Introduction
Despite an increasing incidence, particularly in
duodenal adenocarcinoma, small intestine malignancies are rare,
accounting for ~2% of all gastrointestinal tumors. Adenocarcinoma
is the most common histopathological subtype (1), followed by carcinoid tumors, lymphomas
and sarcomas (2,3).
In accordance with the Robert Koch Institute in
Germany, ~0.33 per 105 males and 0.24 per 105
females are diagnosed with primary adenocarcinoma of the small
intestine each year. The incidence rates of small bowel
adenocarcinoma (SBA) in the U.S. population are 1.45 and 1.00 for
males and females per 105 individuals each year,
respectively (4).
SBA is 40–50 times less common than colorectal
carcinoma, although the small intestine accounts for 70–80% of the
total length and ~90% of the overall surface of the
gastrointestinal tract (5). The
reason for this distinct difference in incidence remains unclear.
In total, ~50% of all SBA are located in the duodenum, most
commonly in the second portion near to the papilla of Vater, 30%
arise in the jejunum and the remaining fifth occur within the
ileum. Diagnosis is mainly determined in middle-aged to elderly
patients (in their fifth and sixth decades of life) with higher
prevalence rates in individuals of African descent than in
Caucasians (1).
The most important predisposing condition recognized
for SBA is Crohn’s disease, followed by celiac disease, Meckel’s
diverticulum, intestinal duplication and hereditary cancer
syndromes [i.e. hereditary intestinal polyposis syndrome
(Peutz-Jeghers syndrome), familial adenomatous polyposis,
hereditary non-polyposis colon cancer syndrome and familial
colorectal polyposis (Gardner syndrome)]. Known independent
prognostic factors indicating poor outcome are lymph node
metastasis ratio and distal tumor location (i.e. jejunum and ileum)
(3,6,7).
Following a review of 491 cases of SBA, the Mayo
Clinic reported that higher age, male gender, increased TNM stage
and grade, residual disease following resection and a lymph node
ratio of ≥50% predict decreased overall survival (OS) in univariate
analysis, with age and TNM staging being predictive for survival in
multivariate analysis (8).
Previously, Overman et al demonstrated a distinctly poorer
cancer-specific survival in SBA than in large bowel adenocarcinoma
(9).
The ongoing poor prognosis of SBA with an overall
five-year survival rate of ~25%, even following complete surgical
(R0) resection and adequate lymphadenectomy, is mainly attributable
to vague, non-specific symptoms, varying accessibility to endoscopy
and the lack of evidence-based diagnostic procedures resulting in
long latency time to diagnosis (10). Thus, despite increasing advantages
in radiographic imaging, early detection of small bowel neoplasms
remains infrequent and the majority of patients present with
already unresectable or metastatic disease (1). Due to the rarity of these tumors,
there is an ongoing lack of sufficient data characterizing this
patient population adequately.
The molecular characterization of colorectal cancer
has led to a differentiated understanding of tumorigenesis and has
resulted in a revolution and individualization of therapy options.
Recent literature provides little data on the etiopathogenesis,
tumor biology and molecular pathways of SBA. A more sophisticated
understanding of carcinogenesis is essential for further hypothesis
generation and the development of new individualized and targeted
therapeutic approaches. To establish treatment guidelines and
define predictors of prognosis, translational study on SBA is
highly warranted. Surgery is the mainstay of therapy. Concerning
adjuvant chemotherapy (CTX), only a few recommendations with higher
levels of evidence are available and its role for resectable
carcinoma remains unclear (5).
Thus, data on first line CTX regimen in advanced stages are also
scarce.
The current study presents a consecutively collected
case series of 33 SBA patients. The aim of the study was to share
our experience of SBA treatment as a high-volume center, and to
provide a potential basis for multinational data pooling and
cross-national research collaboration.
Materials and methods
Patient characteristics
A database of all patients with histologically
verified malignancy of the small intestine, who were diagnosed at
the Department of Surgery, Medical University of Vienna (Vienna,
Austria), between 1994 and 2012, was established. All tumors others
than primary adenocarcinoma of the small intestine were excluded.
Since primary adenocarcinoma of the major duodenal papilla
represent a separate tumor entity, those tumors were also excluded.
This led to an inclusion of 33 patients, who were reviewed for
demographic data (age, gender and comorbidities), baseline
characteristics (clinical manifestation and primary complaints),
predisposing conditions and prognostic factors, tumor features,
preoperative diagnostics, surgical and medical treatment patterns,
and outcome parameters. The study protocol was approved by the
ethics committee of the Medical University of Vienna (no. EC
242/2009).
Pathohistological analysis
Final SBA diagnosis was determined by
pathohistological analysis performed at the Clinical Institute of
Pathology, Medical University of Vienna.
Statistical analysis
Statistical calculations were performed using IBM
SPSS® statistics 19.0 (SPSS, Inc., Chicago, IL, USA).
Data are presented as the means ± SD or the median and
interquartile range (IQR), respectively. OS rates were calculated
using the Kaplan-Meier method and defined as the time from surgical
resection to mortality or last follow-up visit. A two-sided P-value
of <0.05 was considered to indicate a statistically significant
difference.
Results
Patient analysis
A total of 33 patients (20 males and 13 females)
were diagnosed with primary SBA at a median age of 63 years (IQR,
24–83 years) between 1994 and 2012 at the Department of Surgery,
Medical University of Vienna. The most common tumor sites were the
duodenum (n=20; 60.6%) and jejunum (n=8; 24.2%) (Table I). Frequent observations at initial
admission were sporadic abdominal discomfort (n=21; 63.6%),
including abdominal pain and fullness and meteorism, as well as
hypo- or normochromatic anemia (mean hemoglobin level, 10.4 mg/dl)
due to occult blood loss (n=15; 45.5%). Overall weight loss was
documented in 14 cases (42.4%; mean loss, 11.5 kg over 3 weeks). In
total, four patients presented with clinical signs of gastric
orifice stenosis and three patients were admitted to our
institution complaining of vomiting, nausea and abdominal pain
caused by mechanical bowel obstruction. One patient suffered from
chest pain and dyspnea due to thromboembolic disease, likely caused
by underlying malignant disease.
 | Table IClinicopathohistological and
therapeutic data: Survivors vs. non-survivors. |
Table I
Clinicopathohistological and
therapeutic data: Survivors vs. non-survivors.
| Variables (n=33) | Survivors (n=16) | Non-survivors
(n=17) |
|---|
| Median age (IQR) | 63 (24–78) | 63 (42–83) |
| Gender, n (%) |
| Male | 8 (50.0) | 12 (70.6) |
| Female | 8 (50.0) | 5 (29.4) |
| Tumor site, n
(%) |
| Duodenum | 9 (56.3) | 11 (64.7) |
| Duodenojejunal
junction | 1 (6.3) | 1 (5.9) |
| Jejunum | 5 (31.3) | 3 (17.6) |
| Ileum | 0 | 1 (5.9) |
| NOS | 1 (6.3) | 1 (5.9) |
| TNM grading, n
(%) |
| Poor (G3) | 4 (25.0) | 8 (47.1) |
| Moderate (G2) | 9 (56.3) | 9 (52.9) |
| High (G1) | 3 (18.8) | 0 |
| Carcinosis peritonei,
n (%) | 0 | 9 (60.0) |
| R-status, n (%) |
| R0 | 14 (87.5) | 9 (52.9) |
| R1 | 1 (6.3) | 0 |
| No R-status
(palliative GE) | 0 | 5 (29.4) |
| NOS | 1 (6.3) | 3 (17.6) |
| pT, n (%) |
| pT1 | 1 (6.3) | 0 |
| pT2 | 0 | 0 |
| pT3 | 11 (68.8) | 1 (5.9) |
| pT4 | 3 (18.8) | 10 (58.8) |
| No surgery | 0 | 2 (11.8) |
| NOS | 1 (6.3) | 4 (23.5) |
| pN, n (%) |
| pN0 | 8 (50.0) | 5 (29.4) |
| pN1/2 | 5 (31.3) | 9 (52.9) |
| NOS | 3 (18.8) | 3 (17.6) |
| Chemotherapy, n
(%) |
| Adjuvant CTX | 8 (50.0) | 4 (23.2) |
| Palliative CTX | 0 | 5 (29.4) |
| None | 8 (50.0) | 8 (47.1) |
| Surgical treatment, n
(%) |
| Segmental
resection | 14 (87.5) | 6 (35.3) |
| Whipplea | 2 (12.5) | 4 (23.5) |
| Palliative GE | 0 | 5 (29.4) |
| No surgery | 0 | 2 (11.8) |
Patient diagnosis
Initial diagnosis was determined mainly by
high-resolution computed tomography of the abdomen, followed by or
based on an esophagogastroduodenoscopy procedure.
SBA predisposing conditions were found in three
cases, consisting of Morbus Crohn, celiac disease and familial
adenomatous polyposis. With regard to metachronous malignant
neoplasms, one gastric cancer and two types of colorectal cancer
were identified.
Elevated β2-microglobulin levels were found in nine
of the 12 patients (mean, 2.0 mg/l; reference range, 0–1.9 mg/l),
pathological CA 19-9 values were measured in seven of the 24
patients (mean, 506.4 kU/l; reference range, 0–37 kU/l) and an
increased CEA level was observed in only five out of 25 patients
(mean, 24.8 μg/l; reference range, 0–3.4 μg/l).
Treatment and tumor classification
All patients, with the exception of two, underwent
surgical treatment. In total, 26 patients were treated with primary
curative intent, including adequate lymphadenectomy, of whom the
majority received small bowel segmental resection (n=20; 60.0%) and
six (18.2%) patients received partial pancreatoduodenectomy with
Y-Roux anastomosis. In five cases (15.2%), a palliative
gastroenterostomy was accomplished (Table II).
 | Table IITherapeutic procedures: Survivors vs.
non-survivors. |
Table II
Therapeutic procedures: Survivors vs.
non-survivors.
| Therapeutic
procedures | Survivors (n=16), n
(%) | Non-survivors (n=17),
n (%) |
|---|
| Whipplea | 1 (6.3) | 3 (17.6) |
| Whipplea + CTX | 1 (6.3) | 1 (5.9) |
| Segmental
resection | 7 (43.8) | 3 (17.6) |
| Segmental resection +
CTX | 7 (43.8) | 3 (17.6) |
| GE | 0 | 2 (11.8) |
| GE + CTX | 0 | 3 (17.6) |
| Palliative CTX | 0 | 2 (11.6) |
R0 resection was performed in 23 patients (88.5%),
while in one case (3.8%), only incomplete resection was achieved.
Regardless of surgical procedure, lymph node metastases were found
in 14 patients (45.2%), while 13 patients (41.9%) were staged as
pN0 (Table I).
According to the Union for International Cancer
Control TNM classification (11),
75.8% of patients were staged as pT3 (36.4%) or pT4 (39.4%) and
nine patients suffered from carcinosis peritonei (27.3%).
Histopathologically, adenocarcinoma were classified into
well-differentiated (G1; n=3; 9.1%), moderately differentiated (G2;
n=18; 54.5%) and poorly differentiated (G3; n=12; 36.4%)
groups.
Postoperative complications occurred in six patients
(19.4%), including four anastomotic leaks (12.9%) and two wound
infections (6.5%). According to the Clavien-Dindo classification,
four patients were staged as IIIb, two patients as stage I and the
remaining 25 surgically treated individuals were staged as 0.
CTX was performed in 17 cases (51.5%). The majority
of patients were subjected to oxaliplatin and capecitabine (XELOX
or CAPOX), fluorouracil, leucovorin and irinotecan (FOLFIRI) and
folinic acid, fluorouracil and oxaliplatin (FOLFOX) regimens with
no detectable differences in terms of survival.
One patient received gemcitabine neoadjuvantly,
followed by performance of a gastroenterostomy with
hepaticojejunostomy and adjuvant XELOX application.
Follow-up
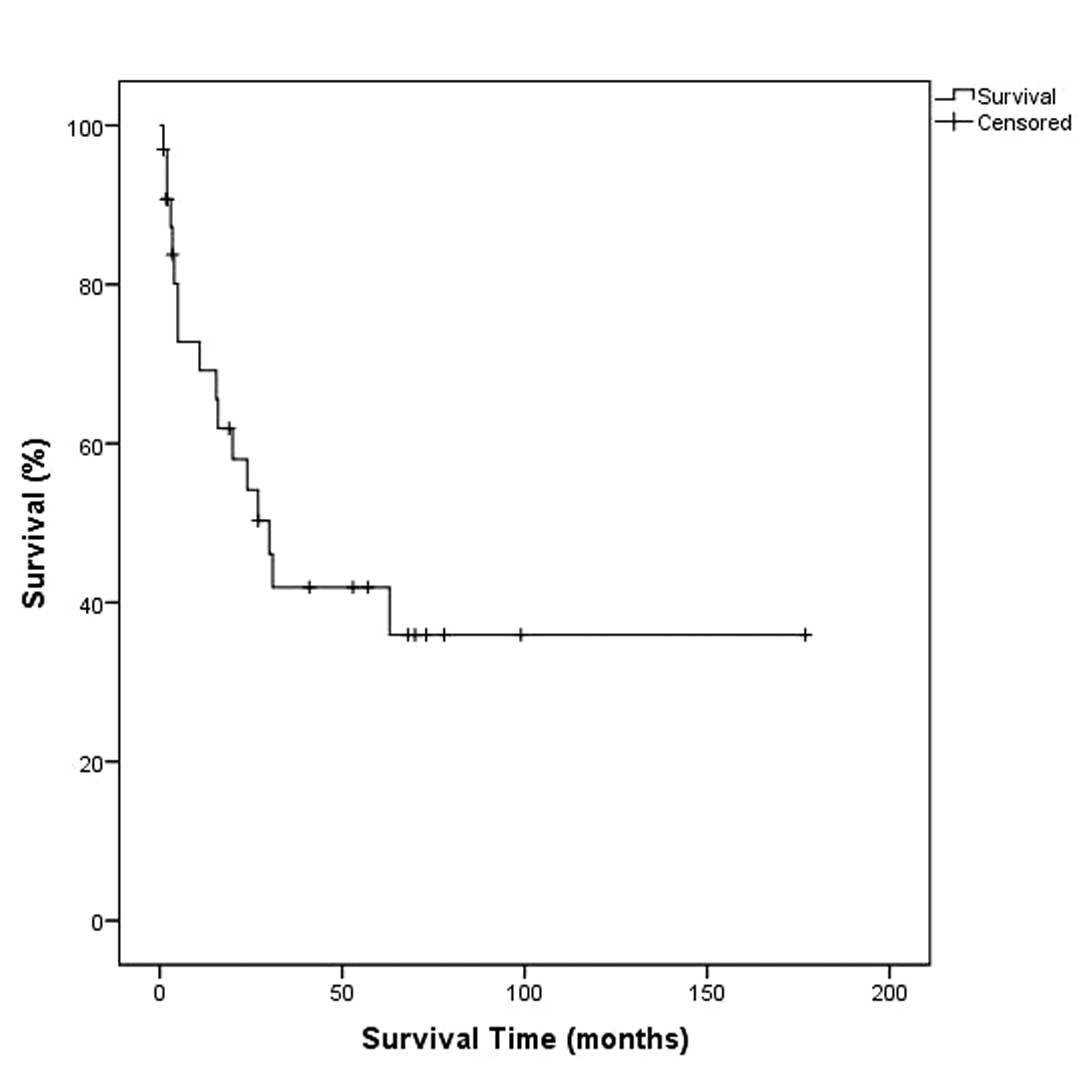
Within a mean follow-up period of 31.4 months and
following a median survival time of 11 months, 17 patients (51.5%)
succumbed to the disease, of whom five had received primary
palliative surgical care (gastroenterostomy) while two patients had
not undergone any surgical therapy.
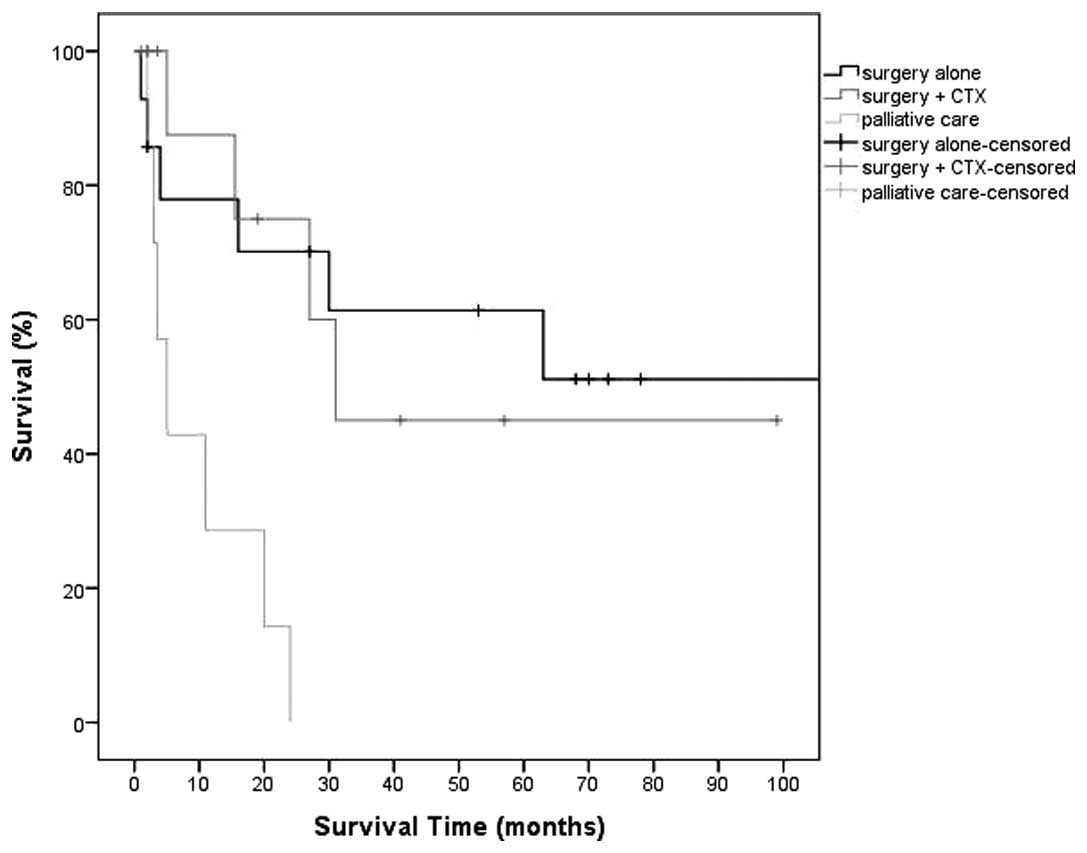
Following selective surgery, the mean OS was 47.4
months and the mean survival time was 19.3 months, while the mean
OS was 25.3 months and the mean survival time was 19.6 months for
those who had received adjuvant CTX. The remaining patients, who
all underwent palliative care and all succumbed to the disease
within the follow-up period, exhibited a mean OS of 9.8 months
(Table III; Figs. 1 and 2).
 | Table IIIPatient characteristics and outcome
data with regard to treatment performed. |
Table III
Patient characteristics and outcome
data with regard to treatment performed.
| Characteristics
(n=33) | Surgery (n=14) | Surgery + CTX
(n=12) | Palliative therapy
(n=7) |
|---|
| Age, years, n | 67.5 | 57.3 | 59.7 |
| Males, n (%) | 7 (50.0) | 8 (66.7) | 5 (71.4) |
| pT3, n (%) | 7 (50.0) | 5 (41.7) | 0 |
| pT4, n (%) | 4 (28.6) | 6 (50.0) | 3 (42.9) |
| pN >0, n
(%) | 5 (35.7) | 5 (41.6) | 4 (57.1) |
| R0, n (%) | 13 (92.9) | 10 (83.3) | 0 |
| Carcinosis, n
(%) | 1 (7.7) | 3 (25) | 5 (83.3) |
| Mean OS,
months | 47.4 | 25.3 | 9.8 |
| Mean survival time,
months | 19.3 | 19.6 | 9.8 |
Discussion
SBA carries a poor prognosis and diagnosis is
usually determined extremely late at advanced stage. At present, no
established screening methods or diagnosis protocols exist and SBA
remains a diagnostic and therapeutic challenge with a markedly
increasing incidence during the last 50 years (2). The non-specificity of initial clinical
complaints and, in part, the endoscopic inaccessibility are major
factors substantially contributing to the delayed diagnosis with an
average latency time of ~8.2 months, resulting in a distribution of
pT staging of ~90% pT3 or pT4 at initial diagnosis (3). The implementation of evidence-based
procedures for detection at an early stage is urgently required to
enhance resectability rates.
In the current series, patients mostly suffered from
fatigue or dyspnea due to anemia or from non-specific abdominal
discomfort. Accordingly, Poddar et al recently emphasized
the importance of considering SBA as a possible underlying cause of
unexplained iron deficiency anemia (1).
Early surgery represents the mainstay in therapy and
the only opportunity for cure (10). There is an overt lack of
evidence-based therapeutic recommendations, but no consensus exists
on the adequate treatment of these malignancies. This is largely
due to the rarity of SBA. Consecutively, studies performed to date,
comprising only small sample sizes, are less conclusive.
Furthermore, the role of adjuvant CTX in curatively
resectable SBA remains unclear and no standard first line CTX
regimen for advanced disease has yet been established. According to
the previous literature, only non-significant survival benefits
have been shown for adjuvant CTX (7,8,10,12–17)
and, to date, no prospective trials addressing this issue have been
published. In 2010, a retrospective study performed by Overman
et al demonstrated an improvement in disease-free survival
(P=0.05), but not in OS (P=0.23) following R0 resection (9).
The only factor which has been found to
significantly correlate with OS is the clinical tumor stage
(7). The application of adjuvant
CTX in the present series was associated with lower OS than that
following selective surgery alone (mean OS, 25.3, vs. 47.4
months).
By contrast, for palliative CTX, a beneficial effect
has been demonstrated in various trials (10,12),
particularly for fluoropyrimidine/oxaliplatin and modified FOLOFOX
regimens (18,19). Furthermore, in late 2011, FOLFOX and
CAPOX were confirmed effective with tolerable toxicity for advanced
SBA (20,21).
Palliative surgical and/or chemotherapeutical
treatment was applied to 21.2% of patients, either to reduce
tumor-related intestinal obstruction or to slow down progression.
Overall prognosis was poor and palliative treated patients showed a
mean OS of only 9.8 months.
In 2007, studies on neoadjuvant radiochemotherapy
showed the therapy had a tendency to improve OS rates in patients
undergoing R0 resection compared with those who received selective
surgical treatment (five-year OS, 83 vs. 53%; P=0.07) (13). Only one of the current patients
received neoadjuvant CTX by application of gemcitabine, but a
combined radiation therapy was not performed.
With regard to targeted therapies, particularly
anti-epidermal growth factor receptor drugs, current literature
only provides case reports (22,23).
In 2008, Tsang et al described that the use of bevacizumab
combined with gemcitabine and oxaliplatin in a patient with
advanced unresectable SBA was beneficial. This lead to disease
stabilization with a survival time of at least one year following
diagnosis, considering a mean survival time of only 8–9 months in
advanced SBA treated with standard chemotherapeutical care
(22). One of the patients of the
present study received bevacizumab combined with FOLFIRI, followed
by FOLFOX regimen, in a palliative setting leading to a survival
rate of 24 months.
In 2010, Overman et al reported cetuximab to
be a promising candidate in the future SBA-treatment (9). However, prospective trials covering
this topic are required.
Concordant with the current literature, the present
study found that the presence of peritoneal carcinosis, lymph node
metastases and increased disease stage impaired patient outcome.
Furthermore, even following adequate surgical treatment, including
R0 resection and sufficient lymphadenectomy, recurrence remained
high leading to low survival rates. Although a multimodal
therapeutic approach has been confirmed as the gold standard in the
majority of solid malignancies, its value in the treatment of SBA
has not yet been defined. With the exception of palliative CTX in
unresectable stages, adjuvant CTX showed no survival benefit in the
current series. The mean OS was longer in patients who had
undergone selective surgery compared with patients who had received
additive CTX.
Since the current literature does not provide any
recommendations on tumor marker determination in SBA patients our
records are incomplete. Due to non-specific initial symptoms and
irregular observations in tumor marker levels, screening
improvements were not detected in the present study.
SBA is rare but presents malignancies with extremely
poor prognosis and often delayed diagnosis due to non-specific
clinical signs and a lack of sufficient screening methods. As
surgery remains the mainstay of treatment, the most important
factor with regard to survival is R0 resection based on early
diagnosis followed by early performed surgery. The role of adjuvant
CTX in SBA treatment remains a matter of debate since no
significant survival benefit has yet been confirmed. Encouraging
translational study is inevitable for further hypothesis generation
and for the development of new therapeutic approaches.
The results of the present study highlighted the
urgent requirement for international collaboration and
cross-national data provision to gain sufficient information on
this specific patient population. We therefore demand a
multinational data pooling to make a first step towards
comprehensive information gathering, enabling us to offer the best
evidence-based medical care to patients suffering from primary SBA
in the future.
References
|
1
|
Poddar N, Raza S, Sharma B, Liu M, Gohari
A and Kalavar M: Small bowel adenocarcinoma presenting with
refractory iron deficiency anemia - case report and review of
literature. Case Rep Oncol. 4:458–463. 2011. View Article : Google Scholar
|
|
2
|
Lu Y, Fröbom R and Lagergren J: Incidence
patterns of small bowel cancer in a population-based study in
Sweden: increase in duodenal adenocarcinoma. Cancer Epidemiol.
36:e158–e163. 2012. View Article : Google Scholar
|
|
3
|
Chang HK, Yu E, Kim J, et al:
Adenocarcinoma of the small intestine: a multi-institutional study
of 197 surgically resected cases. Hum Pathol. 41:1087–1096. 2010.
View Article : Google Scholar
|
|
4
|
Pan SY and Morrison H: Epidemiology of
cancer of the small intestine. World J Gastrointest Oncol. 3:33–42.
2011.PubMed/NCBI
|
|
5
|
Overman MJ: Recent advances in the
management of adenocarcinoma of the small intestine. Gastrointest
Cancer Res. 3:90–96. 2009.PubMed/NCBI
|
|
6
|
Koo DH, Yun SC, Hong YS, et al: Adjuvant
chemotherapy for small bowel adenocarcinoma after curative surgery.
Oncology. 80:208–213. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cao J, Zuo Y, Lv F, Chen Z and Li J:
Primary small intestinal malignant tumors: survival analysis of 48
postoperative patients. J Clin Gastroenterol. 42:167–173. 2008.
View Article : Google Scholar
|
|
8
|
Halfdanarson TR, McWilliams RR, Donohue JH
and Quevedo JF: A single-institution experience with 491 cases of
small bowel adenocarcinoma. Am J Surg. 199:797–803. 2010.
View Article : Google Scholar
|
|
9
|
Overman MJ, Kopetz S, Lin E, Abbruzzese JL
and Wolff RA: Is there a role for adjuvant therapy in resected
adenocarcinoma of the small intestine. Acta Oncol. 49:474–479.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dabaja BS, Suki D, Pro B, Bonnen M and
Ajani J: Adenocarcinoma of the small bowel: presentation,
prognostic factors, and outcome of 217 patients. Cancer.
101:518–526. 2004. View Article : Google Scholar
|
|
11
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: the 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hong SH, Koh YH, Rho SY, et al: Primary
adenocarcinoma of the small intestine: presentation, prognostic
factors and clinical outcome. Jpn J Clin Oncol. 39:54–61. 2009.
View Article : Google Scholar
|
|
13
|
Kelsey CR, Nelson JW, Willett CG, et al:
Duodenal adenocarcinoma: patterns of failure after resection and
the role of chemoradiotherapy. Int J Radiat Oncol Biol Phys.
69:1436–1441. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bakaeen FG, Murr MM, Sarr MG, et al: What
prognostic factors are important in duodenal adenocarcinoma? Arch
Surg. 135:635–641; discussion 641–632. 2000. View Article : Google Scholar
|
|
15
|
Poultsides GA, Huang LC, Cameron JL, et
al: Duodenal adenocarcinoma: clinicopathologic analysis and
implications for treatment. Ann Surg Oncol. 19:1928–1935. 2012.
View Article : Google Scholar
|
|
16
|
Sasaki Y, Natsuizaka M, Takano M, et al: A
case of primary adenocarcinoma of small intestine with multiple
liver metastases successfully treated with mFOLFOX6. Gan To Kagaku
Ryoho. 36:1927–1929. 2009.(In Japanese).
|
|
17
|
Yamano T, Morii E, Arai I, Takada T and
Aozasa K: Successful treatment of recurrent small bowel
adenocarcinoma by cytoreductive surgery and chemotherapy: a case
report and review of the literature. J Med Case Rep. 4:2132010.
View Article : Google Scholar
|
|
18
|
Tsushima T, Taguri M, Honma Y, et al:
Multicenter retrospective study of 132 patients with unresectable
small bowel adenocarcinoma treated with chemotherapy. Oncologist.
17:1163–1170. 2012. View Article : Google Scholar
|
|
19
|
Xiang XJ, Liu YW, Zhang L, et al: A phase
II study of modified FOLFOX as first-line chemotherapy in advanced
small bowel adenocarcinoma. Anticancer Drugs. 23:561–566. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang L, Wang LY, Deng YM, et al: Efficacy
of the FOLFOX/CAPOX regimen for advanced small bowel
adenocarcinoma: a three-center study from China. J BUON.
16:689–696. 2011.
|
|
21
|
Zaanan A, Gauthier M, Malka D, et al:
Second-line chemotherapy with fluorouracil, leucovorin, and
irinotecan (FOLFIRI regimen) in patients with advanced small bowel
adenocarcinoma after failure of first-line platinum-based
chemotherapy: a multicenter AGEO study. Cancer. 117:1422–1428.
2011. View Article : Google Scholar
|
|
22
|
Tsang H, Yau T, Khong PL and Epstein RJ:
Bevacizumab-based therapy for advanced small bowel adenocarcinoma.
Gut. 57:1631–1632. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
De Dosso S, Molinari F, Martin V, Frattini
M and Saletti P: Molecular characterisation and cetuximab-based
treatment in a patient with refractory small bowel adenocarcinoma.
Gut. 59:1587–1588. 2010.PubMed/NCBI
|