Introduction
Laryngeal squamous cell carcinoma (LSCC) is the most
frequent type of head and neck cancer, the risk of which results
from complex interactions between numerous genetic and
environmental factors (1).
Effective treatments include radiotherapy and chemotherapy,
although surgery is currently the only treatment that consistently
prolongs survival time (2). The
most effective approaches to achieving an improved prognosis in
LSCC patients are prevention and early diagnosis. Accumulating
evidence indicates that genetic polymorphisms are associated with
laryngeal carcinoma, and extensive investigations have been
conducted to identify inherited genetic risks for this disease
(3).
Vocal leukoplakia is a mucosal epithelial
hyperplastic keratosis lesion of the vocal cords and is often
considered to be a pre-cancerous lesion of laryngeal carcinoma.
Interleukin (IL)-10 is an anti-inflammatory cytokine, which is
involved in suppressing T-helper (Th)1 lymphocytes and stimulating
B lymphocytes and Th2 lymphocytes to mediate immune responses,
downregulating the production of pro-inflammatory mediators,
including IL-1β, tumor necrosis factor-α, interferon-γ and other
pro-inflammatory cytokines (4–6), and
suppressing the antigen presentation capacity of antigen-presenting
cells (7). The gene encoding IL-10
is located on chromosome 1 (1q31–1q32) and consists of five exons
and four introns.
Numerous polymorphisms of the IL-10 gene promoter
have been identified; primarily single nucleotide polymorphisms
(SNPs), with three polymorphisms in the 5-flanking region of IL-10
at positions −1082 (A-G), −819 (T-C) and −592 (A-C) (8). These polymorphisms are in strong
linkage disequilibrium and are associated with various serum levels
of IL-10 in vivo. The GCC haplotypes are associated with a
high IL-10 production in peripheral blood cell cultures, while the
ATA haplotypes are associated with low levels of IL-10 (9,10).
Furthermore, numerous studies have indicated that environmental
factors, including cigarette smoking and alcohol consumption,
induce cytokine expression and are associated with certain cytokine
genotypes (11). Cigarette smoking
and alcohol consumption may act independently or in synergy with
cytokine genotypes to confer individual susceptibility to
developing LSCC.
In previous years, several studies have indicated
that the IL-10 promoter polymorphisms are associated with an
increased risk of cancers, including lung, thyroid, prostate,
cervical and gastric cancers (12–16).
Nevertheless, studies concerning the interaction of cytokine genes
and environmental factors in LSCC are scarce.
In the present case-control association study, the
effects of three polymorphisms of IL-10 and their allele
frequencies on disease susceptibility and the severity of LSCC were
investigated. The interaction between these cytokine genotypes and
plasma IL-10 levels, and the association with environmental risks,
including cigarette smoking and alcohol consumption, was further
investigated.
Material and methods
Ethical approval
The study protocol was approved by the Medical
Research Council of the Eye, Ear, Nose and Throat Hospital, Fudan
University (Shanghai, China; no. KJ2008-01). Informed consent was
obtained from each patient and healthy (control) individual.
Patients and controls
Between October 2012 and February 2013, 146 patients
with LSCC and 61 with vocal leukoplakia were enrolled in the study
at the Eye, Ear, Nose and Throat Hospital. All patients were of
Chinese Han origin and were recruited from various geographical
regions of China. Healthy volunteers of equivalent ethnicity,
gender and age were enrolled as the control group (n=119) in the
study. Informed consent was obtained according to the Declaration
of Helsinki. The clinicopathological findings of the cancer group
were collected. All the pathological cell types in the cancer group
were squamous cell carcinoma. Smoking habits were defined as
non-smoker (<100 cigarettes in their lifetime) and smoker
(>20 cigarettes per day for ≥1 year), and alcohol consumption
was defined as non-drinker and drinker (>200 ml per day). The
characteristics of various subgroups are shown in Table I.
 | Table IDistribution of cases and controls
according to selected sociodemographic characteristics. |
Table I
Distribution of cases and controls
according to selected sociodemographic characteristics.
| Characteristics | Controls | LSCC | Vocal
leukoplakia | OR (95% CI)a | P-valuea | OR (95% CI)b | P-valueb |
|---|
| Mean age ± SD,
years | 62.32±7.9 | 60.91±8.7 | 56.54±10.7 | | | | |
| Gender, n |
| Female | 5 | 4 | 2 | | | | |
| Male | 114 | 142 | 59 | | | | |
| Smoking, n |
| No | 83 | 39 | 20 | Reference | | Reference | |
| Yes | 36 | 107 | 41 | 6.33 (3.7–10.8) | <0.01 | 4.73 (2.4–9.1) | <0.01 |
| Alcohol consumption,
n |
| No | 95 | 60 | 25 | Reference | | Reference | |
| Yes | 24 | 86 | 36 | 5.67 (3.3–9.9) | <0.01 | 5.70 (2.9–11.2) | <0.01 |
| LSCC type, n (%) |
| Glottic | | 98 (67.1) | | | | | |
| Supraglottic | | 47 (32.2) | | | | | |
| Subglottic | | 1 (0.7) | | | | | |
| LSCC stage, n
(%) |
| Advanced III +
IV | | 50 (34.2) | | | | | |
| Early I + II | | 96 (65.8) | | | | | |
| Lymph node, n
(%) |
| N0 | | 110 (75.3) | | | | | |
| N1+N2 | | 36 (24.7) | | | | | |
Blood collection and DNA and plasma
extraction
Blood (5 ml) was collected from each participant in
an EDTA tube and centrifuged for 10 min at 900 × g. Plasma was
isolated from peripheral blood and stored at −80°C within 30 min of
collection. Genomic DNA (100 ng/μl) was prepared from peripheral
blood using a TIANamp Blood DNA kit [Tiangen Biotech (Beijing),
Co., Inc., Beijing, China].
Analysis of plasma IL-10 levels and IL-10
−1082/−819/−592 polymorphisms
The plasma IL-10 levels were analyzed using a
standard enzyme-linked immunosorbent assay (ELISA); Human IL-10
Platinum ELISA (74540061; eBioscience, San Diego, CA, USA).
The IL-10 (−592, −819 and −1082) polymorphisms were
analyzed by PCR amplification of the promoter or coding regions
using specifically designed pairs of oligonucleotide primers
followed by direct sequencing (ABI Prism 3730xl DNA sequencer; PE
Applied Biosystems, Foster City, CA, USA). For the IL-10
genotyping, the PCR conditions were as follows: 30 cycles of 98°C
for 10 sec, 55°C for 15 sec and 72°C for 1 min. All the laboratory
assays were conducted and interpreted blindly without any knowledge
of the case or control status. The primer sequences (17) used in the present study were: Sense,
5′-ATCCAAGACAACACTACTAA-3′ and antisense,
5′-TAAATATCCTCAAAGTTCC-3′; and direct sequencing with primer,
5′-TAAATATCCTCAAAGTTCC-3′.
Statistical analysis
The demographic characteristics and environmental
factors, and the gene frequencies of IL-10 in patients and controls
were compared and tested using χ2 tests. Since
environmental variables, including cigarette smoking and alcohol
consumption, were the main risk factor for LSCC in the present
study, these factors were also dichotomized and their effects on
the risk of LSCC were investigated. Logistic regression analyses
were used to evaluate the effects of genotypes, plasma IL-10 and
cigarette smoking.
The data were analyzed using the SPSS statistical
package (SPSS, Inc., Chicago, IL, USA). Odds ratios (ORs) and 95%
confidence intervals (CIs) are presented. P<0.05 was considered
to indicate a statistically significant difference.
Results
Demographic details
The characteristics and risk factors in the patients
with LSCC and vocal leukoplakia and in the controls are presented
in Table I. There was no
statistical difference between the ages of the controls (62.32±7.9
years), the LSCC patients (60.91±8.7 years) and the vocal
leukoplakia patients (56.54±10.7 years). However, smoking was a
risk factor of LSCC (OR, 6.33; 95% CI, 3.7–10.8; P<0.01) and
vocal leukoplakia (OR, 4.73; 95% CI, 2.4–9.1; P<0.01). Alcohol
consumption was also a risk factor of LSCC (OR, 5.67; 95% CI,
3.3–9.9; P<0.01) and vocal leukoplakia (OR, 5.70; 95% CI,
2.9–11.2; P<0.01). The type of carcinoma, stage of LSCC and
lymph node metastasis status are also presented in Table I.
Genotype and haplotype frequency
distribution between controls and cases
The distribution of the cytokine gene polymorphisms
among the subjects with LSCC and vocal leukoplakia and the controls
is summarized in Table II. The
results show that the risk for LSCC is associated with the IL-10
polymorphism. The IL-10 genotype containing the G allele at
position −1082 or the C allele at positions −819 or −592 was more
frequent in cases of LSCC or vocal leukoplakia when compared with
the controls (Table II).
Unexpectedly, it was observed that the genotypes at position −592
were changed synchronously with that of −819 in the patients. A
1.82-fold increased susceptibility to LSCC was observed with the
presence of AC at IL-10 −592 and −819 (P=0.024), while the OR for
vocal leukoplakia was 1.93 (P=0.050). Compared with individuals
with the AA genotype, the relative risk (OR) of the development of
LSCC for heterozygotes with AG at position −1082 was 2.20 (95% CI,
1.04–4.67; P=0.037; Table II) and
for vocal leukoplakia this was 2.14 (95% CI, 0.87–5.27; P=0.092;
Table II). However, the OR of the
CC genotype at positions −592 and −819 in LSCC was 0.83 (CI,
0.37–1.86; P=0.642) and for vocal leukoplakia this was 1.91 (CI,
0.78–4.72; P=0.164).
 | Table IIAssociation between IL-10 genotypes
and development of LSCC and vocal leukoplakia. |
Table II
Association between IL-10 genotypes
and development of LSCC and vocal leukoplakia.
| Genotype | Controls
(n=119) | LSCC (n=146) | Vocal leukoplakia
(n=61) |
|---|
|
|
|---|
| n | OR (95% CI) | P-value | n | OR (95% CI) | P-value |
|---|
| IL-10 −592 |
| AA | 64 | 63 | Reference | | 23 | Reference | |
| AC | 39 | 70 | 1.82
(1.08–3.08) | 0.024 | 27 | 1.93
(0.97–3.81) | 0.050 |
| CC | 16 | 13 | 0.83
(0.37–1.86) | 0.642 | 11 | 1.91
(0.78–4.72) | 0.164 |
| Alleles |
| A | 167 | 196 | Reference | | 73 | Reference | |
| C | 71 | 96 | 1.15
(0.80–1.67) | 0.453 | 49 | 1.58
(1.00–2.49) | 0.049 |
| IL-10 −819 |
| TT | 64 | 63 | Reference | | 23 | Reference | |
| TC | 39 | 70 | 1.82
(1.08–3.08) | 0.024 | 27 | 1.93
(0.97–3.81) | 0.050 |
| CC | 16 | 13 | 0.83
(0.37–1.86) | 0.642 | 11 | 1.91
(0.78–4.72) | 0.164 |
| Alleles |
| T | 167 | 196 | Reference | | 73 | Reference | |
| C | 71 | 96 | 1.15
(0.80–1.67) | 0.453 | 49 | 1.58
(1.00–2.49) | 0.049 |
| IL-10 −1082 |
| AA | 107 | 115 | Reference | | 50 | Reference | |
| AG | 11 | 26 | 2.20
(1.04–4.67) | 0.037 | 11 | 2.14
(0.87–5.27) | 0.092 |
| GG | 1 | 5 | 4.65
(0.54–40.47) | 0.127 | 0 | -- | |
| Alleles |
| A | 225 | 256 | Reference | | 111 | Reference | |
| G | 13 | 36 | 2.43
(1.26–4.70) | 0.007 | 11 | 1.72
(0.75–3.95) | 0.201 |
Genotype and haplotype frequency
distribution based on stages of cancer
For the genotypic comparison of the patients with
differing stages of cancer, individuals were categorized into three
groups: Control, early (stages I and II) and advanced (stages III
and IV). The present results did not show any risk for LSCC
associated with CC polymorphisms at IL-10 −592 and −819. With
regard to the IL-10 −1082 polymorphism, a 1.80-fold increased risk
for early-stage LSCC associated with AC at −592 and −819 (P=0.046)
and a 1.88-fold increased risk for advanced LSCC (P=0.080) was
observed. A 2.06-fold increased susceptibility for early-stage LSCC
associated with the G allele (GA/GG) at IL-10 −1082 (P=0.068) was
also observed, while for advanced LSCC the OR was 3.13 (P=0.008).
Therefore, patients with advanced LSCC had a significantly higher
OR when compared with those with early-stage LSCC (Table III).
 | Table IIIPrevalence of IL-10 polymorphism in
controls and patients in regard to early (I, II) and advanced (III,
IV) cancer stages. |
Table III
Prevalence of IL-10 polymorphism in
controls and patients in regard to early (I, II) and advanced (III,
IV) cancer stages.
| | Early (I+II) | Advanced
(III+IV) |
|---|
| |
|
|
|---|
| Genotype | Controls, n | n | OR (95% CI) | P-value | n | OR (95% CI) | P-value |
|---|
| −592 |
| AA | 64 | 42 | Reference | | 21 | Reference | |
| AC | 39 | 46 | 1.80
(1.01–3.20) | 0.046 | 24 | 1.88
(0.92–3.81) | 0.080 |
| CC | 16 | 8 | 0.76
(0.30–1.94) | 0.567 | 5 | 0.95
(0.31–2.92) | 0.932 |
| −819 |
| TT | 64 | 42 | Reference | | 21 | Reference | |
| TC | 39 | 46 | 1.80
(1.01–3.20) | 0.046 | 24 | 1.88
(0.92–3.81) | 0.080 |
| CC | 16 | 8 | 0.76
(0.30–1.94) | 0.567 | 5 | 0.95
(0.31–2.92) | 0.932 |
| −1082 |
| AA | 107 | 78 | Reference | | 37 | Reference | |
| AG | 11 | 16 | 2.00
(0.88–4.54) | 0.095 | 10 | 2.63
(1.03–6.69) | 0.038 |
| GG | 1 | 2 | 2.74
(0.24–30.80) | 0.396 | 3 | 8.68
(0.88–86.00) | 0.060 |
| AG+GG | 12 | 18 | 2.06
(0.94–4.52) | 0.068 | 13 | 3.13
(1.31–7.47) | 0.008 |
Genotype and haplotype frequency
distribution based on lymph node metastasis
The association between the IL-10 −592, −819 and
−1082 gene variants and the lymph node metastasis status was also
determined. No significant association for metastasis risk with the
IL-10 −592 and −819 polymorphisms was observed. However, the OR for
patients with lymph node metastasis at −1082 GA/GG was 2.97
(P=0.048), and 2.23 (P=0.035) (Table
IV) in the no lymph node metastasis group. These data indicated
that the patients with the IL-10 −1082 GA/GG genotypes were at an
increased risk of lymph node metastasis.
 | Table IVAffect of IL-10 polymorphism on lymph
node metastasis. |
Table IV
Affect of IL-10 polymorphism on lymph
node metastasis.
| | Lymph node
metastasis (−) | Lymph node
metastasis (+) |
|---|
| |
|
|
|---|
| Genotype | Controls, n | n | OR (95% CI) | P-value | n | OR (95% CI) | P-value |
|---|
| −592 |
| AA | 64 | 47 | Reference | | 16 | Reference | |
| AC+CC | 55 | 63 | 1.56
(0.93–2.63) | 0.094 | 20 | 1.46
(0.69–3.08) | 0.326 |
| −819 |
| TT | 64 | 47 | Reference | | 16 | Reference | |
| TC+CC | 55 | 63 | 1.56
(0.93–2.63) | 0.094 | 20 | 1.46
(0.69–3.08) | 0.326 |
| −1082 |
| AA | 107 | 88 | Reference | | 27 | Reference | |
| AG+GG | 12 | 22 | 2.23
(1.05–4.76) | 0.035 | 9 | 2.97
(1.14–7.78) | 0.048 |
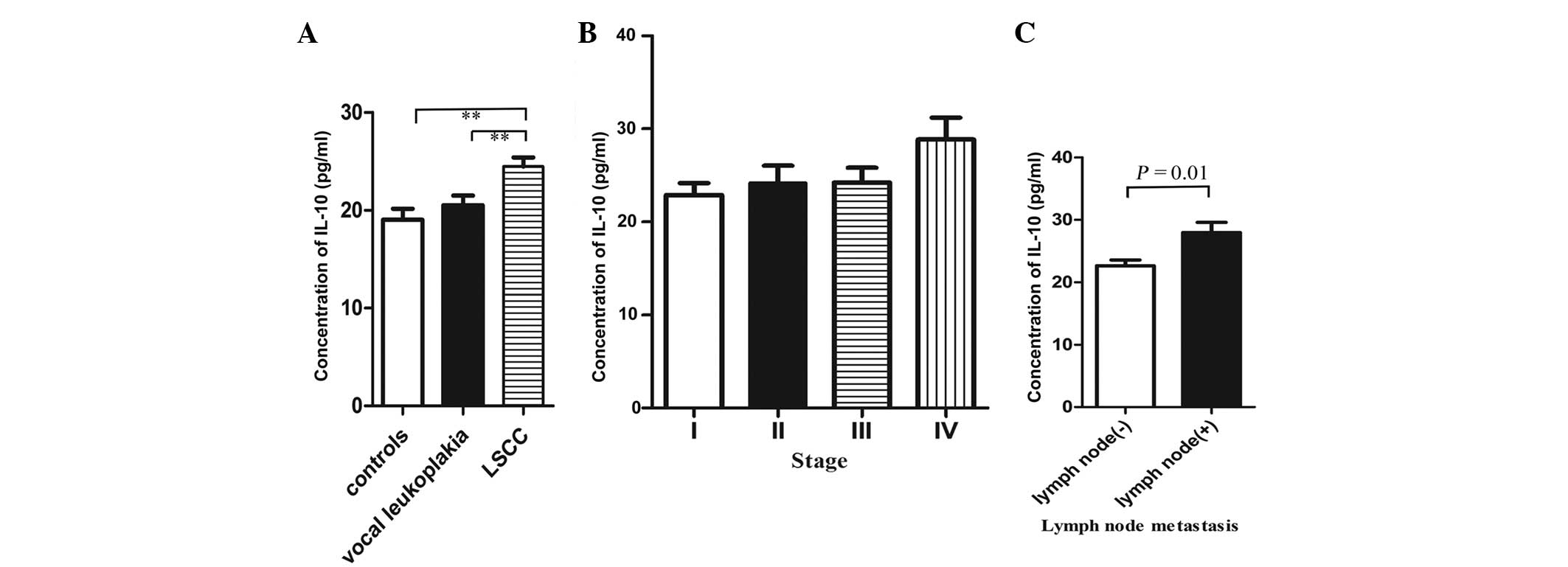
Plasma IL-10 concentrations in controls
and cases
Variations in plasma IL-10 concentrations were
observed in the patients with LSCC and vocal leukoplakia and in the
control group (Fig. 1A). In the
LSCC patients, the plasma IL-10 concentrations (24.47±5.4 pg/ml)
were significantly higher compared with those in the controls
(19.02±7.01 pg/ml; P<0.01) and the vocal leukoplakia patients
(20.33±3.1 pg/ml; P=0.001), although the concentrations in the
vocal leukoplakia patients were also higher than those in the
controls.
Association of plasma IL-10
concentrations and stages of cancer and lymph node metastasis
The variations in plasma IL-10 concentrations were
observed in the patients with differing stages of cancer (Fig. 1B). The concentration of plasma IL-10
was found to increase with the cancer staging. The concentration of
plasma IL-10 at stage IV (28.84±5.8 pg/ml) was significantly higher
than at other stages (I: 22.88±4.8 pg/ml, P=0.028; II: 24.19±6.1
pg/ml, P=0.121; III: 24.23±4.8 pg/ml, P=0.051). The concentrations
of plasma IL-10 in the patients with lymph node metastasis
(27.95±5.7 pg/ml) were significantly higher compared with the
patients with no lymph node metastasis (22.66±4.4 pg/ml) (P=0.01;
Fig. 1C).
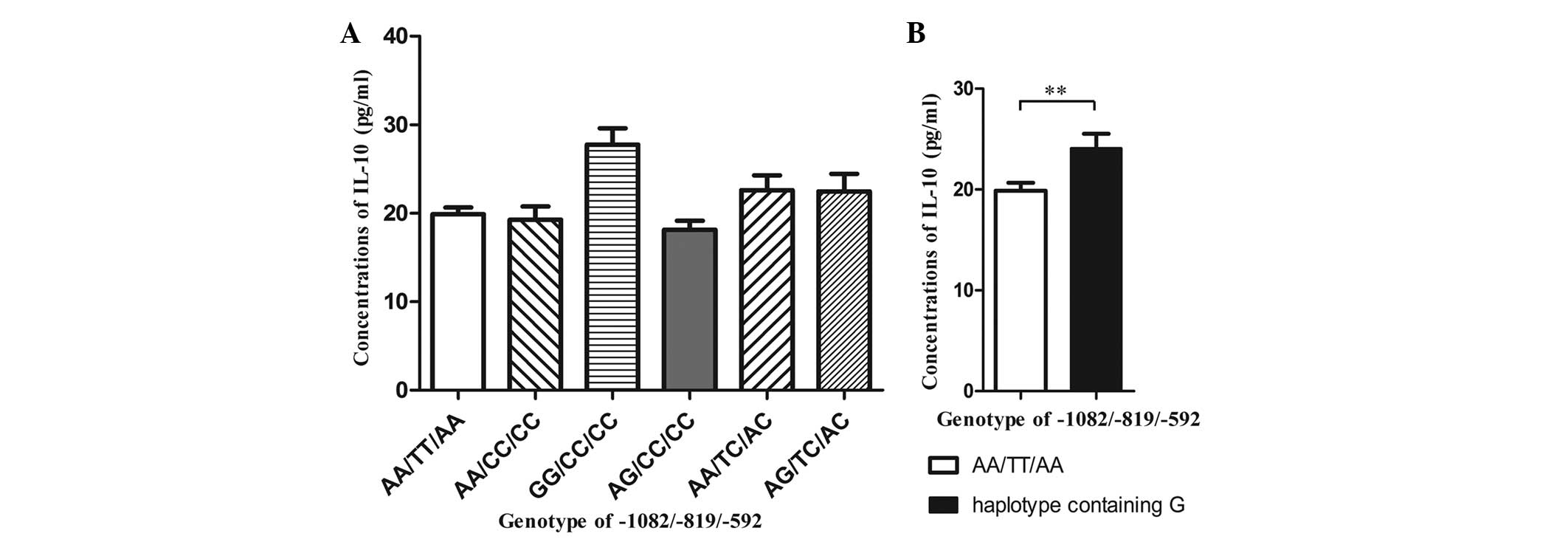
Plasma concentration of IL-10 in
association with IL-10 genotype polymorphism
Variations in plasma IL-10 concentrations were
observed in the different IL-10 genotypes (Fig. 2A). It was found that the GG/CC/CC
genotype had significantly higher plasma concentrations of IL-10
(27.76±5.0 pg/ml) than the other genotypes. Other studies have
documented that the IL-10 haplotype containing the G allele is
associated with increased production of IL-10 (9,10),
therefore, the plasma IL-10 concentrations of the haplotype based
on the IL-10 producing capability were analyzed further. The
haplotypes containing the G allele had significantly higher plasma
IL-10 concentrations (24.33±5.7 pg/ml) compared with the ATA
haplotype (19.88±4.7 pg/ml) (P=0.008; Fig. 2B).
Discussion
IL-10 polymorphisms have been widely studied and are
reported to be associated with certain other cancers and diseases;
however, to the best of our knowledge, this is the first analysis
of IL-10 polymorphisms and plasma IL-10 levels in patients with
LSCC.
Immunity is mediated by Th1, Th2, Th3 and Treg
responses, the balance of which affects the course of
cytokine-mediated inflammation. IL-10 is a potent and pleiotropic
cytokine that plays a crucial role in immunological and
inflammatory responses, as it regulates B-cell proliferation and
differentiation, and exhibits immunoregulatory activities and
anti-inflammatory properties (18).
Increased levels of IL-10 have been observed in patients with solid
tumors, including oral squamous cell carcinoma, indicating that
this cytokine has a significant role in carcinoma (19–21).
It has been speculated that IL-10 contributes to the escape of
tumor cells from immune surveillance and that it favors tumor
growth.
The IL-10 gene is located on chromosome 1
(1q31–1q32). In recent decades, several SNPs have been investigated
in the IL-10 gene region (17,22).
It has been identified that there are three important polymorphisms
in the 5-flanking region of the IL-10 gene at positions −1082, −819
and −592, which are associated with high transcriptional promoter
activity (8). Numerous studies have
reported associations between IL-10 polymorphisms and cancer
susceptibility (23,24).
Different IL-10 promoter genotypes are associated
with different levels of IL-10 production. Numerous studies have
investigated the association between IL-10 promoter polymorphisms
and the susceptibility to various types of cancers, but this
association has not been examined in LSCC (12–16).
The purpose of the present study was to examine
whether the IL-10 promoter polymorphisms and plasma IL-10 levels
are associated with a risk for LSCC. The individuals under
investigation were patients with LSCC or vocal leukoplakia, whose
genotypes were compared with those of age- and gender-matched
healthy controls.
In the study, it was demonstrated that the presence
of the IL-10 −819 C, −592 C and −1082 G alleles were associated
with an increased risk for LSCC development and vocal leukoplakia.
The risk factors (OR) calculated in the present study indicated a
1.82-fold increased susceptibility to LSCC with the presence of AC
at IL-10 −592 and −819 (P=0.024), while the OR for vocal
leukoplakia was 1.93 (P=0.050). The relative risk (OR) of LSCC
development for AG at −1082 heterozygotes was 2.20 (95% CI,
1.04–4.67; P=0.037; Table II), and
the vocal leukoplakia OR was 2.14 (95% CI, 0.87–5.27; P=0.092;
Table II). The −819 T/C SNP IL-10
polymorphism was in complete linkage disequilibrium with the −592
A/C SNP, which was in accordance with the observations of another
study (18). Additionally, it was
found that the OR for advanced LSCC was higher than for early-stage
LSCC at the three positions, −1082, −819 and −592. Furthermore, the
OR was significantly higher at −1082 GA/GG in the patients with
lymph node metastasis, compared with those without (2.97 vs. 2.23;
Table IV). Increased plasma IL-10
levels were detected in the patients with LSCC (P<0.01) and
vocal leukoplakia (P<0.01) (Fig.
1A), while the level of plasma IL-10 was found to increase with
the cancer staging. Furthermore, plasma IL-10 concentrations were
significantly higher in the patients with lymph node metastasis
(27.95±5.7 pg/ml) compared with those without metastasis (22.66±4.4
pg/ml) (P=0.01; Fig. 1C). The
present findings indicate that the GG/CC/CC genotype is more common
in patients with high IL-10 production, whereas AA/TT/AA is
associated with low IL-10 production (Fig. 2A), which is consistent with the
observations of McCarron et al (25). In addition, the haplotype containing
the G allele was associated with higher plasma IL-10 concentrations
(24.33±5.7 pg/ml) compared with the ATA haplotype (19.88±4.7 pg/ml)
(P=0.008; Fig. 2B).
The association of cigarette smoking with an
increased risk of LSCC and vocal leukoplakia was also found in the
present study (LSCC: OR, 6.33; 95% CI, 3.7–10.8; P<0.01; vocal
leukoplakia: OR, 4.73; 95% CI, 2.4–9.1; P<0.01). A similar
association was identified for heavy alcohol consumption (LSCC: OR,
5.67; 95% CI, 3.3–9.9; P<0.01; vocal leukoplakia: OR, 5.70; 95%
CI, 2.9–11.2; P<0.01). Thus, the present data indicates that
environmental factors play a significant role in the pathogenesis
of LSCC and vocal leukoplakia.
Certain limitations are present in the current
study. First, other laryngeal carcinoma pathological types were not
taken into account. Second, the sample size in the study was not
large. Therefore, further studies should be conducted to further
verify the results. However, the data further confirm that IL-10
levels are affected by numerous factors associated with the age and
lifestyle of a patient, in accordance with the results of a
previous study (26), although, the
underlying mechanisms remain to be elucidated.
In conclusion, the present study indicates that the
−592 A/C, −819 T/C and −1082 A/G polymorphisms of the IL-10 gene
and increased plasma IL-10 levels are associated with an increased
risk for LSCC and vocal leukoplakia. In addition, with the
development of LSCC, higher IL-10 plasma levels and higher OR
values of the −592 A/C, −819 T/C, −1082 A/G polymorphisms were
found in the advanced LSCC patients and the patients with lymph
node metastasis.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (30801283 and 30972691), the Shanghai
Science and Technology Development Funds (09QA1401000 and
10QA1405900), the Training Program of the Excellent Young Talents
of the Shanghai Municipal Health System (XYQ2011055 and XYQ2011015)
and the Shanghai Municipal Science and Technology Foundation
(11JC1410802).
References
|
1
|
Hashibe M, Brennan P, Benhamou S, et al:
Alcohol drinking in never users of tobacco, cigarette smoking in
never drinkers, and the risk of head and neck cancer: pooled
analysis in the International Head and Neck Cancer Epidemiology
Consortium. J Natl Cancer Inst. 99:777–789. 2007. View Article : Google Scholar
|
|
2
|
Moyer JS, Wolf GT and Bradford CR: Current
thoughts on the role of chemotherapy and radiation in advanced head
and neck cancer. Curr Opin Otolaryngol Head Neck Surg. 12:82–87.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Boccia S, Cadoni G, Sayed-Tabatabaei FA,
et al: CYP1A1, CYP2E1, GSTM1, GSTT1, EPHX1 exons 3 and 4, and NAT2
polymorphisms, smoking, consumption of alcohol and fruit and
vegetables and risk of head and neck cancer. J Cancer Res Clin
Oncol. 134:93–100. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
de Waal Malefyt R, Abrams J, Bennett B,
Figdor CG and de Vries JE: Interleukin 10(IL-10) inhibits cytokine
synthesis by human monocytes: an autoregulatory role of IL-10
produced by monocytes. J Exp Med. 174:1209–1220. 1991.PubMed/NCBI
|
|
5
|
Moore KW, de Waal Malefyt R, Coffman RL
and O’Garra A: Interleukin-10 and the interleukin-10 receptor. Ann
Rev Immunol. 19:683–765. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Akdis CA and Blaser K: Mechanisms of
interleukin-10-mediated immune suppression. Immunology.
103:131–136. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu MS, Huang SP, Chang YT, et al: Tumor
necrosis factor-alpha and interleukin-10 promoter polymorphisms in
Epstein-Barr virus-associated gastric carcinoma. J Infect Dis.
185:106–109. 2002. View
Article : Google Scholar
|
|
8
|
Turner DM, Williams DM, Sankaran D,
Lazarus M, Sinnott PJ and Hutchinson IV: An investigation of
polymorphism in the interleukin-10 gene promoter. Eur J
Immunogenet. 24:1–8. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Crawley E, Kay R, Sillibourne J, Patel P,
Hutchinson I and Woo P: Polymorphic haplotypes of the
interleukin-10 5′ flanking region determine variable interleukin-10
transcription and are associated with particular phenotypes of
juvenile rheumatoid arthritis. Arthritis Rheum. 42:1101–1108.
1999.
|
|
10
|
Edwards-Smith CJ, Jonsson JR, Purdie DM,
Bansal A, Shorthouse C and Powell EE: Interleukin-10 promoter
polymorphism predicts initial response of chronic hepatitis C to
interferon alfa. Hepatology. 30:526–530. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shimoyama T, Everett SM, Fukuda S, Axon
AT, Dixon MF and Crabtree JE: Influence of smoking and alcohol on
gastric chemokine mRNA expression in patients with Helicobacter
pylori infection. J Clin Pathol. 54:332–334. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Seifart C, Plagens A, Dempfle A, et al:
TNF-alpha, TNF-beta, IL-6, and IL-10 polymorphisms in patients with
lung cancer. Dis Markers. 21:157–165. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Erdogan M, Karadeniz M, Ozbek M, Ozgen AG
and Berdeli A: Interleukin-10 gene polymorphism in patients with
papillary thyroid cancer in Turkish population. J Endocrinol
Invest. 31:750–754. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kesarwani P, Ahirwar DK, Mandhani A, et
al: IL-10 −1082 G>A: a risk for prostate cancer but may be
protective against progression of prostate cancer in North Indian
cohort. World J Urol. 27:389–396. 2009.
|
|
15
|
Matsumoto K, Oki A, Satoh T, et al:
Interleukin-10 −1082 gene polymorphism and susceptibility to
cervical cancer among Japanese women. Jpn J Clin Oncol.
40:1113–1116. 2010.
|
|
16
|
Zhou Y, Hu W, Zhuang W and Wu X:
Interleukin-10 −1082 promoter polymorphism and gastric cancer risk
in a Chinese Han population. Mol Cell Biochem. 347:89–93. 2011.
|
|
17
|
Wu MS, Wu CY, Chen CJ, Lin MT, Shun CT and
Lin JT: Interleukin-10 genotypes associate with the risk of gastric
carcinoma in Taiwanese Chinese. Int J Cancer. 104:617–623. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gibson AW, Edberg JC, Wu J, Westendorp RG,
Huizinga TW and Kimberly RP: Novel single nucleotide polymorphisms
in the distal IL-10 promoter affect IL-10 production and enhance
the risk of systemic lupus erythematosus. J Immunol. 166:3915–3922.
2001. View Article : Google Scholar
|
|
19
|
Fortis C, Foppoli M, Gianotti L, et al:
Increased interleukin-10 serum levels in patients with solid
tumours. Cancer Lett. 104:1–5. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Karcher J, Reisser C, Daniel V and
Herold-Mende C: Cytokine expression of transforming growth
factor-beta2 and interleukin-10 in squamous cell carcinomas of the
head and neck. Comparison of tissue expression and serum levels.
HNO. 47:879–884. 1999.(In German).
|
|
21
|
Fujieda S, Sunaga H, Tsuzuki H, Fan GK and
Saito H: IL-10 expression is associated with the expression of
platelet-derived endothelial cell growth factor and prognosis in
oral and oropharyngeal carcinoma. Cancer Lett. 136:1–9. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zambon CF, Basso D, Navaglia F, et al:
Pro- and anti-inflammatory cytokines gene polymorphisms and
Helicobacter pylori infection: interactions influence
outcome. Cytokine. 29:141–152. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rad R, Dossumbekova A, Neu B, et al:
Cytokine gene polymorphisms influence mucosal cytokine expression,
gastric inflammation, and host specific colonisation during
Helicobacter pylori infection. Gut. 53:1082–1089. 2004.
View Article : Google Scholar
|
|
24
|
Suzuki S, Muroishi Y, Nakanishi I and Oda
Y: Relationship between genetic polymorphisms of drug-metabolizing
enzymes (CYP1A1, CYP2E1, GSTM1, and NAT2), drinking habits,
histological subtypes, and p53 gene point mutations in Japanese
patients with gastric cancer. J Gastroenterol. 39:220–230. 2004.
View Article : Google Scholar
|
|
25
|
McCarron SL, Edwards S, Evans PR, Gibbs R,
Dearnaley DP, Dowe A, Southgate C, Easton DF, Eeles RA and Howell
WM: Influence of cytokine gene polymorphisms on the development of
prostate cancer. Cancer Res. 62:3369–3372. 2002.PubMed/NCBI
|
|
26
|
Gravitt PE, Hildesheim A, Herrero R, et
al: Correlates of IL-10 and IL-12 concentrations in cervical
secretions. J Clin Immunol. 23:175–183. 2003. View Article : Google Scholar : PubMed/NCBI
|