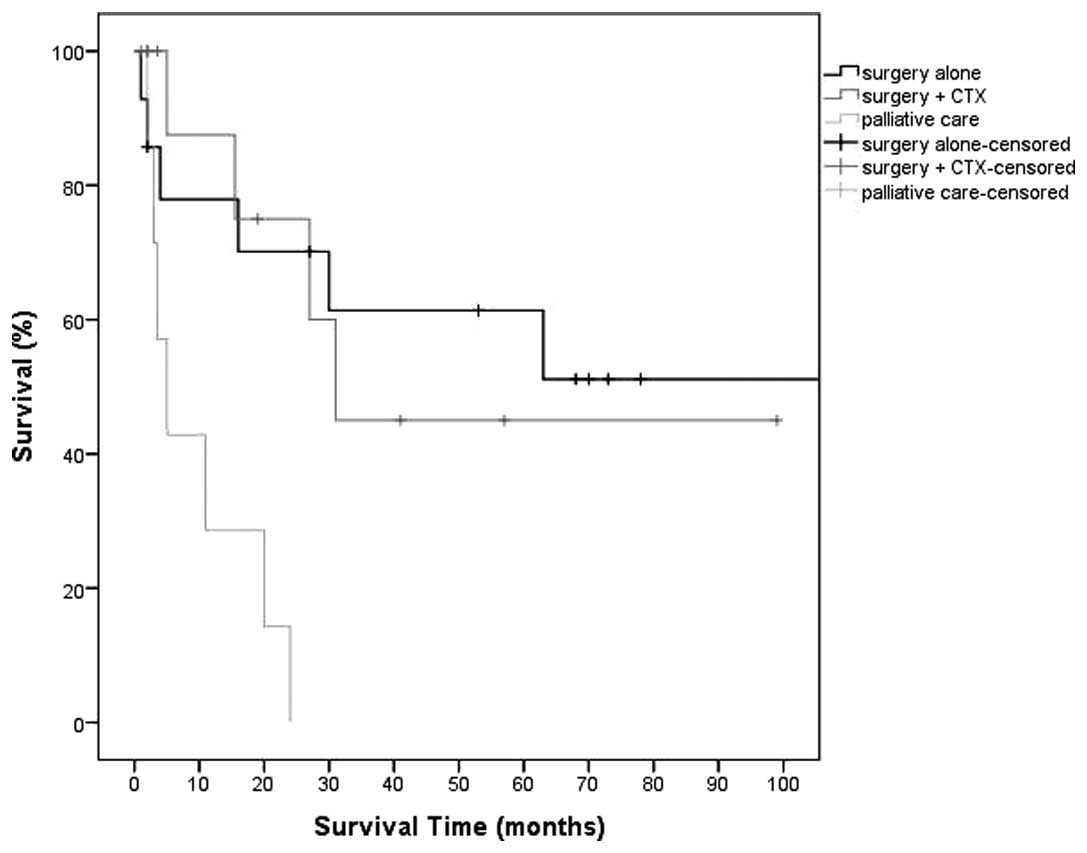
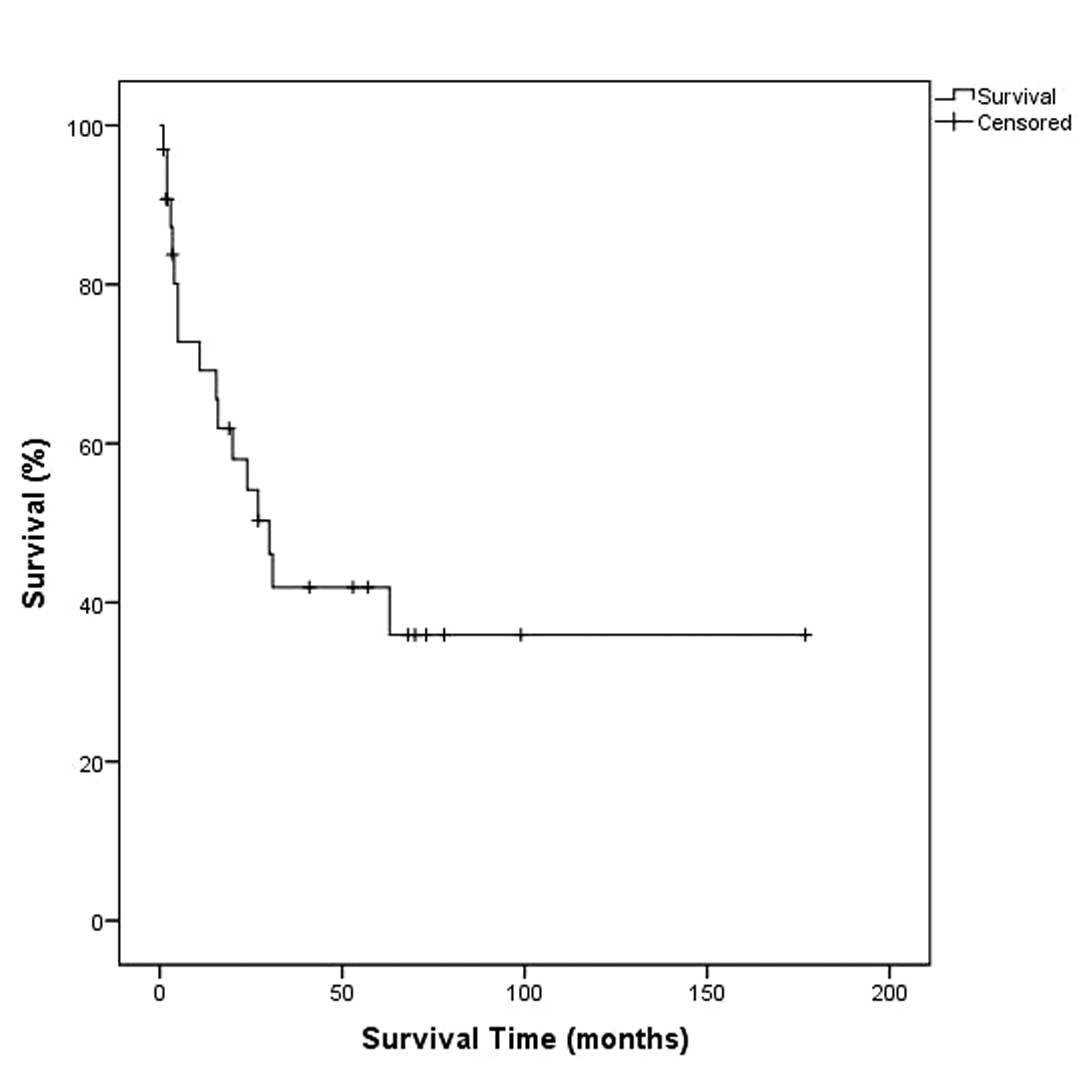
|
1
|
Poddar N, Raza S, Sharma B, Liu M, Gohari
A and Kalavar M: Small bowel adenocarcinoma presenting with
refractory iron deficiency anemia - case report and review of
literature. Case Rep Oncol. 4:458–463. 2011. View Article : Google Scholar
|
|
2
|
Lu Y, Fröbom R and Lagergren J: Incidence
patterns of small bowel cancer in a population-based study in
Sweden: increase in duodenal adenocarcinoma. Cancer Epidemiol.
36:e158–e163. 2012. View Article : Google Scholar
|
|
3
|
Chang HK, Yu E, Kim J, et al:
Adenocarcinoma of the small intestine: a multi-institutional study
of 197 surgically resected cases. Hum Pathol. 41:1087–1096. 2010.
View Article : Google Scholar
|
|
4
|
Pan SY and Morrison H: Epidemiology of
cancer of the small intestine. World J Gastrointest Oncol. 3:33–42.
2011.PubMed/NCBI
|
|
5
|
Overman MJ: Recent advances in the
management of adenocarcinoma of the small intestine. Gastrointest
Cancer Res. 3:90–96. 2009.PubMed/NCBI
|
|
6
|
Koo DH, Yun SC, Hong YS, et al: Adjuvant
chemotherapy for small bowel adenocarcinoma after curative surgery.
Oncology. 80:208–213. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cao J, Zuo Y, Lv F, Chen Z and Li J:
Primary small intestinal malignant tumors: survival analysis of 48
postoperative patients. J Clin Gastroenterol. 42:167–173. 2008.
View Article : Google Scholar
|
|
8
|
Halfdanarson TR, McWilliams RR, Donohue JH
and Quevedo JF: A single-institution experience with 491 cases of
small bowel adenocarcinoma. Am J Surg. 199:797–803. 2010.
View Article : Google Scholar
|
|
9
|
Overman MJ, Kopetz S, Lin E, Abbruzzese JL
and Wolff RA: Is there a role for adjuvant therapy in resected
adenocarcinoma of the small intestine. Acta Oncol. 49:474–479.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dabaja BS, Suki D, Pro B, Bonnen M and
Ajani J: Adenocarcinoma of the small bowel: presentation,
prognostic factors, and outcome of 217 patients. Cancer.
101:518–526. 2004. View Article : Google Scholar
|
|
11
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: the 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hong SH, Koh YH, Rho SY, et al: Primary
adenocarcinoma of the small intestine: presentation, prognostic
factors and clinical outcome. Jpn J Clin Oncol. 39:54–61. 2009.
View Article : Google Scholar
|
|
13
|
Kelsey CR, Nelson JW, Willett CG, et al:
Duodenal adenocarcinoma: patterns of failure after resection and
the role of chemoradiotherapy. Int J Radiat Oncol Biol Phys.
69:1436–1441. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bakaeen FG, Murr MM, Sarr MG, et al: What
prognostic factors are important in duodenal adenocarcinoma? Arch
Surg. 135:635–641; discussion 641–632. 2000. View Article : Google Scholar
|
|
15
|
Poultsides GA, Huang LC, Cameron JL, et
al: Duodenal adenocarcinoma: clinicopathologic analysis and
implications for treatment. Ann Surg Oncol. 19:1928–1935. 2012.
View Article : Google Scholar
|
|
16
|
Sasaki Y, Natsuizaka M, Takano M, et al: A
case of primary adenocarcinoma of small intestine with multiple
liver metastases successfully treated with mFOLFOX6. Gan To Kagaku
Ryoho. 36:1927–1929. 2009.(In Japanese).
|
|
17
|
Yamano T, Morii E, Arai I, Takada T and
Aozasa K: Successful treatment of recurrent small bowel
adenocarcinoma by cytoreductive surgery and chemotherapy: a case
report and review of the literature. J Med Case Rep. 4:2132010.
View Article : Google Scholar
|
|
18
|
Tsushima T, Taguri M, Honma Y, et al:
Multicenter retrospective study of 132 patients with unresectable
small bowel adenocarcinoma treated with chemotherapy. Oncologist.
17:1163–1170. 2012. View Article : Google Scholar
|
|
19
|
Xiang XJ, Liu YW, Zhang L, et al: A phase
II study of modified FOLFOX as first-line chemotherapy in advanced
small bowel adenocarcinoma. Anticancer Drugs. 23:561–566. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang L, Wang LY, Deng YM, et al: Efficacy
of the FOLFOX/CAPOX regimen for advanced small bowel
adenocarcinoma: a three-center study from China. J BUON.
16:689–696. 2011.
|
|
21
|
Zaanan A, Gauthier M, Malka D, et al:
Second-line chemotherapy with fluorouracil, leucovorin, and
irinotecan (FOLFIRI regimen) in patients with advanced small bowel
adenocarcinoma after failure of first-line platinum-based
chemotherapy: a multicenter AGEO study. Cancer. 117:1422–1428.
2011. View Article : Google Scholar
|
|
22
|
Tsang H, Yau T, Khong PL and Epstein RJ:
Bevacizumab-based therapy for advanced small bowel adenocarcinoma.
Gut. 57:1631–1632. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
De Dosso S, Molinari F, Martin V, Frattini
M and Saletti P: Molecular characterisation and cetuximab-based
treatment in a patient with refractory small bowel adenocarcinoma.
Gut. 59:1587–1588. 2010.PubMed/NCBI
|