Introduction
Pancreatic ductal adenocarcinoma (PDA), also known
as pancreatic cancer, is the fourth leading cause of cancer-related
mortality in the United States (1).
Survival has not markedly improved despite the routine use of
surgery, chemotherapy and radiotherapy. The overall five-year
survival rate is <5% and the median overall survival is less
than six months (1,2). In addition, only <20% of patients
present with potentially curable localized resectable tumors
(3). However, the majority of
patients are likely to develop local recurrence or metastasis
following surgery. For patients with metastatic disease, PDA is
lethal and notoriously difficult to treat and such individuals
exhibit a poor median survival of three to six months (4). Over the past decade, gemcitabine
(Gem)-based chemotherapy or chemoradiation have been the standard
regimen, although, the overall therapeutic efficacy of these
methods is considered to be minimal (5,6). In
order to improve the current treatment status to achieve greater
efficacy and to improve prognosis, novel treatment strategies must
be investigated.
Multiple new agents with diverse mechanisms of
action in combination with Gem have been previously assessed in
randomized clinical trials of pancreatic cancer, with no
improvement in outcome observed (2,7,8). To
date, the laboratory results of targeted therapies have been
significant and only erlotinib, an epidermal growth factor receptor
(EGFR)-tyrosine kinase inhibitor, has achieved a modest survival
benefit in combination with Gem in a previous phase III clinical
trial (9). Nimotuzumab (Nimo) is a
humanized monoclonal antibody that recognizes the EGFR
extracellular domain. Based on the results of previous phase I/II
trials for pancreatic cancer, the recommended dose of Nimo has been
established at 200 mg per week. In addition, Nimo is safe and well
tolerated, although, the efficacy of monotherapy is minimal. At
present, a randomized, placebo-controlled trial of Gem plus Nimo
has been initiated, of which the results are of interest (10).
Immunotherapeutic approaches are becoming promising
strategies for effectively inducing antitumor immune responses with
reduced toxicity (11–13). However, the manner in which
immunotherapy may be optimally integrated with existing
non-immunological therapies for optimal synergy remains to be
elucidated (14). In addition, an
approach should be established to arrange the order of the various
combination treatments, including immunotherapy, chemotherapy and
monoclonal antibodies (15–18).
Expanded activated autologous lymphocyte (EAAL)
therapy with cluster of differentiation (CD)3(+) and CD8(+)
cytotoxic T lymphocytes, and CD3(−) and CD56(+) natural killer
cells as the major effector cells is a type of adoptive cell
therapy. It has previously been shown that EAAL therapy has the
ability to enrich potential antitumor responses and that it is safe
for early- and late-stage cancer patients (19,20). A
randomized trial sponsored by Takayama et al (21) demonstrated that adoptive
immunotherapy lowers postsurgical recurrence rates of
hepatocellular carcinoma with significantly longer recurrence-free
(P=0.01) and disease-specific (P=0.04) survival than those of the
control group. Expanded activated allogeneic lymphocyte
(EAAL*) therapy is a type of EAAL therapy with infusion
lymphocytes, which are obtained from a human leukocyte antigen
(HLA)-matched related donor rather than from the patients
themselves.
The present study reports the eight-month follow-up
of a patient with advanced pancreatic cancer with multiple
metastases. The patient was treated with EAAL* therapy
obtained from a related donor in addition to conventional
chemotherapy with Gem and oxaliplatin (L-OHP) plus targeted therapy
with Nimo. Written informed consent was obtained from the family of
the patient.
Case report
A 46-year-old female presented to with cough and
expectoration with no apparent cause in October 2012 at the local
doctor. Positron emission tomography (PET)/computed tomography (CT)
and biopsy revealed a PDA involved in the body of the pancreas with
multiple metastases to the lungs, liver and abdominal lymph nodes.
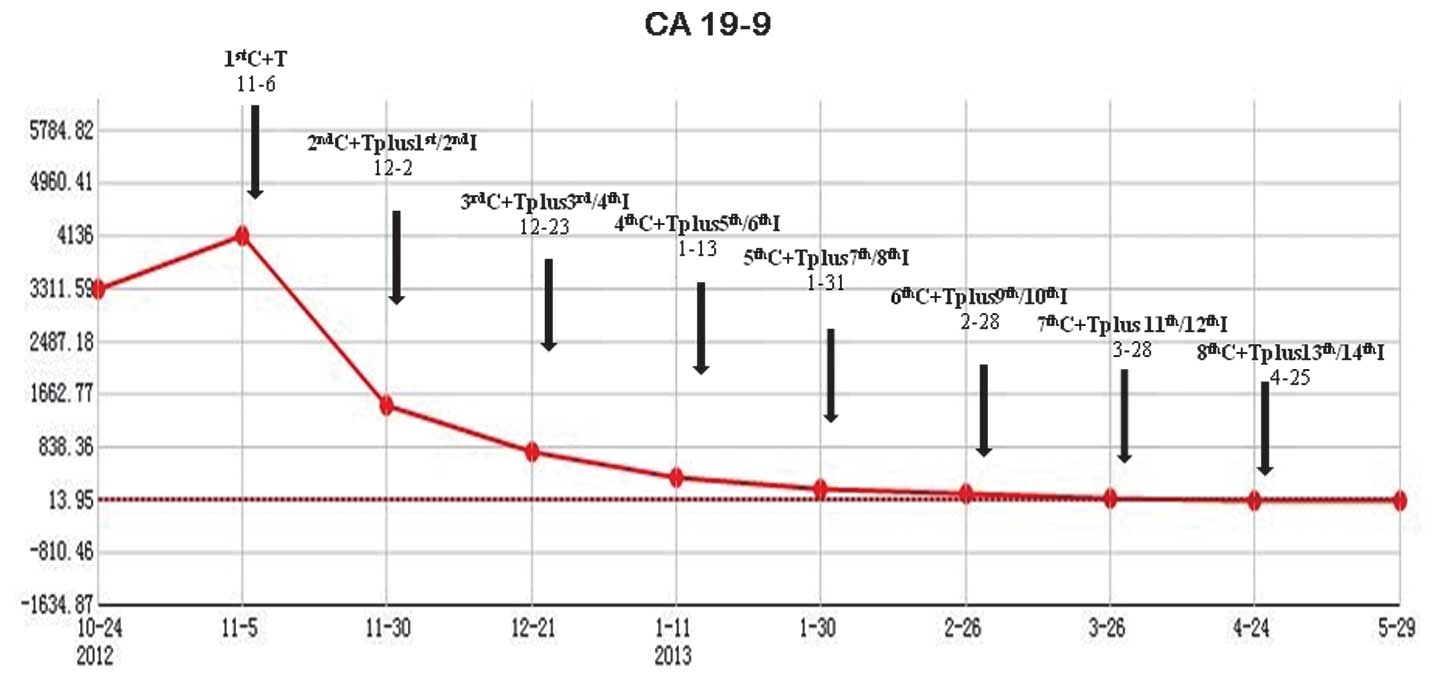
The carbohydrate antigen (CA) 19-9 value was 3,318 U/ml at
diagnosis. Prior to the cell-based immunotherapy, the patient
received one cycle of intravenous chemotherapy with 1,800 mg Gem
(1,000 mg/m2 i.v. on days one and eight, every 21 days)
and 150 mg L-OHP (85 mg/m2 i.v. on day one, every 21
days), and targeted therapy with 200 mg Nimo (i.v. on day seven,
every seven days). The immunotherapy was subsequently initiated. At
diagnosis, the tumor load of the patient was considered to be
large, due to multiple metastases, and the patient had relatively
weak immunity, thus, the EAAL* therapy was designed
(Beijing ImmunoTech Applied Science Ltd., Beijing, China). Written
informed consent was obtained and the patient’s HLA genotype was
matched with that of a related donor, peripheral blood was
collected from the related donor in heparin tubes and transported
to the laboratory under cold conditions.
Activated lymphocytes using anti-CD3 monoclonal
antibody and interleukin-2 were generated as described previously
(22). Briefly, 20–100 ml of
peripheral blood was collected from the related donor and
peripheral blood mononuclear cells (PBMCs) were isolated by
Ficoll-Hypaque gravity centrifugation (ALLEGRA X-12, Beckman
Coulter, Miani, FL, USA) at 400 × g. The isolated PBMCs were washed
and resuspended in serum-free medium (IMSF 100; Immunotech, London,
UK) supplemented with 700 U/ml of interleukin (IL)-2 (CCBIO,
Changchun, China). The PBMC suspension was placed in a flask coated
with immobilized anti-CD3 antibody (eBioscience, San Diego, CA,
USA)and incubated for one week. The lymphocyte suspension was
transferred to a gas-permeable bag to allow the lymphocytes to grow
for two more weeks. The activated lymphocytes were subsequently
harvested, filtered through 100-μm membranes and resuspended in 100
ml of normal saline containing 1% human serum albumin for the
intravenous infusion. Prior to cell transplantation, the cells were
assessed for endotoxin levels using a Limulus Amebocyte Lysate kit
(Associates of Cape Cod, Inc., Falmouth, MA, USA). The average cell
count following the in vitro expansion was
2.04–5.18×109 cells/100 ml. Therefore, the patient was
administrated 100 or 200 ml (large dose) of these activated
lymphocytes up to 14 times a week or every other week. For each
infusion, one part of these cells was recovered, activated and
expanded for two weeks and transferred into the patient as
previously described.
Overall, the patient received 14 infusions of
EAAL*, with an overall cell count of
7.414×1010, in combination with seven cycles of
chemotherapy plus targeted therapy. The initial four infusions of
EAAL* were at doses of 100 ml and were followed by doses
of 200 ml. The specific regimen was as follows: EAAL*,
100 ml/200 ml i.v. on days 2 and 9; Gem, 1,800 mg i.v. on days 4
and 11; L-OHP, 150 mg i.v. on day 4; and Nimo, 200 mg i.v. on days
1, 8 and 15, every 21 days. Of note, during the last two cycles of
chemotherapy, the dosage of Gem was reduced to 1,600 mg and that of
L-OPH was reduced to 125 mg, due to concern regarding the
cumulative toxicities, although, the patient tolerated the
treatments well exhibiting only grade II adverse effects.
Responses were evaluated according to the Response
Evaluation Criteria in Solid Tumors (RECIST) and the toxic effects
were assessed using the National Cancer Institute Common Toxicity
Criteria, version 3.0 (23,24). The responses were recorded during
and following each infusion and the final follow-up was in July
2013.
Following two infusions of EAAL* in
combination with one cycle of chemotherapy plus targeted therapy,
the patient’s response was evaluated. A CT scan in December 2012
revealed that lesions in the pancreas, liver, lungs and abdominal
lymph nodes showed slight shrinkage. In addition, the CA 19-9
levels had decreased from 4,136 U/ml (prior to treatment) to 758.50
U/ml (Fig. 1). The status of the
patient was stable disease according to the RECIST criteria.
The patient continued with six infusions of
EAAL* combined with three cycles of chemotherapy plus
targeted therapy. Responses were evaluated by CT scan, which
revealed that the metastatic lesions in the lungs and liver were
significantly reduced, with lesions in the pancreas being slightly
reduced. In addition, the CA 19-9 levels decreased to 113.6 U/ml
(Fig. 1) and partial remission (PR)
was achieved.
Finally, the patient received a further six
infusions of EAAL* at large doses (200 ml) together with
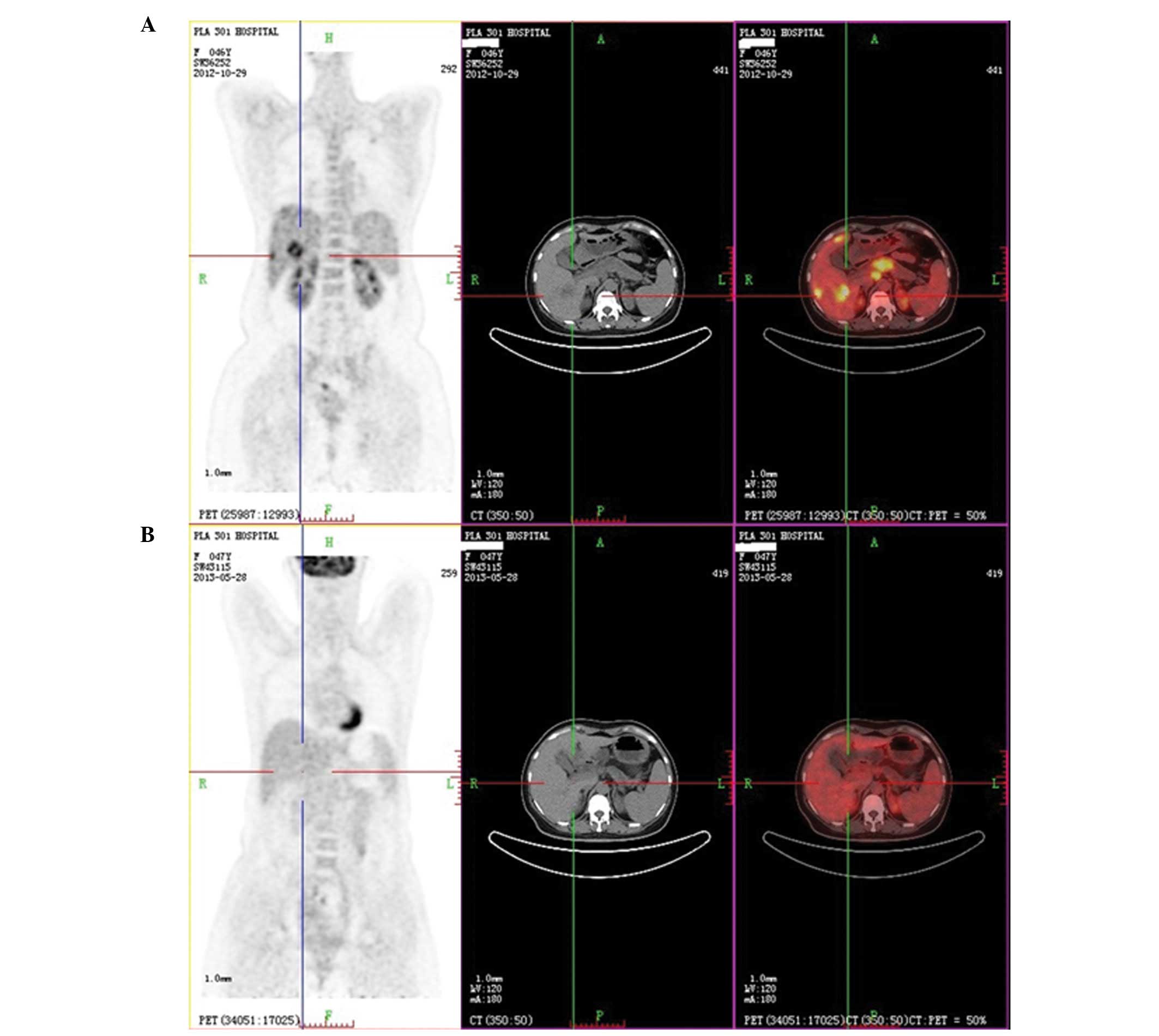
three cycles of chemotherapy plus targeted therapy. PET/CT was
performed to evaluate the responses and showed that almost all the
metabolic values of the deposits had markedly decreased or even
disappeared; in addition, the size of all the lesions had markedly
decreased (Fig. 2). The CA 19-9
levels decreased to 24.09 U/ml (normal range, 0.1–37 U/ml; Fig. 1) and PR and near complete remission
(nCR) were achieved.
The last follow-up in July 2013 revealed a static
non-progressive disease with a progression-free survival (PFS) of
eight months and demonstrated that all of the parameters, including
the tumor markers, were within their normal ranges.
The patient showed an improved quality of life
without a cough or expectoration. Following all 14 infusions of
EAAL*, with an overall cell count of
7.414×1010, mild upset was occasionally identified
following large-dosage lymphocyte transfusions without other severe
adverse effects. For the whole combined modality, the most serious
toxicities observed were grade II hematological and
gastrointestinal toxicities, in addition to grade I liver function
damage and a skin rash.
Discussion
The current study presents a patient with stage IV
pancreatic cancer with multiple metastases for whom curative
surgery was not an option at diagnosis. A novel therapeutic
strategy was administered to the patient, which included several
infusions of EAAL* together with Gem-based chemotherapy
and Nimo-based targeted therapy. Notably, the strategy achieved an
ideal and rare antitumor responses, PR and nCR. The CA 19-9 levels
decreased from 4,136 U/ml to within the normal ranges. In addition,
significant regression of the lesions was observed. The eight-month
follow-up showed a prolonged static non-progressive disease with a
PFS of seven months, which far exceeded the predictions of a
previous study (4). The patient
benefited from the individualized treatment and multimodality
therapy, which is consistent with the previous observations that
immunotherapy combined with other non-immunological therapies
moderately enriches the potential antitumor responses through the
mechanism(s) by which these modalities are synergized. However,
these mechanisms are not fully understood (17).
Of note, the order of administration for the
combined therapeutic approach is a critical factor that affects the
therapeutic outcome. Therefore, establishing the order of
administration to maximize the efficacy and guarantee safety is a
significant problem that remains unsolved. Zhang et al
(16) hypothesized that timely
immune modification of chemotherapy-activated antitumor immunity
results in an enhanced antitumor immune response and complete tumor
eradication. In accordance with this, chemotherapy is commonly
administered prior to autologous cell-based immunotherapy in
clinical practice. However, for safety considerations, the
EAAL* was administered prior to chemotherapy in the
current study. It was hypothesized that allogeneic cell infusion
may be relatively unsafe compared with infusions of autologous
lymphocytes, as it may lead to rejection or unknown adverse
effects. Therefore, the current patient received chemotherapy 48 h
following the allogeneic cell infusion since toxic chemotherapy
agents are likely to eventually remove foreign cells. The present
study showed that EAAL* therapy was safe and the order
of administration was effective.
To date, the patient has achieved nCR with marked
regression of the deposits. Immunotherapy has been planned as a
maintenance treatment to lower the local recurrence and metastasis
rates, and eradicate any minimal residual disease. In addition,
follow-up treatment and prognosis will be tracked for subsequent
studies.
In conclusion, the current study showed that this
type of combined therapy is effective and safe, therefore, we
recommend that this strategy is considered for the treatment of
similar cases.
Acknowledgements
The authors would like to thank the patient and
family for their support and cooperation with the present
study.
References
|
1
|
Jemal A, Siegel R, Ward E, et al: Cancer
statistics, 2008. CA Cancer J Clin. 58:71–96. 2008. View Article : Google Scholar
|
|
2
|
Li D, Xie K, Wolff R and Abbruzzese JL:
Pancreatic cancer. Lancet. 363:1049–1057. 2004. View Article : Google Scholar
|
|
3
|
Wong HH and Lemoine NR: Pancreatic cancer:
molecular pathogenesis and new therapeutic targets. Nat Rev
Gastroenterol Hepatol. 6:412–422. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pancreatric Section, British Society of
Gastroenterology; Pancreatic Society of Great Britain and Ireland;
Association of Upper Gastrointestinal Surgeons of Great Britain and
Ireland; Royal College of Pathologists; Special Interest Group for
Gastro-Intestinal Radiology. Guidelines for the management of
patients with pancreatic cancer periampullary and ampullary
carcinomas. Gut. 54(Suppl 5): v1–v16. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Burris HA III, Moore MJ, Andersen J, et
al: Improvements in survival and clinical benefit with gemcitabine
as first-line therapy for patients with advanced pancreas cancer: a
randomized trial. J Clin Oncol. 15:2403–2413. 1997.PubMed/NCBI
|
|
6
|
Hidalgo M: Pancreatic cancer. N Engl J
Med. 362:1605–1617. 2010. View Article : Google Scholar
|
|
7
|
Sultana A, Smith CT, Cunningham D,
Starling N, Neoptolemos JP and Ghaneh P: Meta-analyses of
chemotherapy for locally advanced and metastatic pancreatic cancer.
J Clin Oncol. 25:2607–2615. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Heinemann V, Boeck S, Hinke A, Labianca R
and Louvet C: Meta-analysis of randomized trials: evaluation of
benefit from gemcitabine-based combination chemotherapy applied in
advanced pancreatic cancer. BMC Cancer. 8:822008. View Article : Google Scholar
|
|
9
|
Moore MJ, Goldstein D, Hamm J, et al:
Erlotinib plus gemcitabine compared with gemcitabine alone in
patients with advanced pancreatic cancer: a phase III trial of the
National Cancer Institute of Canada Clinical Trials Group. J Clin
Oncol. 25:1960–1966. 2007. View Article : Google Scholar
|
|
10
|
Strumberg D, Schultheis B, Scheulen ME, et
al: Phase II study of nimotuzumab, a humanized monoclonal
anti-epidermal growth factor receptor (EGFR) antibody, in patients
with locally advanced or metastatic pancreatic cancer. Invest New
Drugs. 30:1138–1143. 2012. View Article : Google Scholar
|
|
11
|
Vonderheide RH, Bajor DL, Winograd R,
Evans RA, Bayne LJ and Beatty GL: CD40 immunotherapy for pancreatic
cancer. Cancer Immunol Immunother. 62:949–954. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sekine T, Takayama T, Konomi Y and Kakizoe
T: Prevention of cancer recurrence by infusion of activated
autologous lymphocytes. Hum Cell. 7:121–124. 1994.(In
Japanese).
|
|
13
|
Dudley ME and Rosenberg SA:
Adoptive-cell-transfer therapy for the treatment of patients with
cancer. Nat Rev Cancer. 3:666–675. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ostrand-Rosenberg S: Looking to the future
of cancer immunotherapy: many questions to answer and many
therapeutic opportunities. Cancer Immunol Immunother. 62:1–2. 2013.
View Article : Google Scholar
|
|
15
|
Baxevanis CN, Perez SA and Papamichail M:
Combinatorial treatments including vaccines, chemotherapy and
monoclonal antibodies for cancer therapy. Cancer Immunol
Immunother. 58:317–324. 2009. View Article : Google Scholar
|
|
16
|
Zhang L, Feng D, Yu LX, Tsung K and Norton
JA: Preexisting antitumor immunity augments the antitumor effects
of chemotherapy. Cancer Immunol Immunother. 62:1061–1071. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ramakrishnan R and Gabrilovich DI: Novel
mechanism of synergistic effects of conventional chemotherapy and
immune therapy of cancer. Cancer Immunol Immunother. 62:405–410.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mitchell MS: Combining chemotherapy with
biological response modifiers in treatment of cancer. J Natl Cancer
Inst. 80:1445–1450. 1988. View Article : Google Scholar
|
|
19
|
Sekine T, Shiraiwa H, Yamazaki T, Tobisu K
and Kakizoe T: A feasible method for expansion of peripheral blood
lymphocytes by culture with immobilized anti-CD3 monoclonal
antibody and interleukin-2 for use in adoptive immunotherapy of
cancer patients. Biomed Pharmacother. 47:73–78. 1993. View Article : Google Scholar
|
|
20
|
Sun Z, Shi L, Zhang H, et al: Immune
modulation and safety profile of adoptive immunotherapy using
expanded autologous activated lymphocytes against advanced cancer.
Clin Immunol. 138:23–32. 2011. View Article : Google Scholar
|
|
21
|
Takayama T, Sekine T, Makuuchi M, et al:
Adoptive immunotherapy to lower postsurgical recurrence rates of
hepatocellular carcinoma: a randomised trial. Lancet. 356:802–807.
2000. View Article : Google Scholar
|
|
22
|
Schmidt-Wolf IG, Negrin RS, Kiem HP, Blume
KG and Weissman IL: Use of a SCID mouse/human lymphoma model to
evaluate cytokine-induced killer cells with potent antitumor cell
activity. J Exp Med. 174:139–149. 1991. View Article : Google Scholar
|
|
23
|
Therasse P, Arbuck SG, Eisenhauer EA, et
al: New guidelines to evaluate the response to treatment in solid
tumors. European Organization for Research and Treatment of Cancer,
National Cancer Institute of the United States, National Cancer
Institute of Canada. J Natl Cancer Inst. 92:205–216. 2000.
View Article : Google Scholar
|
|
24
|
Holmboe L, Andersen AM, Mørkrid L, Slørdal
L and Hall KS: High dose methotrexate chemotherapy:
pharmacokinetics, folate and toxicity in osteosarcoma patients. Br
J Clin Pharmacol. 73:106–114. 2012. View Article : Google Scholar
|