Introduction
Mucoepidermoid carcinoma (MEC) is common in human
salivary glands. Poorly differentiated MEC is a lethal malignancy
that readily invades nearby tissues and is likely to recur
(1). Conventional surgery is the
most common treatment method for MEC, however, often results in
devastating functional and cosmetic consequences. In order to kill
residual tumor cells and prevent the recurrence of MEC,
chemotherapy is required following surgery. The chemotherapeutic
agent, 5-fluorouracil (5-Fu), is commonly used; however,
chemotherapy is unable to kill all of the remaining tumor cells or
prevent the recurrence of MEC. The underlying mechanisms of MEC
recurrence following chemotherapy have not yet been
investigated.
Cancer stem-like (CSL)-cells are a rare population
of cancer cells exhibiting stem cell properties, constituting a
reservoir of self-sustaining cells with an exclusive ability to
self-renew and maintain the tumor. CSL-cells were identified first
in acute myeloid leukemia (2)
followed by solid tumors and subsequently breast cancer in 2003
(3). CSL-cells have been isolated
from a variety of human malignancies, including leukemia (2,4),
breast cancer (3,5), brain tumors (6–8),
hepatocellular carcinoma (9),
pancreatic (10) and colorectal
cancers (11,12), melanomas (13), prostate cancer (14) and bone sarcomas (15). CSL-cells are significant in tumor
formation and growth (16–18). Potentially quiescent CSL-cells,
which are vital and capable of repopulating under cancer therapies,
may be a source of recurrence and drug resistance (3,19).
The present study aimed to investigate the effects
of chemotherapy on the MC3 MEC cell line and the potential roles of
CSL-cells in recurrent MEC following chemotherapy.
Materials and methods
Cell line and culture
The MC3 MEC cell line was provided and conserved at
the State Key Laboratory of Oral Diseases, Sichuan University
(Chengdu, China). The MC3 cells were maintained in a
serum-containing medium composed of RPMI-1640 (Hyclone, Logan, UT,
USA) and 10% fetal bovine serum (FBS; Gibco-BRL, Grand Island, NY,
USA). The cells were incubated at 37°C in a 5% CO2
humidified atmosphere and passaged once every three days.
MC3 cell culture in 5-Fu-containing
medium
The MC3 cells were incubated in a serum-containing
medium composed of RPMI-1640, 10% FBS and 1 peak plasma
concentration of 100 μg/ml 5-Fu (20) at 37°C in a 5% CO2
humidified atmosphere for 24 h.
Soft agarose assays of clone
formation
The 5-Fu-treated and parent MC3 cells were seeded in
24-well plates. Low melting-point agarose (0.3 ml, 0.6%; Type VII,
Sigma-Aldrich, St. Louis, MO, USA) was poured into each well and
0.3 ml (0.35%) agarose containing 100 cells was subsequently added
to each well. The cells were incubated following the solidification
of agarose at room temperature. The number of clones containing
>50 cells was counted under a microscope after ten days and the
cloning efficiency was calculated using the following formula:
Colony formation rate (%) = no. of clones/no. of cells incubated ×
100.
MTT assay
The 5-Fu-treated and parent MC3 cells were seeded in
96-well plates, each well contained 2,000 cells and was cultured in
complete RPMI-1640 medium with 10% FBS. The cell viability was
measured using the MTT assay (Sigma-Aldrich). The optical density
(OD) values were obtained using a microplate reader (ThermoElectron
3001 Varioskan Flash; USA) on days one, three, five, seven and
nine.
Quantitative polymerase chain reaction
(qPCR)
qPCR was performed using the SYBR® Green
reporter to detect the expression of genes, cluster of
differentiation (CD)44 and octamer-binding transcription factor 4
(Oct4). The primer sequences are summarized in Table I. The cells were harvested and RNA
was extracted from the 5-Fu-treated and parent MC3 cells using
TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA, USA),
then reverse-transcribed into cDNA using PrimeScript RT reagent kit
(Takara, Dalian, China) according to the manufacturer’s
instructions. qPCR was performed according to the standard protocol
of the SYBR Premix Ex Taq™ II kit (Takara) on an ABI 7300 Real Time
PCR system (Applied Biosystems, Foster City, CA, USA). To quantify
the changes in gene expression, the ΔΔCt method was used to
calculate the relative fold changes following normalization using
the internal reference gene, GAPDH.
 | Table IPrimer sequences for quantitative
polymerase chain reaction. |
Table I
Primer sequences for quantitative
polymerase chain reaction.
| Gene | Upstream
primer | Downstream
primer |
|---|
| CD44 |
5′-gagcagcacttcaggaggttaca-3′ |
5′-agtggtagcagggattctgtctg-3′ |
| Oct4 |
5′-gcacaacgagaggattttgagg-3′ |
5′-agggaaagggaccgaggagta-3′ |
| GAPDH |
5′-ctttggtatcgtggaaggactc-3′ |
5′-gtagaggcagggatgatgttct-3′ |
Immunocytochemistry
The 5-Fu-treated and parent MC3 cells were plated on
glass coverslips at 37°C overnight, washed twice with PBS, and
immunostained for CD44, Oct4 and the isotype control. The primary
antibodies included rat monoclonal anti-CD44 (dilution 1:100;
eBiosciences, San Diego, CA, USA) and rabbit monoclonal anti-Oct4
(dilution 1:50; Bioworld Technology, Minneapolis, MN, USA). The
secondary antibodies included goat anti-rat IgG and goat
anti-rabbit IgG (dilutions 1:50; Bios, Beijing, China). The
intensity of 3,3′-diaminobenzidine was analyzed using the
immunohistochemical Avidin Biotin Complex (ABC) method (15). Images were captured using a Nikon
eclipse 80i microscope (Nikon Corp., Tokyo, Japan).
Fluorescence-activated cell sorting
(FACS) of CD44 and Oct4
The 5-Fu-treated and parent MC3 cells were
trypsinized into solitary cell suspensions. The cells were counted,
washed twice with PBS, resuspended in ice-cold PBS (supplemented
with 2% FBS) and labeled with antibodies specific for human cells,
such as rat monoclonal anti-CD44 antibody. The cells were incubated
with their antibodies for 30 min at 4°C in the dark. The unbound
antibodies were removed by washing twice with PBS. The fluorescein
isothiocyanate (FITC)-labeled secondary antibody was added to the
cell suspension and incubated for 30 min at 4°C in the dark. The
cells were washed twice with PBS and FACS analysis (BD Biosciences,
San Jose, CA, USA) was performed.
The 5-Fu-treated and parent MC3 cells were fixed and
perforated, resuspended in ice-cold PBS and labeled with antibodies
specific for human cells, such as rabbit monoclonal anti-Oct4
antibody. The cells were incubated with their antibodies for 30 min
at 4°C in the dark. The unbound antibodies were removed by washing
twice with PBS. The FITC-labeled secondary antibody was added to
the cell suspension and incubated for 30 min at 4°C in the dark.
The cells were washed twice with PBS and FACS analysis was
performed.
Culture of the cells in serum-free
medium
The 5-Fu-treated and parent MC3 cells were washed
three times with PBS to remove all traces of FBS. The cells were
placed in serum-free Dulbecco’s modified Eagle’s medium (DMEM)/F12
(Hyclone), which was composed of 20 ng/ml basic fibroblast growth
factor (PeproTech, Rocky Hill, NJ, USA), 20 ng/ml epidermal growth
factor (PeproTech), 1 mg/ml insulin (Sigma-Aldrich) and 2% B27
(Invitrogen Life Technologies) at a density of 1×102/ml.
The cell suspensions (200 μl) were plated onto ultra-low attachment
96-well plates. The number of clones containing >50 cells was
counted under a microscope on day seven and the cloning efficiency
was calculated using the following formula: Colony formation rate
(%) = no. of clones/no. of cells incubated ×100.
FACS analysis of side population (SP)
cells
SP cell analysis was based on a previously described
method (21) with certain
modifications. Briefly, cells were trypsinized and resuspended in
PBS with 2% FBS at a density of 1×610/ml. Verapamil
(Sigma-Aldrich) at a final concentration of 50 μg/ml was added to
the control group. After 10 min, 10 μg/ml Hoechst 33342
(Sigma-Aldrich) was added to the cell suspension, this was
incubated in the dark for 90 min, centrifuged and resuspended in
ice-cold PBS containing 2% FBS. Propidium iodine (2 μg/ml;
Sigma-Aldrich) was added to separate the dead cells. Analysis and
sorting were performed on a BD FACSAria.
Statistical analysis
Statistical analyses were performed with SPSS
software, version 11.5 (SPSS, Inc., Chicago, IL, USA). All
quantified data present the means of at least three samples and
error bars represent the standard deviation. Student’s t-test was
used to determine the statistical differences between the
experimental and control groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
5-Fu-treated cells
The MC3 cells were exposed to 5-Fu for 24 h
resulting in a large number of cell deaths. The dead cells were
suspended in the medium and the surviving cells adhered to the
plate wall. The viable cells were collected for subsequent
experiments.
5-Fu-treated cells exhibit a higher
cloning efficiency
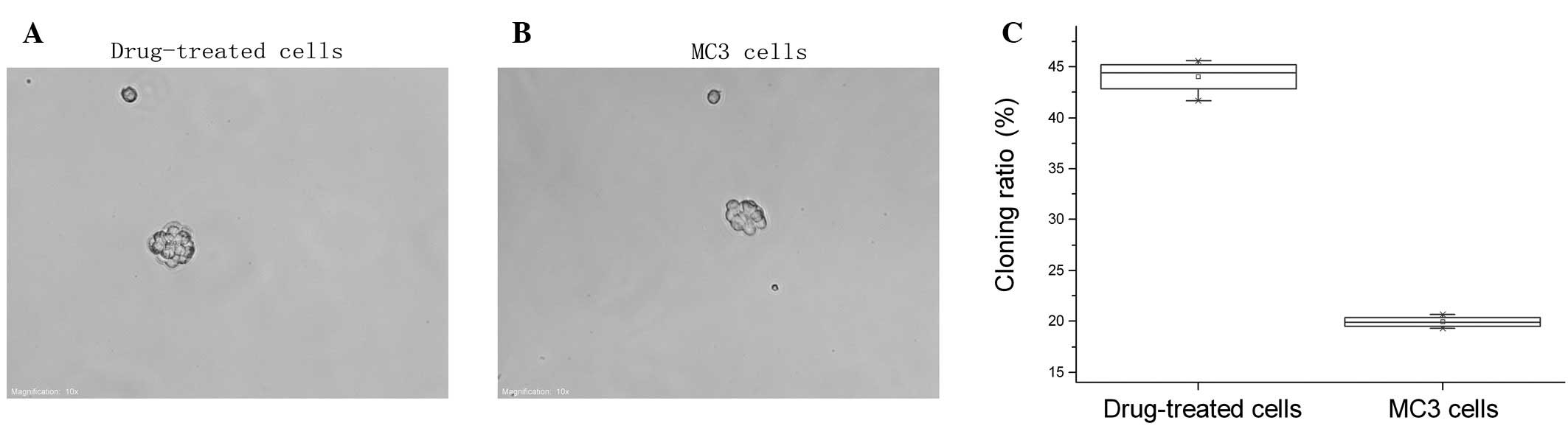
The 5-Fu-treated and parent MC3 cells underwent the
agarose colony formation experiments and showed that the cloning
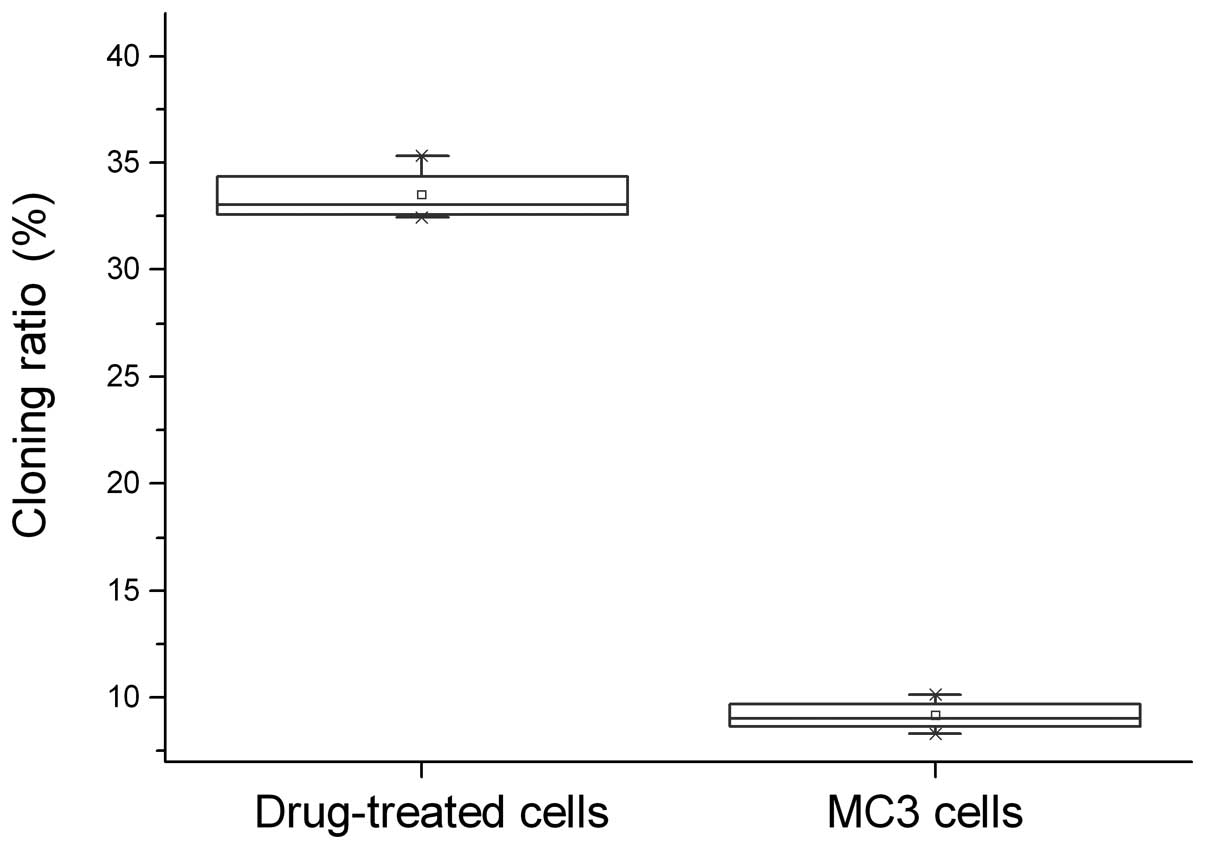
ratio of 5-Fu-treated cells (33.47±1.30%) was significantly higher
compared with the parent MC3 cells (9.14±0.747%, P<0.05;
Fig. 1).
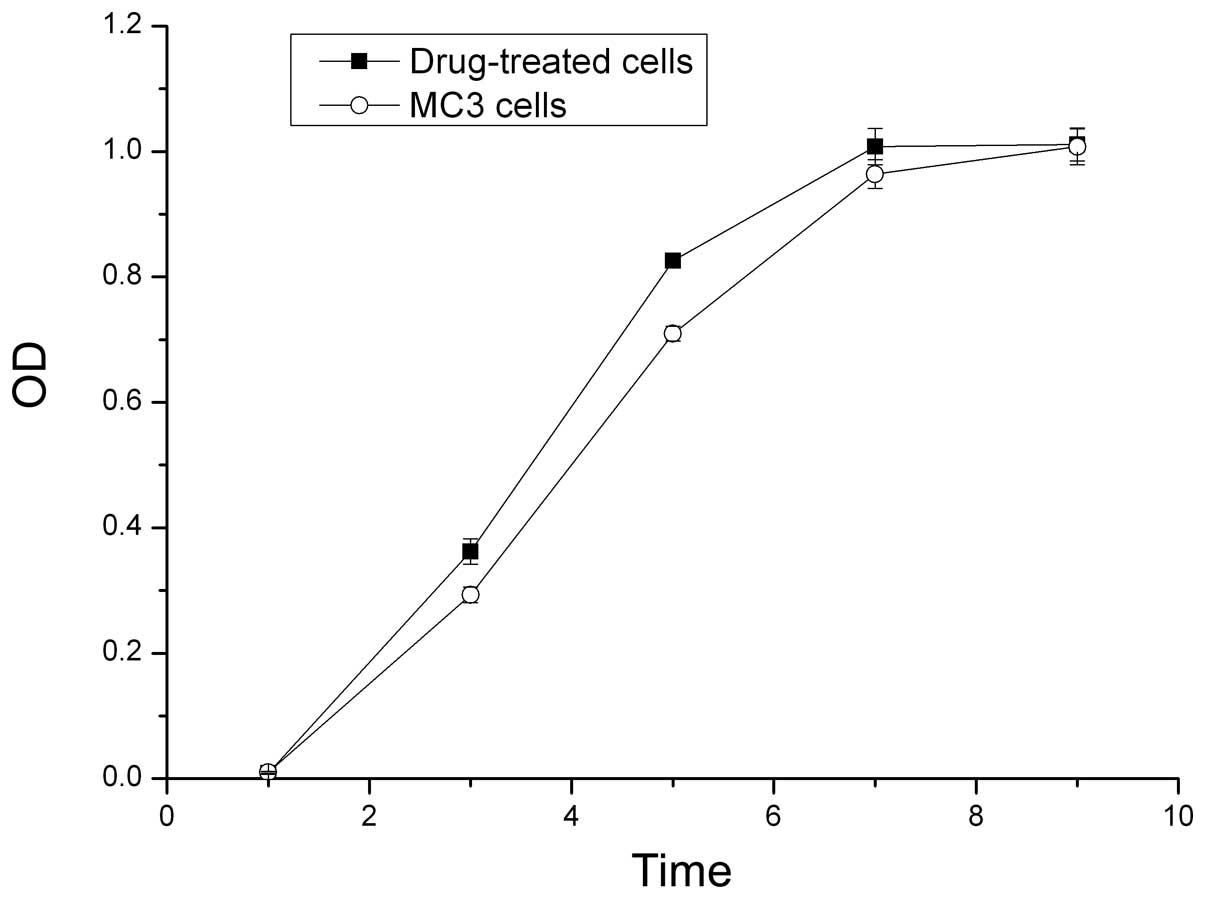
Growth curves of the cells
The OD values from the MTT assay were used to
construct growth curves. The proliferative ability of the
5-Fu-treated cells was higher compared with the parent MC3 cells in
the first seven days. The 5-Fu-treated cells reached the plateau
phase on day seven, whereas the parent MC3 cells reached the
plateau phase on day nine (P<0.05; Fig. 2).
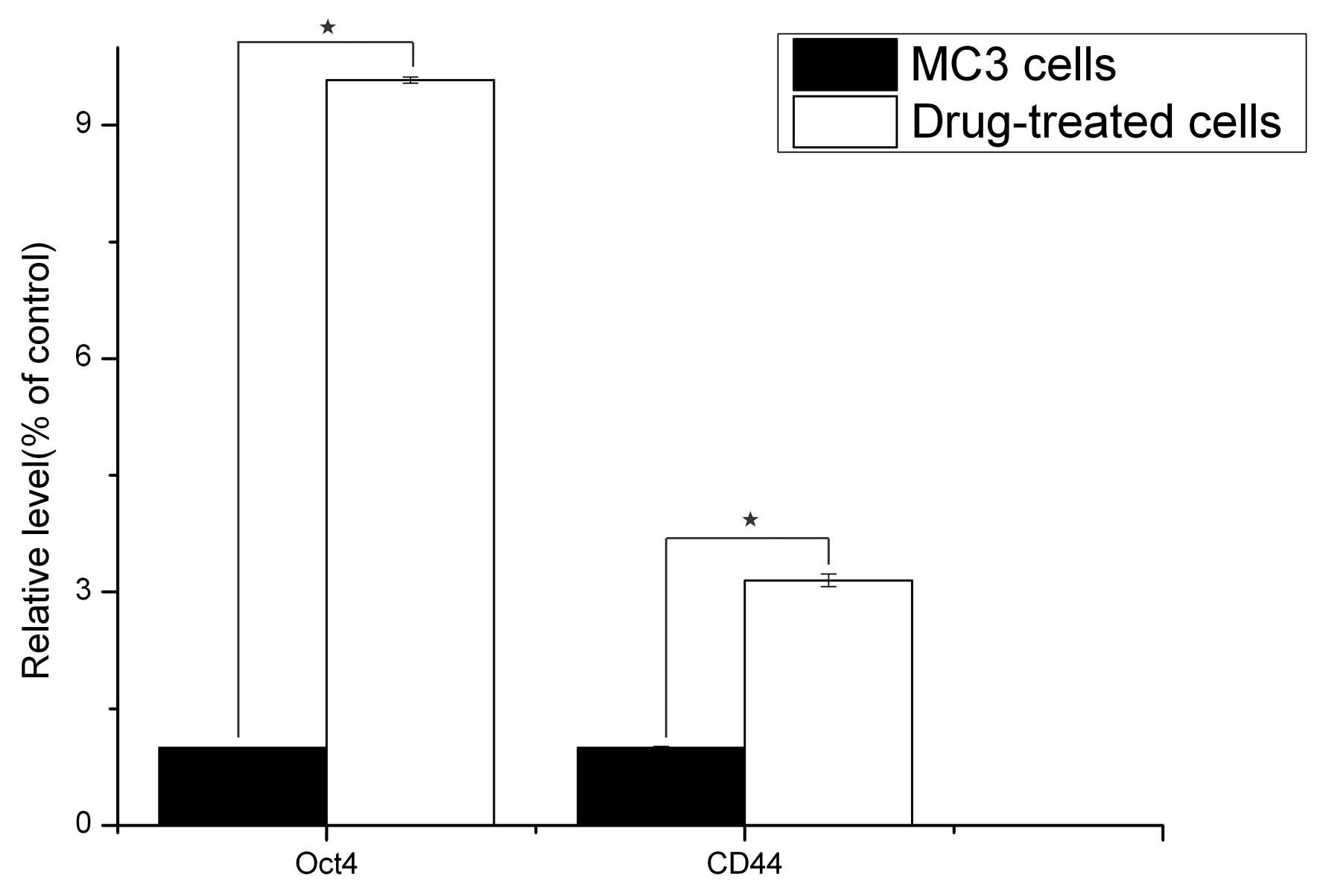
qPCR analysis
The gene expression status of CD44 and Oct4 were
compared between the 5-Fu-treated and the parent MC3 cells via
qPCR. The results revealed that the reference gene, GAPDH, was
stably expressed in all the samples, and CD44 and Oct4 were
significantly expressed in the 5-Fu-treated cells compared with the
parent MC3 cells (P<0.05; Fig.
3).
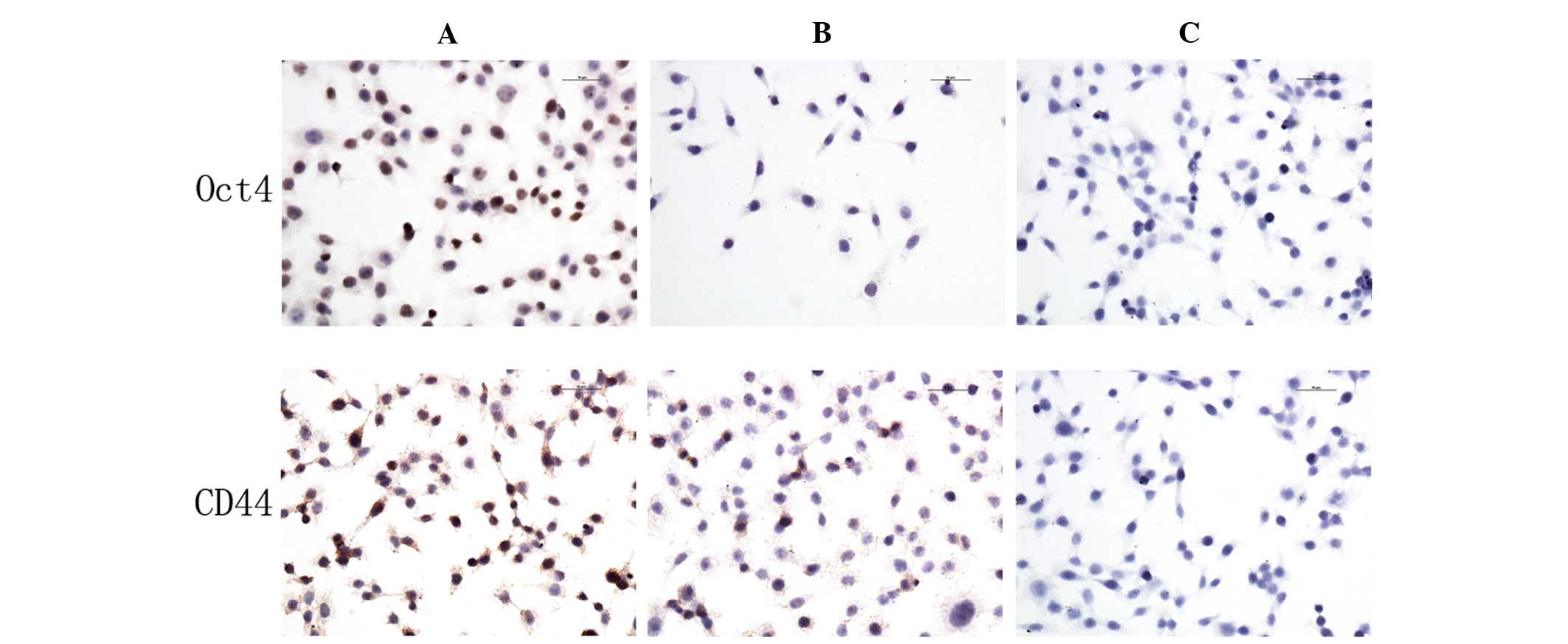
CD44 and Oct4 protein expression
Immunocytochemistry assays were used to analyze the
expression of CD44 and Oct4 in 5-Fu-treated and parent MC3 cells.
The expression levels of CD44 and Oct4 in 5-Fu-treated cells were
higher compared with the parent MC3 cells. CD44 was expressed in
the cell membrane and cytoplasm, whereas Oct4 was expressed in the
nucleus (Fig. 4).
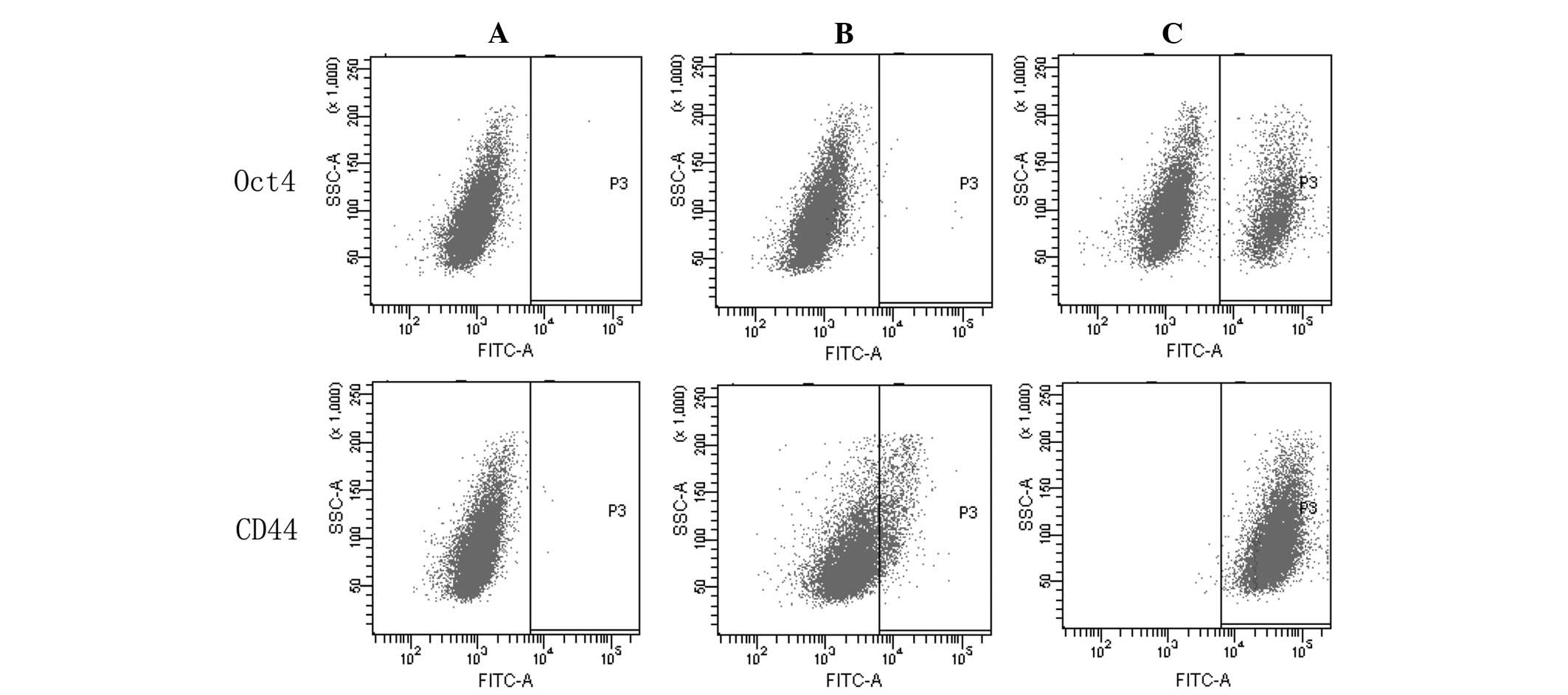
Furthermore, the expression of CD44 and Oct4 was
analyzed by FACS. According to three independent experiments, the
expression levels of CD44 and Oct4 were 99.50±0.30 and 14.60±0.36%,
respectively in the 5-Fu-treated cells, and 14.47±0.15 and
1.37±0.06%, respectively, in the MC3 cells (Fig. 5). The expression levels of CD44 and
Oct4 were significantly different between the two cell populations
(P<0.05).
Spheroid cells in the serum-free
medium
The 5-Fu-treated and parent MC3 cells that were
incubated in serum-free medium for one day revealed multicellular
spheroids. Spheroids were apparent following cell culture in
serum-free medium for four days (Fig.
6). The number of cells in the spheroids gradually increased in
a time-dependent manner and on day seven spherical bodies
comprising of dozens of cells were observed. The number of
spherical bodies increased by >20% following treatment with
5-Fu. When the spheroid cells were cultured in RPMI-1640 with 10%
FBS they became adherent. These findings identified that under stem
cell culture conditions, MC3 and 5-Fu-treated cells formed
spheroids, and chemotherapy may improve the ratio of the formation
of spheroids.
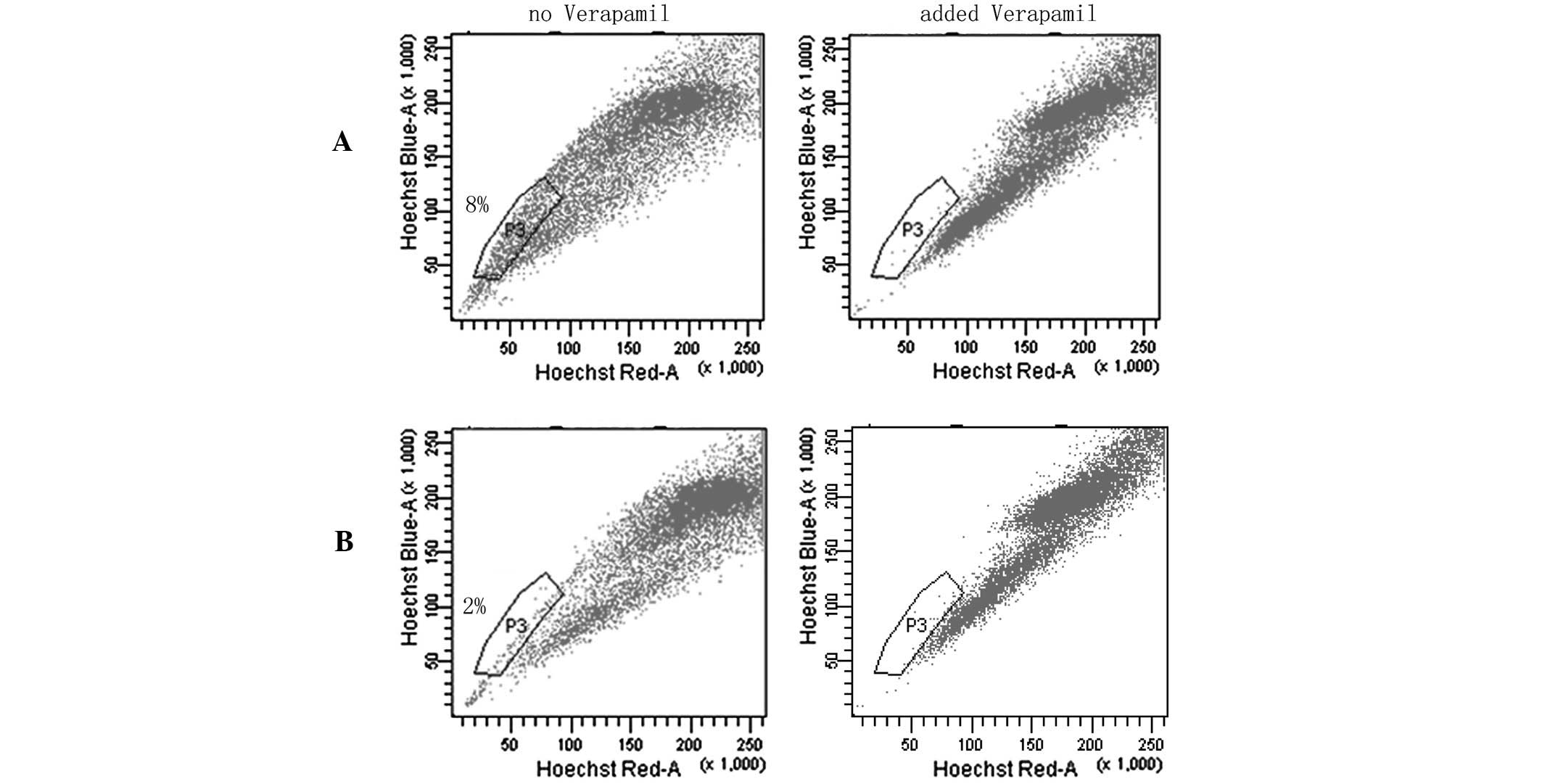
SP cell assays
SP flow cytometry has previously been used to enrich
cancer stem cells (CSCs) from various cancer cell lines and primary
tumors (22–24). SP cells do not fluoresce under the
dual wavelength parameters of FACS as they are able to efflux
Hoechst 33342 by adenosine triphospate-binding cassette
transporters (21,25–29).
In the SP assays, SP cells were located in the area of weak
fluorescence and the ratio of SP to 5-Fu-treated cells was higher
compared with MC3 cells. These data strongly indicated that
chemotherapy may significantly increase the number of CSL-cells in
MC3 cells (Fig. 7).
Discussion
Previous studies have identified CSL-cells within
tumors and that the injection of CSL-cells into nude mice induces
the development of tumors. CSL-cells are considered to be
comparable to normal tissue stem cells as they possess the ability
to divide asymmetrically and symmetrically, and undergo
multilineage differentiation (30,31).
Similar to the activity of normal stem cells in the maintenance of
tissue architecture, CSL-cells are regarded as a resource of tumor
formation, progression, recurrence and drug resistance (11,32).
CSL-cells are able to self-renew and differentiate into a diverse
range of cells that form tumor masses (33,34).
CSL-cells have a stronger resistance to traditional treatments,
such as chemotherapy and radiation, compared with other types of
tumor cells due to their high expression of drug resistant
transporter proteins (such as ABC) (35–37),
DNA repair enzymes (38,39) and anti-apoptotic proteins (40–42).
The present study indicated that the CSC phenotype
may be induced by 5-Fu as cancer cells are able to acquire a
stemness state, which is characterized by the increased stemness
gene expression of Oct4. Oct4 is a typical stem-cell associated
gene (43) and may be able to
reprogram adult cells into induced pluripotent stem cells (iPS)
(44,45). Despite the transcription factors of
c-Myc, kruppel-like factor 4 and NANOG, Oct4 is an important gene
as its expression is significant in the production of iPS (44,46,47).
Previous studies identified a high expression of Oct4 in human
embryonic stem cells compared with differentiated tissues and a
high expression in CSL-cells compared with other types of cancer
cells (18,48,49).
In certain cell lines, the increased expression of Oct4 results in
enhanced stemness and acquisition of a stem cell-like phenotype
(50,51), which is associated with an increase
in sphere formation and resistance to chemotherapy and
radiotherapy. Knockdown of Oct4 may increase the sensitivity to
chemotherapy and radiotherapy due to the restriction of the factors
that lead to self-renewal. Therefore, the expression of Oct4 is
important in the identification of CSL-cells.
As a type of transmembrane glycoprotein, CD44 is
widely distributed on the cell surface of lymphocytes and
fibroblasts (52). CD44 is
predominantly involved in specific adhesion processes, such as
cell-cell and cell-matrix. Thus, CD44 may be used as a surface
marker of CSL-cells. In addition to breast cancer (3), CD44 was considered to be a CSL-cell
marker in ovarian (53), prostate
(54) and pancreatic cancer
(10), and head and neck squamous
cell carcinoma (55).
The present study demonstrated that the expression
of Oct4 and CD44 increased following treatment with 5-Fu,
particularly Oct4 expression in 5-Fu-treated cells, which was
markedly higher compared with the parent MC3 cells. These findings
were consistent with the increased stem cell-like phenotype, as the
cloning ratio of the cells in the soft agarose increased from
9.14±0.747 to 33.47±1.30%. To examine this further, the
5-Fu-treated cells were cultured under stem cell culture
conditions, which were selective for CSL-cell enrichment. The
results indicated that chemotherapy was associated with a
significant increase in sphere-formation ability, reflecting a
greater self-renewal and proliferation ability of the 5-Fu-treated
cells; furthermore, no difference in morphology was observed
between the two types of spheroids. In addition, the 5-Fu-treated
cells grew faster, reaching the plateau phase more rapidly than the
parent MC3 cells in the MTT assays. These findings were consistent
with previous studies, demonstrating that the drug resistance of
tumor cells is associated with CSL-cells in tumors (17,56,57).
Over the past century, chemotherapy has been used
extensively as a curative or adjuvant cancer treatment,
particularly for metastatic tumors. However, the majority of human
malignancies, including MEC, are resistant to this important
therapeutic method. Resistance to chemotherapy is the primary
obstacle for patient survival, particularly for those with
metastatic tumors (58). In the
present study, chemotherapy induced stem cell-like properties, such
as sphere formation, clone formation and stemness-related gene
expression, demonstrating that chemotherapy may enrich CSL-cells in
the MC3 cell line. To further explore the number of CSL-cells in
the 5-Fu-treated MC3 cells, flow cytometry using Hoechst 33342 dye
exclusion was performed to isolate the SP cells that were enriched
in CSCs. Notably, the drug-treated cells exhibited a higher
percentage of SP cells compared with the parent MC3 cells; the CSC
component in the MC3 cell line increased from 2 to 8% of the total
cell population, indicating that they were more enriched for the
CSC phenotype.
In conclusion, CSL-cells are considered to be a
cause of tumors due to their similar characteristics to stem cells
(self-renewal and multilineage differentiation). The present study
indicated that 5-Fu may induce MC3 cells into a stem-like phenotype
and that the remaining CSL-cells of MEC following chemotherapy were
significant in tumor recurrence, as well as in promoting tumor
survival. These findings demonstrated the mechanisms involved in
the resistance of cancer cells to chemotherapy and implied that
targeting CSL-cells may improve the efficacy of chemotherapy.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 30973345 and
81172578).
References
|
1
|
Spiro RH, Huvos AG, Berk R and Strong EW:
Mucoepidermoid carcinoma of salivary gland origin. A
clinicopathologic study of 367 cases. Am J Surg. 136:461–468. 1978.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bonnet D and Dick JE: Human acute myeloid
leukemia is organized as a hierarchy that originates from a
primitive hematopoietic cell. Nat Med. 3:730–737. 1997. View Article : Google Scholar
|
|
3
|
Al- Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc NatI Acad Sci USA.
100:3983–3988. 2003.
|
|
4
|
Lapidot T, Sirard C, Vormoor J, et al: A
cell initiating human acute myeloid leukaemia after transplantation
into SCID mice. Nature. 367:645–648. 1994. View Article : Google Scholar
|
|
5
|
Liu S, Dontu G and Wicha MS: Mammary stem
cells, self-renewal pathways, and carcinogenesis. Breast Cancer
Res. 7:86–95. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Singh SK, Hawkins C, Clarke ID, et al:
Identification of human brain tumour initiating cells. Nature.
432:396–401. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Galli R, Binda E, Orfanelli U, et al:
Isolation and characterization of tumorigenic, stem-like neural
precursors from human glioblastoma. Cancer Res. 64:7011–7021. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hemmati HD, Nakano I, Lazareff JA,
Masterman-Smith M, Geschwind DH, Bronner-Fraser M and Kornblum HI:
Cancerous stem cells can arise from pediatric brain tumors. Proc
Natl Acad Sci USA. 100:15178–15183. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chiba T, Kita K, Zheng YW, et al: Side
population purified from hepatocellular carcinoma cells harbors
cancer stem cell-like properties. Hepatology. 44:240–251. 2006.
View Article : Google Scholar
|
|
10
|
Li C, Heidt DG, Dalerba P, et al:
Identification of pancreatic cancer stem cells. Cancer Res.
67:1030–1037. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
O’Brien CA, Pollett A, Gallinger S and
Dick JE: A human colon cancer cell capable of initiating tumour
growth in immunodeficient mice. Nature. 445:106–110.
2007.PubMed/NCBI
|
|
12
|
Ricci-Vitiani L, Lombardi DG, Pilozzi E,
Biffoni M, Todaro M, Peschle C and De Maria R: Identification and
expansion of human colon-cancer-initiating cells. Nature.
445:111–115. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fang D, Nguyen TK, Leishear K, et al: A
tumorigenic subpopulation with stem cell properties in melanomas.
Cancer Res. 65:9328–9337. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Collins AT, Berry PA, Hyde C, Stower MJ
and Maitland NJ: Prospective identification of tumorigenic prostate
cancer stem cells. Cancer Res. 65:10946–10951. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gibbs CP, Kukekov VG, Reith JD, et al:
Stem-like cells in bone sarcomas: implications for tumorigenesis.
Neoplasia. 7:967–976. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Beachy PA, Karhadkar SS and Berman DM:
Tissue repair and stem cell renewal in carcinogenesis. Nature.
432:324–331. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dean M, Fojo T and Bates S: Tumour stem
cells and drug resistance. Nat Rev Cancer. 5:275–284. 2005.
View Article : Google Scholar
|
|
18
|
Kamstrup MR, Gniadecki R and Skovgaard GL:
Putative cancer stem cells in cutaneous malignancies. Exp Dermatol.
16:297–301. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Setoguchi T, Taga T and Kondo T: Cancer
stem cells persist in many cancer cell lines. Cell Cycle.
3:414–415. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sunyou Z, Yi Z, Xu D and Xinghao Z: The
relationship between oral cancer HSP-70 expression and drug
sensitivity test in vitro. Shiyong Yixue Zazhi. 22:1839–1841.
2006.(In Chinese).
|
|
21
|
Goodell MA, Brose K, Paradis G, Conner AS
and Mulligan RC: Isolation and functional properties of murine
hematopoietic stem cells that are replicating in vivo. J Exp Med.
183:1797–1806. 1996. View Article : Google Scholar
|
|
22
|
Kondo T, Setoguchi T and Taga T:
Persistence of a small subpopulation of cancer stem-like cells in
the C6 glioma cell line. Proc Natl Acad Sci USA. 101:781–786. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hirschmann-Jax C, Foster AE, Wulf GG, et
al: A distinct ‘side population’ of cells with high drug efflux
capacity in human tumor cells. Proc Natl Acad Sci USA.
101:14228–14233. 2004.
|
|
24
|
Chen JS, Pardo FS, Wang-Rodriguez J, et
al: EGFR regulates the side population in head and neck squamous
cell carcinoma. Laryngoscope. 116:401–406. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhou S, Schuetz JD, Bunting KD, et al: The
ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem
cells and is a molecular determinant of the side-population
phenotype. Nat Med. 7:1028–1034. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Robinson SN, Seina SM, Gohr JC, Kuszynski
CA and Sharp JG: Evidence for a qualitative hierarchy within the
Hoechst-33342 ‘side population’ (SP) of murine bone marrow cells.
Bone Marrow Transplant. 35:807–818. 2005.PubMed/NCBI
|
|
27
|
Hadnagy A, Gaboury L, Beaulieu R and
Balickin D: SP analysis may be used to identify cancer stem cell
populations. Exp Cell Res. 312:3701–3710. 2006. View Article : Google Scholar
|
|
28
|
Wu C and Alman BA: Side population cells
in human cancers. Cancer Lett. 268:1–9. 2008. View Article : Google Scholar
|
|
29
|
Hirschmann-Jax C, Foster AE, Wulf GG,
Goodwell MA and Brenner MK: A distinct ‘side population’ of cells
in human tumor cells: Implications for tumor biology and therapy.
Cell Cycle. 4:203–205. 2005.
|
|
30
|
Jordan CT, Guzman ML and Noble M: Cancer
stem cells. N Engl J Med. 355:1253–1261. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Visvader JE and Lindeman GJ: Cancer stem
cells in solid tumours: accumulating evidence and unresolved
questions. Nat Rev Cancer. 8:755–768. 2008. View Article : Google Scholar
|
|
32
|
Loebinger MR, Giangreco A, Groot KR, et
al: Squamous cell cancers contain a side population of stem-like
cells that are made chemosensitive by ABC transporter blockade. Br
J Cancer. 98:380–387. 2008. View Article : Google Scholar
|
|
33
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer, and cancer stem cells. Nature.
414:105–111. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Woodruff MF: Cellular heterogeneity in
tumours. Br J Cancer. 47:589–594. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Haraguchi N, Ishii H, Mimori K, et al:
CD13 is a therapeutic target in human liver cancer stem cells. J
Clin Invest. 120:3326–3339. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gottesman MM, Fojo T and Bates SE:
Multidrug resistance in cancer: role of ATP-dependent transporters.
Nat Rev Cancer. 2:48–58. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Doyle LA, Yang W, Abruzzo LV, Krogmann T,
Gao Y, et al: A multidrug resistance transporter from human MCF-7
breast cancer cells. Proc Natl Acad Sci USA. 95:15665–15670. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Martin LP, Hamilton TC and Schilder RJ:
Platinum resistance: the role of DNA repair pathways. Clin Cancer
Res. 14:1291–1295. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang M, Atkinson RL and Rosen JM:
Selective targeting of radiation resistant tumor-initiating cells.
Proc Natl Acad Sci USA. 107:3522–3527. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Madjd Z, Mehrjerdi AZ, Sharifi AM,
Molanaei S, Shahzadi SZ and Asadi-Lari M: CD44+ cancer cells
express higher levels of the anti-apoptotic protein Bcl-2 in breast
tumours. Cancer Immun. 9:42009.
|
|
41
|
Zobalova R, McDermott L, Stantic M,
Prokopova K, Dong LF and Neuzil J: CD133-positive cells are
resistant to TRAIL due to up-regulation of FLIP. Biochem Biophys
Res Commun. 373:567–571. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu G, Yuan X, Zeng Z, et al: Analysis of
gene expression and chemoresistance of CD133+ cancer stem cells in
glioblastoma. Mol Cancer. 5:672006.
|
|
43
|
Pan GJ, Chang ZY, Scholer HR and Pei D:
Stem cell pluripotency and transcription factor Oct4. Cell Res.
12:321–329. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Takahashi K and Yamanaka S: Induction of
pluripotent stem cells from mouse embryonic and adult fibroblast
cultures by defined factors. Cell. 126:663–676. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kim JB, Sebastiano V, Wu G, et al:
Oct4-induced pluripotency in adult neural stem cells. Cell.
136:411–419. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yu J, Vodyanik MA, Smuga-Otto K, et al:
Induced pluripotent stem cell lines derived from human somatic
cells. Science. 318:1917–1920. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kim JB, Zaehres H, Wu G, et al:
Pluripotent stem cells induced from adult neural stem cells by
reprogramming with two factors. Nature. 454:646–650. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Jones TD, Ulbright TM, Eble JN, Baldridge
LA and Cheng L: OCT-4 staining in testicular tumors: a sensitive
and specific marker for seminoma and embryonal carcinoma. Am J Surg
Pathol. 28:935–940. 2004. View Article : Google Scholar
|
|
49
|
Tai MH, Chang CC, Kiupel M, Webster JD,
Olson LK and Trosko JE: Oct4 expression in adult human stem cells:
evidence in support of the stem cell theory of carcinogenesis.
Carcinogenesis. 26:495–502. 2005.PubMed/NCBI
|
|
50
|
Beltran AS, Rivenbark AG, Richardson BT,
et al: Generation of tumor-initiating cells by exogenous delivery
of OCT4 transcription factor. Breast Cancer Res. 13:R942011.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ghisolfi L, Keates AC, Hu X, Lee DK and Li
CJ: Ionizing radiation induces stemness in cancer cells. PLoS One.
7:e436282012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Haynes BF, Liao HX and Patton KL: The
transmembrane hyaluronate receptor (CD44): multiple functions,
multiple forms. Cancer Cells. 3:347–350. 1991.
|
|
53
|
Zhang S, Balch C, Chan MW, et al:
Identification and characterization of ovarian cancer-initiating
cells from primary human tumors. Cancer Res. 68:4311–4320. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hurt EM, Kawasaki BT, Klarmann GJ, Thomas
SB and Farrar WL: CD44+ CD24(−) prostate cells are early cancer
progenitor/stem cells that provide a model for patients with poor
prognosis. Br J Cancer. 98:756–765. 2008.
|
|
55
|
Pries R, Witrkopf N, Trenkle T, Nitsch SM
and Wollenberg B: Potential stem cell marker CD44 is constitutively
expressed in permanent cell lines of head and neck cancer. In Vivo.
22:89–92. 2008.PubMed/NCBI
|
|
56
|
Kawabata S, Oka M, Soda H, et al:
Expression and functional analyses of breast cancer resistance
protein in lung cancer. Clin Cancer Res. 9:3052–3057.
2003.PubMed/NCBI
|
|
57
|
Al-Hajj M, Becker MW, Wicha M, Weissman I
and Clarke MF: Therapeutic implications of cancer stem cells. Curr
Opin Genet Dev. 14:43–47. 2004. View Article : Google Scholar
|
|
58
|
Dy GK, Hobday TJ, Nelson G, et al:
Long-term survivors of metastatic colorectal cancer treated with
systemic chemotherapy alone: a North Central Cancer Treatment Group
review of 3811 patients, N0144. Clin Colorectal Cancer. 8:88–93.
2009. View Article : Google Scholar
|