chromatography was performed on silica gel 60 (230–400 mesh;
Merck).
GM3 (30 mg, 0.025 mmol) in 10 ml 0.1 M KOH in
H2O/butanol (1:9) solution was stirred at 80°C for 5 h.
The mixture was neutralized by 6 M HCl (12–14)
and concentrated in vacuo. The resulting residue was
purified by flash column chromatography (CHCl3/MeOH,
2:1) to yield crude intermediate 3 (Fig. 1). To a solution of crude
intermediate 3 (in 3 ml MeOH and 3 ml
CH2Cl2), Et3N (74 μl, 0.53 mmol)
and chloroacetyl chloride (40 μl, 0.50 mmol) were added. The
mixture was stirred at room temperature (r.t). for 2 h and
concentrated in vacuo. The resulting residue was purified by
flash column chromatography (CHCl3/MeOH, 3:1) to produce
compound 1. Compound 2 was prepared by the same procedure, except
that dichloroacetyl chloride (48 μl, 0.50 mmol) was used instead of
chloroacetyl chloride.
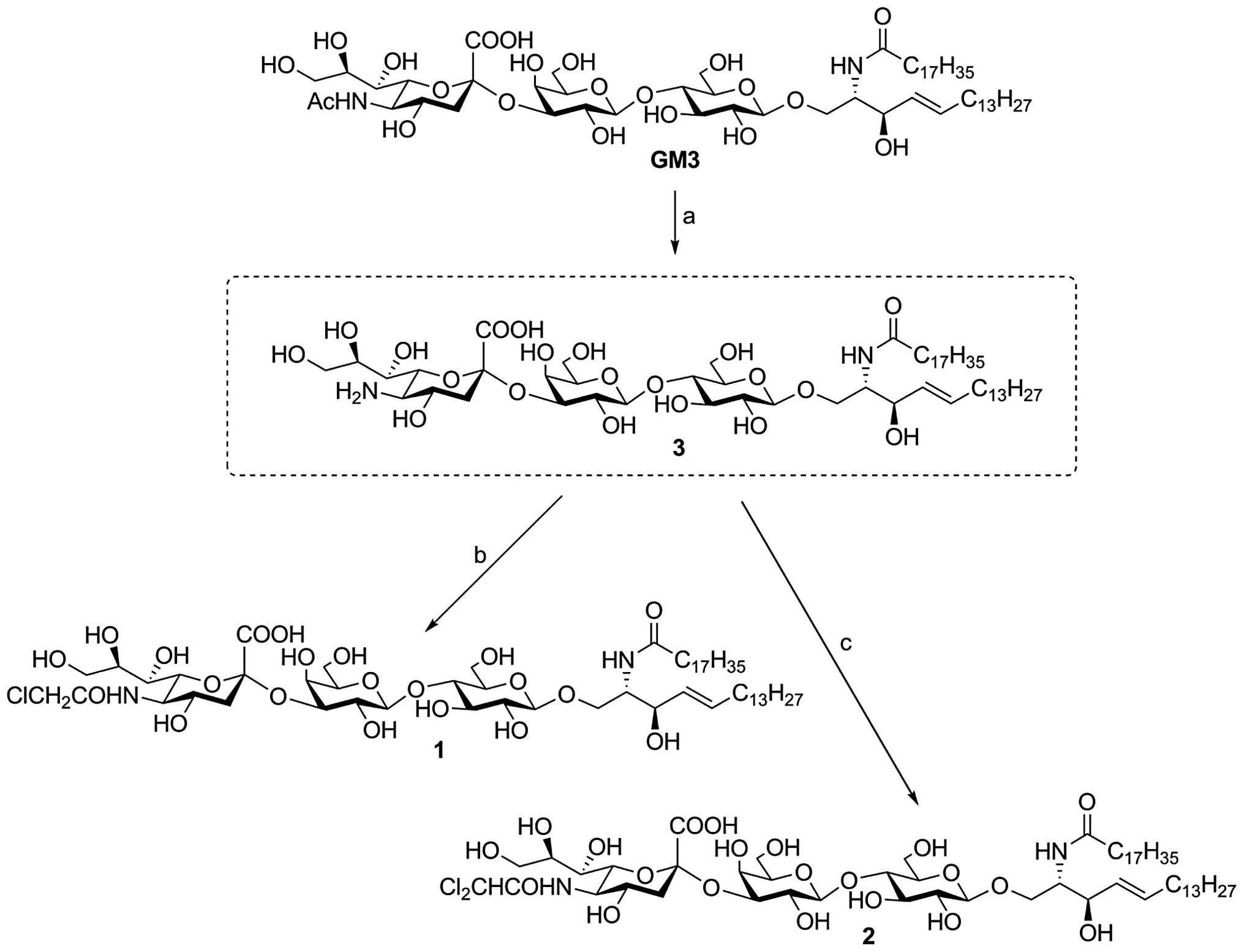 | Figure 1Method for synthesis of GM3
chloro-derivatives. Reagents and conditions: (A) 0.1 M KOH, 80°C, 5
h; (B) MeOH, CH2Cl2, Et3N,
ClCH2COCl, r.t., 2 h, 49% (two steps from GM3); (C)
MeOH, CH2Cl2, Et3N,
Cl2CHCOCl, r.t., 2 h, 26% (two steps from GM3). Compound
1, monochloro-GM3; and compound 2, dichloro-GM3. GM3,
sialosyllactosylceramide. |
Cell lines and culture
The human ovarian epidermoid cancer A431 cells were
purchased from the American Type Culture Collection (Rockville, MD,
USA). The human glioblastoma U87MG cell line and its stable
transfectants, expressing wild-type EGFR (U87MG.wtEGFR) or mutant
ΔEGFR (U87MG.ΔEGFR) (15,16), were from the Cavanee laboratory,
Ludwig Institute for Cancer Research (San Diego, USA). Cells were
grown in Dulbecco’s modified Eagle’s medium (DMEM) containing 10%
heat-inactivated fetal bovine serum, 100 U/ml penicillin and 100
μg/ml streptomycin in 5% CO2 at 37°C in a humidified
atmosphere.
Reagents and antibodies
The GM3 used for the biological analyses was
purchased from Matreya Inc., (Pleasant Gap, PA, USA) and dissolved
in chloroform/methanol (C/M; 2:1) to make a stock solution (1
mg/ml). The antibodies used for western blotting were rabbit
anti-EGFR monoclonal antibody (mAb; sc-03; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA), rabbit anti-phospho-EGFR
(PY1068) mAb (Epitomics, Burlingame, CA, USA) and mouse anti-GAPDH
mAb (Millipore, Billerica, MA, USA).
EGFR activation assay
EGFR autophosphorylation and the effects of GM3 and
the chloro-derivatives were analyzed as described previously
(17). GM3, dichloro-GM3 and
monochloro-GM3 in C/M (2:1) were dried completely under
N2 stream. The dried gangliosides were added with
serum-free DMEM and sonicated for 10 min. The A431, U87MG.wtEGFR
and U87MG.ΔEGFR cells were cultured in 24-well plates until ~90%
confluency and starved in serum-free DMEM for 24 h. The starved
cells were incubated in serum-free DMEM containing GM3,
dichloro-GM3 or monochloro-GM3 for 16 h at 37°C. EGF (100 or 1
ng/ml) was added to the culture media, and the cells were further
incubated for 30 min at 37°C. The cells were washed with Dulbecco’s
phosphate-buffered saline (PBS) containing 500 nM
Na3VO4, 5 mM EDTA, 5 mM NaF and 10 mM
Na4O7P2 and then lysed with 100 μL
radioimmunoprecipitation assay lysis buffer [1% Nonidet P-40, 25 mM
Tris-HCl (pH 7.6), 150 mM NaCl, 1% deoxycholic acid and 0.1% SDS]
containing 1% aprotinin, 1% phenylmethanesulfonyl fluoride, 500 nM
Na3VO4 and Halt Protease and Phosphatase
Inhibitor cocktail (Thermo Fisher Scientific, Inc., Waltham, MA,
USA) for 30 min at 4°C. The solutions were centrifuged at 13,225 ×
g for 10 min at 4°C, and the supernatants were collected and used
as cell lysates. The protein concentration of the cell lysates was
determined using a Micro Bicinchoninic Acid Protein Assay kit
(Pierce Biotechnology, Inc., Rockford, IL, USA).
SDS-PAGE and western blot analysis
The cell lysates were analyzed by western blotting,
as described previously (18–21).
In brief, subsequent to boiling for 5 min at 98°C in SDS sample
buffer, 5 μg protein of each cell lysate was resolved by 7.5%
SDS-PAGE and transferred onto polyvinylidene fluoride membranes
(Thermo Fisher Scientific, Inc.). The membranes were blocked with
3% bovine serum albumin/Tris-buffered saline (TBS) containing 0.1%
Tween-20 (TBS-T) for 1 h at r.t., incubated with specific primary
antibodies in TBS-T for 2 h at r.t. or overnight at 4°C and washed.
The membranes were then incubated with appropriate secondary
antibodies conjugated with horseradish peroxidase in TBS-T for 1 h
at r.t. and washed 3 times with TBS-T. Detection was performed by
enhanced chemiluminescence using SuperSignal West Pico
chemiluminescent substrate (Thermo Fisher Scientific, Inc.). The
intensity of western blotting was determined by densitometry using
the ImageJ program (http://rsb.info.nih.gov/ij/).
[3H]-thymidine cell
incorporation assay
[3H]-thymidine incorporation into DNA was
used as a measure of the DNA replication level, following the
method of Gabelman and Emerman (22). The A431, U87MG.wtEGFR and
U87MG.ΔEGFR cells were cultured in 48-well plates in DMEM
containing 10% fetal bovine serum until 70–80% confluence, and then
starved in serum-free DMEM for 24 h. The starved cells were
incubated in serum-free DMEM containing GM3, monochloro-GM3 or
dichloro-GM3 for 16 h at 37°C. EGF (100 or 1 ng/ml) was added to
the culture media and the cells were further incubated for 2 or 24
h at 37°C. The cells were then incubated with 0.8 μCi of
[3H]-thymidine (PerkinElmer, Waltham, MA, USA) for 4 h
at 37°C, washed 3 times with PBS and detached with trypsin/EDTA.
The cell suspension was mixed with Ecoscint (1:30; National
Diagnostics, Atlanta, GA, USA), and [3H]-thymidine
incorporation was determined by a liquid scintillation β-counter
(Beckman Instruments, Fullerton, CA, USA).
Statistical analysis
The data were analyzed by Student’s t-test. P≤0.05
was considered to indicate a statistically significant
difference.
Results
Synthesis of GM3 chloro-derivatives
GM3 was synthesized, as described previously
(11), at the Institute of Paris
Molecular Chemistry (University Pierre & Marie Curie Paris 6,
Paris, France). A suitably protected lactoside diol was
glycosylated with sialyl xanthate to exclusively produce the
α-sialyl trisaccharide at a good yield based on a highly
stereoselective and regioselective sialylation. Following chemical
modification, this trisaccharide was reacted with 3-O-benzoylated
azidosphingosine to form a GSL, which subsequent to a reduction of
azide followed by condensation with stearic acid and deprotection,
yielded GM3.
Under strongly basic conditions (0.1 M KOH, 80°C),
the N-acetyl group of GM3 was hydrolyzed to yield the key
intermediate 3 (Fig. 1), in which
the free amino functionality could be subjected to derivatization.
Two N-modified GM3 analogues, compound 1 (monochloro-GM3)
and compound 2 (dichloro-GM3) (Fig.
1), were synthesized from intermediate 3.
Compound 1 was obtained (14.9 mg, 49% for two steps)
as a white foam with a retention factor (Rf) value of 0.45
(EtOAc-iPrOH-H2O, 3:2:1) and an [α]D of −0.3
(c, 0.5; and CHCl3:MeOH, 1:1). The NMR spectral data
were in good agreement with results reported previously (23). The ESI-HRMS (m/z) for
C59H106ClN2O21
[M-H]− was calculated as 1213.6982 m/z and found to be
1213.7015 m/z.
Compound 2 was obtained (8.1 mg, 26% for two steps)
as a white foam, with an Rf of 0.53 (EtOAc-iPrOH-H2O,
3:2:1); and an [α]D of −0.4 (c, 0.5; and
CHCl3:MeOH, 1:1). 1H NMR (400 MHz;
CDCl3:CD3OD, 1:1): δ 6.16 [singlet (s), 1H,
Cl2CH], 5.63 [triple doublet (td), coupling constants
(J)=15.0, 6.8 Hz, 1H, H-5cer], 5.38 [double doublet (dd), J=15.3,
7.6 Hz, 1H, H-4cer], 4.36 (d, J=7.8 Hz, 1H, H-1Gal), 4.24 (d, J=7.8
Hz, 1H, H-1Glu), 4.14 (dd, J=9.9, 4.0 Hz, 1H, Ha-1cer), 4.04 (dd,
J=8.5, 5.9 Hz, 1H, H-3cer), 4.01-3.89 [multiplet (m), 3H, H-3Gal,
H-2cer, H-4Gal], 3.89-3.78 (m, 4H, Ha-6Gal, Hb-6Gal, Ha-6Glu,
H-6Neu), 3.77-3.68 (m, 4H, Ha-9Neu, H-4Neu, H-5Neu, H-5Gal),
3.66-3.46 (m, 7H, Hb-6Glu, H-5Glu, Hb-9Neu, H-8Neu, H-2Gal, H-3Glu,
H-4Glu), 3.44 (d, J=9.0 Hz, 1H, Hb-1cer), 3.40-3.34 (m, 1H,
H-7Neu), 3.24 (d, J=10.2 Hz, 1H, H-2Glu), 2.88-2.67 (m, 1H,
Heq-3Neu), 2.11 [triplet (t), J=7.6 Hz, 2H, CH2C(O)],
1.96 (dd, J=12.7, 5.8 Hz, 2H, H2-6cer), 1.76-1.66 (m, 1H,
Hax-3Neu), 1.56-1.48 (m, 2H, CH2CH2C(O)),
1.25-1.19 (m, 50H, alkane CH2) and 0.82 (t, J=6.8 Hz,
6H, 2xCH3). 13C NMR (100 MHz;
CDCl3:CD3OD, 1:1): δ 134.92 (C-5cer), 130.25
(C-4cer), 104.48 (C-1Gal), 103.73 (C-1Glu), 80.36, 76.95, 76.21,
75.66, 75.39, 74.05, 73.95, 72.51, 72.37, 70.10, 69.42 (C-1cer),
68.43, 68.31, 66.94 (Cl2CH), 64.05, 63.91, 62.25, 61.18,
53.99 (C-2cer), 53.74 (C-5Neu), 41.30 (C-3cer), 37.04, 33.01,
32.53, 30.31, 30.24, 30.22, 30.12, 29.97, 29.91, 26.64, 23.24 and
14.34 (2xCH3). ESI-HRMS (m/z) for
C59H105Cl2N2O21
[M-H]− was calculated as 1247.6592 m/z and found to be
1247.6595 m/z.
Inhibitory effect of GM3
chloro-derivatives on EGFR activation
The inhibitory effects of monochloro- and
dichloro-GM3 on EGF-induced EGFR activation were evaluated and
compared with that of GM3 using A431 cells, which express a high
level of EGFR at the cell surface. In agreement with the findings
of our previous studies (3,4,18,19,24),
pre-incubation of the cells with GM3 at either 0.1 or 0.2 mM caused
~65% inhibition of EGFR activation (as assessed by its
autophosphorylation) induced by 100 ng/ml EGF (Fig. 2A, columns c and d). The degree of
inhibition by the chloro-derivatives at concentrations of 0.1 and
0.2 mM was ~50 and ~70% for dichloro-GM3 (Fig. 2A, columns e and f) and ~85 and ~90%
for monochloro-GM3 (Fig. 2A,
columns g and h), respectively. For EGFR activation induced by 1
ng/ml EGF, 0.2 mM GM3 caused ~40% inhibition and 0.1 mM GM3
exhibited no significant effect (Fig.
2B, columns c and d). The degree of inhibition at
concentrations of 0.1 and 0.2 mM was ~37 and ~32% for dichloro-GM3
(Fig. 2B, columns e and f) and ~65
and ~75% for monochloro-GM3 (Fig.
2B, columns g and h), respectively. The inhibitory effects of
GM3 and the chloro-derivatives on EGFR activation in the
U87MG.wtEGFR cells were quite similar for EGF concentrations of 100
(Fig. 2C, columns c–h) and 1 ng/ml
(Fig. 2D, columns c–h).
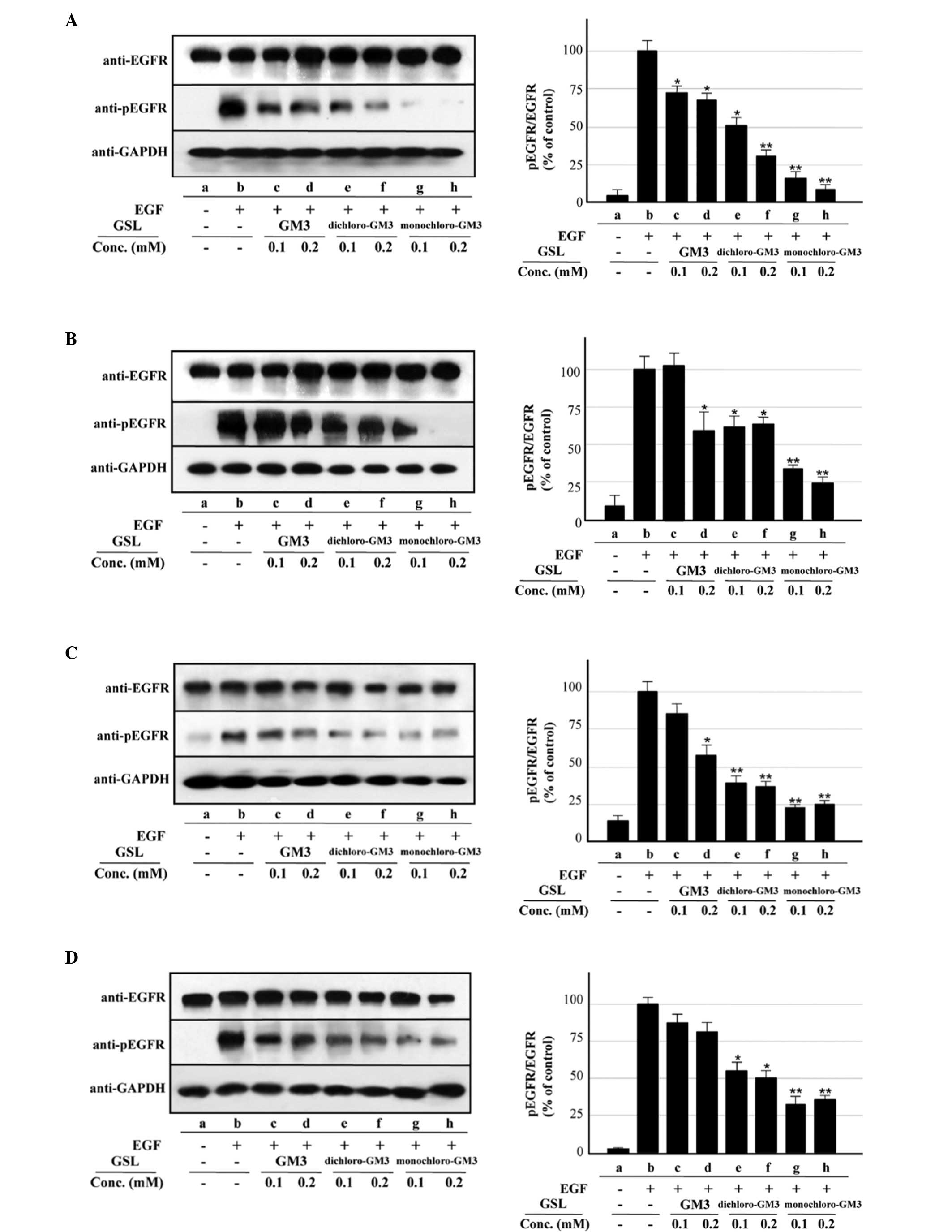 | Figure 2Inhibitory effects of pre-incubation
with GM3, monochloro-GM3 and dichloro-GM3 on EGFR activation
(autophosphorylation) in the A431, U87MG.wtEGFR and U87MG.ΔEGFR
cells. (A) A431 cells, 100 ng/ml EGF. (B) A431 cells, 1 ng/ml EGF.
(C) U87MG.wtEGFR cells, 100 ng/ml EGF. (D) U87MG.wtEGFR cells, 1
ng/ml EGF. Left, representative western blotting results from
triplicate experiments. GAPDH signals are shown to confirm the
protein amounts in the cell lysates. Right, the y-axis indicates
the ratio of phosphorylated EGFR (pEGFR) to EGFR as a percentage of
the control value. The control is b in panels A–F and a in panel G.
(E) U87MG.ΔEGFR cells, 100 ng/ml EGF. (F) U87MG.ΔEGFR cells, 1
ng/ml EGF. (G) U87MG.ΔEGFR cells, no EGF. The results are presented
as the mean ± dtandard deviation. *P<0.05;
**P<0.01. GM3, sialosyllactosylceramide; EGFR,
epidermal growth factor receptor; GSL, glycosphingolipids. |
The inhibitory effects of GM3 and its
chloro-derivatives were also evaluated in U87MG.ΔEGFR cells, which
overexpress the highly oncogenic mutant ΔEGFR gene, a commonly
occurring variant of EGFR that lacks exons 2–7 (25,26).
These exons code for the N-terminal region that includes the EGF
binding site, and the deleted form of EGFR is therefore
constitutively autophosphorylated without EGF stimulation in
U87MG.ΔEGFR cells (15,16). In the present study, two types of
EGFR (endogenous wtEGFR and transfected ΔEGFR) were detected in
these cells as expected, and ΔEGFR autophosphorylation was detected
at similar levels regardless of the presence or absence of EGF. In
contrast to the results for wtEGFR, ΔEGFR autophosphorylation was
not significantly inhibited by 0.1 mM GM3, but was ~25% inhibited
by 0.2 mM GM3 at 100 (Fig. 2Eb,
columns b–d) and 1 ng/ml EGF (Fig.
2Fb, columns b–d). The chloro-derivatives showed notable
inhibitory effects under all three conditions: 100 ng/ml EGF
(Fig. 2E), 1 ng/ml EGF (Fig. 2F) and no EGF (Fig. 2G). Monochloro-GM3 produced ~73 and
~80% inhibition at concentrations of 0.1 and 0.2 mM, respectively
(Fig. 2Eb and Fb, columns b, g and
h; Fig. 2G, columns a, f and g).
The inhibitory effects of dichloro-GM3 were stronger than those of
GM3, but less than those of monochloro-GM3 (Fig. 2E–G). The inhibitory effects of GM3
and the chloro-derivatives on endogenous wtEGFR expressed in the
U87MG.ΔEGFR cells were extremely similar to those observed for the
A431 and U87MG.wtEGFR cells (Fig. 2Ec
and Fc).
Inhibitory effects of GM3 and its
chloro-derivatives on cell proliferation
The inhibitory effects of GM3 and its
chloro-derivatives on cell proliteration were analyzed by the
[3H]-thymidine incorporation assay. Treatment with EGF
at 100 ng/ml, the concentration used for analysis of EGFR
activation in the A431 cells, inhibited rather than promoted cell
proliferation (data not shown). As indicated in our previous study
(24), this result may be due to
the excessive expression of EGFR on the surface of the A431 cells.
As 1 ng/ml EGF induced cell proliferation (Fig. 3B, columns a and b), this
concentration was used for the analysis of the inhibitory effects
of GM3 and the chloro-derivatives. The chloro-derivatives,
particularly monochloro-GM3, significantly inhibited
[3H]-thymidine incorporation into the A431 cells
(Fig. 3A and B, columns b, e–h),
whereas GM3 showed an enhancing effect (Fig. 3A and B, columns b–d). The mechanism
of this enhancing effect remains unclear, but it may be associated
with the excessive expression of EGFR on the surface of the A431
cells, as aforementioned. Similar inhibitory effects of the
chloro-derivatives were observed in the U87MG.wtEGFR cells at the
EGF concentrations used (Fig. 3C and
D, columns a–h). The chloro-derivatives significantly inhibited
[3H]-thymidine incorporation into the U87MG.ΔEGFR cells
in the presence and absence of EGF, whereas GM3 exhibited no
significant inhibitory effect (Fig. 3E
and F, columns a–h; Fig. 3G,
columns a–g). These findings were consistent with those from the
analysis of ΔEGFR autophosphorylation, as described in the
preceding section.
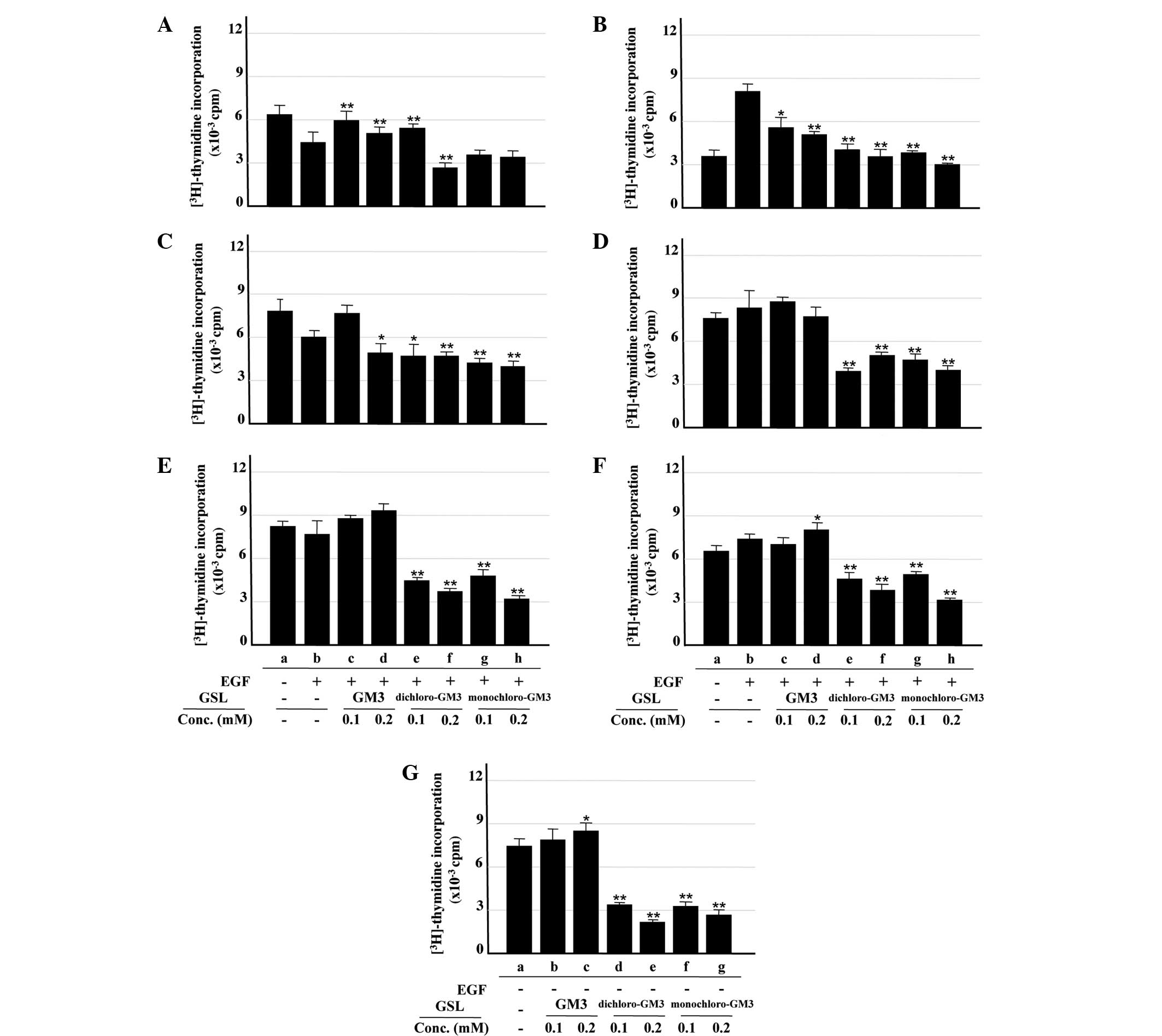 | Figure 3Inhibitory effects of GM3,
monochloro-GM3 and dichloro-GM3 on cell proliferation, assessed by
[3H]-thymidine incorporation assay. (A) A431 cells, 100
ng/ml EGF. (B) A431 cells, 1 ng/ml EGF. (C) U87MG.wtEGFR cells, 100
ng/ml EGF. (D) U87MG.wtEGFR cells, 1 ng/ml EGF. (E) U87MG.ΔEGFR
cells, 100 ng/ml EGF. (F) U87MG.ΔEGFR cells, 1 ng/ml EGF. (G)
U87MG.ΔEGFR cells, no EGF. [3H]-thymidine incorporation
was expressed as cpm. All experiments were performed in triplicate
and results are presented as the mean ± standard deviation. The
control is b in panels A–F and a in panel G. *P<0.05;
**P<0.01. GM3, sialosyllactosylceramide; EGF,
epidermal growth factor; GSL, glycosphingolipids; cpm, counts per
minute. |
Discussion
The expression of glycosyl epitopes, carried as
glycoproteins or GSLs on the surface of mammalian cells, is known
to vary quantitatively and qualitatively in association with
various cell phenotypes. GM3, a sialic acid-containing GSL whose
expression is reduced in transformed cells, inhibits EGFR
activation, a process associated with cancer cell growth and
motility (4).
Studies utilizing synthetic molecules that resemble
natural GSLs provide important information that is not available
from studies utilizing molecules from natural sources. In the
present study, to elucidate the inhibitory effects of the
chloro-derivatives of GM3 on EGFR activation, a simple and
efficient synthetic route was developed for the preparation of
monochloro- and dichloro-GM3. The key step is a highly
regioselective and stereoselective sialylation from a suitably
protected lactoside diol with a sialyl xanthate, to exclusively
provide the α-sialyl trisaccharide at a good yield. Selective
hydrolysis of the N-acetyl group of GM3 was achieved under
basic conditions (0.1 M KOH, 80°C) to yield key intermediate 3
(Fig. 1), from which the two
chloro-derivatives were prepared.
The finding that two halogen-(chloro-) derivatives
of GM3 have stronger inhibitory effects on EGFR activation than
GM3, indicates that the chemical synthesis approach described in
the present study can be applied for the development of more potent
inhibitors. The effects of trichloro-derivatives and
fluoro-derivatives of GM3 should also be evaluated. It is
noteworthy that monochloro-GM3 inhibited the activation of ΔEGFR,
which is commonly expressed in glioblastomas (the most aggressive
type of brain tumor in humans) and whose continuous EGF-independent
activation is associated with greatly enhanced malignant behavior.
The inhibitory effects of the two chloro-derivatives on EGFR
activation (autophosphorylation) and cell proliferation
([3H]-thymidine incorporation) were stronger than that
of GM3. Monochloro-GM3 also exhibited a significant inhibitory
effect on ΔEGFR, which is a mutant form of EGFR often found in
glioblastomas.
Our previous studies have shown that: (i) GM3
inhibits EGFR activation through carbohydrate-to-carbohydrate
interaction; (ii) GM3 interacts with N-linked glycans
carrying multiple GlcNAc termini carried by EGFR; and (iii) such
interaction is the molecular mechanism whereby GM3 inhibits EGFR
activation (18,19). Studies are in progress to determine
whether monochloro- and dichloro-GM3 bind more strongly than GM3 to
N-linked glycans carrying multiple GlcNAc termini.
The chemical synthesis of other GM3 derivatives
using approaches similar to that described in the present study has
the potential to create more potent EGFR inhibitors.
Acknowledgements
The present study was funded by the Université
Pierre et Marie Curie-Paris 6 for the program of LIA, The
Biomembrane Institute and P01-CA95616. Support was also accorded by
a PhD fellowship from the China Scholarship Council. The authors
are grateful to Dr S. Anderson for editing the English of the
manuscript.
Abbreviations:
|
DMEM
|
Dulbecco’s modified Eagle’s medium
|
|
GSL
|
glycosphingolipid
|
|
EGF
|
epidermal growth factor
|
|
EGFR
|
epidermal growth factor receptor
|
|
GM3
|
sialosyllactosylceramide
|
|
HRMS
|
high-resolution mass spectrometry
|
|
NMR
|
nuclear magnetic resonance
|
|
PBS
|
phosphate-buffered saline
|
|
r.t.
|
room temperature
|
|
TBS
|
Tris-buffered saline
|
References
|
1
|
Hakomori S: Tumor malignancy defined by
aberrant glycosylation and sphingo(glyco)lipid metabolism. Cancer
Res. 56:5309–5318. 1996.PubMed/NCBI
|
|
2
|
Bremer EG and Hakomori S: GM3 ganglioside
induces hamster fibroblast growth inhibition in chemically-defined
medium: ganglioside may regulate growth factor receptor function.
Biochem Biophys Res Commun. 106:711–718. 1982. View Article : Google Scholar
|
|
3
|
Bremer EG, Hakomori S, Bowen-Pope DF,
Raines E and Ross R: Ganglioside-mediated modulation of cell
growth, growth factor binding, and receptor phosphorylation. J Biol
Chem. 259:6818–6825. 1984.PubMed/NCBI
|
|
4
|
Bremer EG, Schlessinger J and Hakomori S:
Ganglioside-mediated modulation of cell growth. Specific effects of
GM3 on tyrosine phosphorylation of the epidermal growth factor
receptor. J Biol Chem. 261:2434–2440. 1986.
|
|
5
|
Miljan EA, Meuillet EJ, Mania-Farnell B,
et al: Interaction of the extracellular domain of the epidermal
growth factor receptor with gangliosides. J Biol Chem.
277:10108–10113. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Noll EN, Lin J, Nakatsuji Y, Miller RH and
Black PM: GM3 as a novel growth regulator for human gliomas. Exp
Neurol. 168:300–309. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nakatsuji Y and Miller RH: Selective
cell-cycle arrest and induction of apoptosis in proliferating
neural cells by ganglioside GM3. Exp Neurol. 168:290–299. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hakomori S, Young WW Jr, Patt LM, Yoshino
T, Halfpap L and Lingwood CA: Cell biological and immunological
significance of ganglioside changes associated with transformation.
Adv Exp Med Biol. 125:247–261. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nishikawa R, Ji XD, Harmon RC, et al: A
mutant epidermal growth factor receptor common in human glioma
confers enhanced tumorigenicity. Proc Natl Acad Sci USA.
91:7727–7731. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Furnari FB, Fenton T, Bachoo RM, et al:
Malignant astrocytic glioma: genetics, biology, and paths to
treatment. Genes Dev. 21:2683–2710. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhu ZY and Zhang YM: An efficient method
for ganglioside GM3 preparation. Acta Chim Sinica. 65:2909–2916.
2007.(In Chinese).
|
|
12
|
Tanaka H, Adachi M and Takahashi T:
One-pot synthesis of sialo-containing glycosyl amino acids by use
of an N-trichloroethoxycarbonyl-beta-thiophenyl sialoside.
Chemistry. 11:849–862. 2005. View Article : Google Scholar
|
|
13
|
Lin CC, Adak AK, Horng JC and Lin CC:
Phosphite-based sialic acid donors in the synthesis of α(2→9)
oligosialic acids. Tetrahedron. 65:4714–4725. 2009.
|
|
14
|
Byramova NE, Tuzikov AB and Bovin NV:
Studies on the synthesis of sialosides and sialic acid analogs. 2.
A simple procedure for the synthesis of the methyl and benzyl
glycosides of Neu5Ac and 4-epi-Neu5Ac - Conversion of the benzyl
and methyl glycosides of Neu5Ac into N-trifluoroacetylneuraminic
acid benzyl glycosides. Carbohydr Res. 237:161–175. 1992.
|
|
15
|
Fernandes H, Cohen S and Bishayee S:
Glycosylation-induced conformational modification positively
regulates receptor-receptor association: a study with an aberrant
epidermal growth factor receptor (EGFRvIII/DeltaEGRF) expressed in
cancer cells. J Biol Chem. 276:5375–5383. 2001. View Article : Google Scholar
|
|
16
|
Huang HS, Nagane M, Klingbeil CK, et al:
The enhanced tumorigenic activity of a mutant epidermal growth
factor receptor common in human cancers is mediated by threshold
levels of constitutive tyrosine phosphorylation and unattenuated
signaling. J Biol Chem. 272:2927–2935. 1997. View Article : Google Scholar
|
|
17
|
Zhou Q, Hakomori S, Kitamura K and
Igarashi Y: GM3 directly inhibits tyrosine phosphorylation and
de-N-acetyl-GM3 directly enhances serine phosphorylation of
epidermal growth factor receptor, independently of
receptor-receptor interaction. J Biol Chem. 269:1959–1965.
1994.
|
|
18
|
Yoon SJ, Nakayama K, Hikita T, Handa K and
Hakomori SI: Epidermal growth factor receptor tyrosine kinase is
modulated by GM3 interaction with N-linked GlcNAc termini of the
receptor. Proc Natl Acad Sci USA. 103:18987–18991. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kawashima N, Yoon SJ, Itoh K and Nakayama
K: Tyrosine kinase activity of epidermal growth factor receptor is
regulated by GM3 binding through carbohydrate to carbohydrate
interactions. J Biol Chem. 284:6147–6155. 2009. View Article : Google Scholar
|
|
20
|
Todeschini AR, Dos Santos JN, Handa K and
Hakomori S: Ganglioside GM2-tetraspanin CD82 complex inhibits met
and its cross-talk with integrins, providing a basis for control of
cell motility through glycosynapse. J Biol Chem. 282:8123–8133.
2007. View Article : Google Scholar
|
|
21
|
Mitsuzuka K, Handa K, Satoh M, Arai Y and
Hakomori S: A specific microdomain (“glycosynapse 3”) controls
phenotypic conversion and reversion of bladder cancer cells through
GM3-mediated interaction of alpha3beta1 integrin with CD9. J Biol
Chem. 280:35545–34553. 2005.
|
|
22
|
Gabelman BM and Emerman JT: Effects of
estrogen, epidermal growth factor, and transforming growth
factor-alpha on the growth of human breast epithelial cells in
primary culture. Exp Cell Res. 201:113–118. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zheng M and Ye XS: Synthesis of N-modified
ganglioside GM3 derivatives. Tetrahedron. 68:1475–1482. 2012.
View Article : Google Scholar
|
|
24
|
Hanai N, Nores GA, MacLeod C,
Torres-Mendez CR and Hakomori S: Ganglioside-mediated modulation of
cell growth. Specific effects of GM3 and lyso-GM3 in tyrosine
phosphorylation of the epidermal growth factor receptor. J Biol
Chem. 263:10915–10921. 1988.PubMed/NCBI
|
|
25
|
Ekstrand AJ, Sugawa N, James CD and
Collins VP: Amplified and rearranged epidermal growth factor
receptor genes in human glioblastomas reveal deletions of sequences
encoding portions of the N- and/or C-terminal tails. Proc Natl Acad
Sci USA. 89:4309–4313. 1992. View Article : Google Scholar
|
|
26
|
Sugawa N, Ekstrand AJ, James CD and
Collins VP: Identical splicing of aberrant epidermal growth factor
receptor transcripts from amplified rearranged genes in human
glioblastomas. Proc Natl Acad Sci USA. 87:8602–8606. 1990.
View Article : Google Scholar
|

















