Introduction
Prostate cancer is a common disease among elderly
men and the prostate-specific antigen (PSA) is a valuable tumor
marker for the detection of this cancer. However, improvement of
the detection specificity is required as, in addition to prostate
cancer, serum PSA levels are also increased in patients with
prostate benign hyperplasia and prostatitis (1). Furthermore, it has been recognized
that the serum PSA levels are influenced by other drugs, such as
statins and non-steroidal anti-inflammatory drugs (2–4). As
elderly individuals are frequently prescribed drugs for the
treatment of other chronic and underlying illnesses, there is a
considerable chance that these drugs may influence the serum PSA
levels, subsequently leading to false-positive or false-negative
PSA test results (5).
In cases of abnormal growth of prostate cancer
cells, the PSA is expressed in prostate epithelial cells and
released into the blood by disruption of the basement membrane.
Therefore, the potential mechanism underlying medication-induced
changes in the blood PSA levels may be that certain drugs induce
the release of PSA from the prostate gland or the expression of PSA
itself. The present study used prostate cancer LNCaP cells to
investigate whether the drugs that are frequently prescribed to
patients, and collected at the pharmacy of Gifu Pharmaceutical
University (Gifu, Japan), altered the expression level of PSA in
prostate cancer LNCaP cells.
Materials and methods
Materials
Betamethasone, amlodipine, insulin, lansoprazole,
loxoprofen, metformin and warfarin were purchased from Wako Pure
Chemical Industries, Ltd. (Osaka, Japan); allopurinol, famotidine,
magnesium oxide and D-pantothenic acid were obtained from Nacalai
Tesque, Inc. (Kyoto, Japan); aspirin was obtained from Merck Hoei
Ltd. (Osaka, Japan); candesartan was purchased from Toronto
Research Chemicals Inc. (North York, ON, Canada); and rebamipide
was purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo,
Japan). All other chemicals used were of analytical grade.
Investigation of prescription drugs
The prescriptions received at the Gifu
Pharmaceutical University pharmacy for one year (between April 1,
2010 and March 31, 2011) were investigated for generic name, dosing
period, and patient age and gender. Patients aged between 50 and 75
years at the prescription issue date were the focus of the present
study.
Cell culture
Human prostate carcinoma LNCaP cells were obtained
from the American Type Culture Collection (Rockville, MD, USA). The
cells were cultured in RPMI-1640 medium containing 10% fetal bovine
serum (FBS) and 1% penicillin-streptomycin, under a humidified 5%
CO2 atmosphere at 37°C.
Cell viability
Cell viability was evaluated by measuring the
fluorescence intensity of cells using the alamarBlue viability
assay (Invitrogen Life Technologies, Carlsbad, CA, USA) (6). LNCaP cells were seeded in 96-well
plates (Sumilon, Tokyo, Japan) at a density of 8×103
cells/well in RPMI-1640 medium supplemented with 10% FBS. On the
following day, cells were treated with various concentrations of
each compound and the incubation was continued for three days.
AlamarBlue solution was subsequently added to the wells and the
plates were incubated for 1 h. Next, the fluorescence intensity of
the cells was measured using a POLARstar Galaxy microplate reader
(BMG Labtech Ltd., Offenburg, Germany) using excitation and
emission wavelengths of 544 and 612 nm, respectively.
Real-time reverse transcription
polymerase chain reaction (qPCR)
qPCR was performed according to previously described
protocols with minor modifications (7). Total RNA was extracted using the
TRIzol reagent (Invitrogen Life Technologies) and first-strand
complementary DNA was synthesized from 1 μg of total RNA using
PrimeScript reverse transcriptase (Takara Bio, Inc., Otsu, Japan).
Real-time monitoring of the PCR was performed using the Thermal
Cycler Dice Real-Time system (Takara Bio, Inc.) with Thunderbird
SYBR qPCR mix (Toyobo Corporation, Osaka, Japan). At the end of the
reaction, a dissociation curve analysis was performed to examine
the specificity of the product. The PCR was performed using the
following conditions: 35 Cycles of 15 sec at 95°C and 60 sec at
60°C. The β-actin (ACTB) housekeeping gene was used for the
normalization of the target mRNA expression. The primers used were
as follows: Sense, 5′-GAGGTCCACACACTGAAGTT-3′ and antisense,
5′-CCTCCTGAAGAATCGATTCCT-3′ for PSA (KLK3) ; and sense,
5′-CAAGTACTCCGTGTGGATCG-3′ and antisense,
5′-AGTCCGCCTAGAAGCATTTG-3′ for β-actin (ACTB).
Luciferase assay
The luciferase assay was performed as described
previously (6). LNCaP cells
(1×105 cells/well) were incubated in a 24-well culture
plate (Sumilon) for one day and cotransfected with 0.76 μg of the
androgen-responsive MMTV-luc firefly luciferase reporter plasmid
and 0.04 μg of the Renilla luciferase plasmid, phRL-TK,
using Lipofectamine 2000 (Invitrogen Life Technologies). Cells were
treated for 24 h with various concentrations of betamethasone or
dexamethasone. Cell lysates were prepared and the luciferase
activities were measured using the Dual-Luciferase Reporter assay
system (Promega Corporation, Madison, WI, USA). The firefly
luciferase activity was normalized to the activity of
Renilla luciferase.
Statistical analysis
Statistical significance was assessed by one-way
analysis of variance followed by Dunnett’s test, using PRISM 4
software (Graphpad Software, San Diego, CA, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Effect of the drugs on PSA expression in
prostate cancer LNCaP cells
Table I shows the
most frequently prescribed drugs for elderly men, including the
drugs for the treatment of chronic diseases (such as hypertension
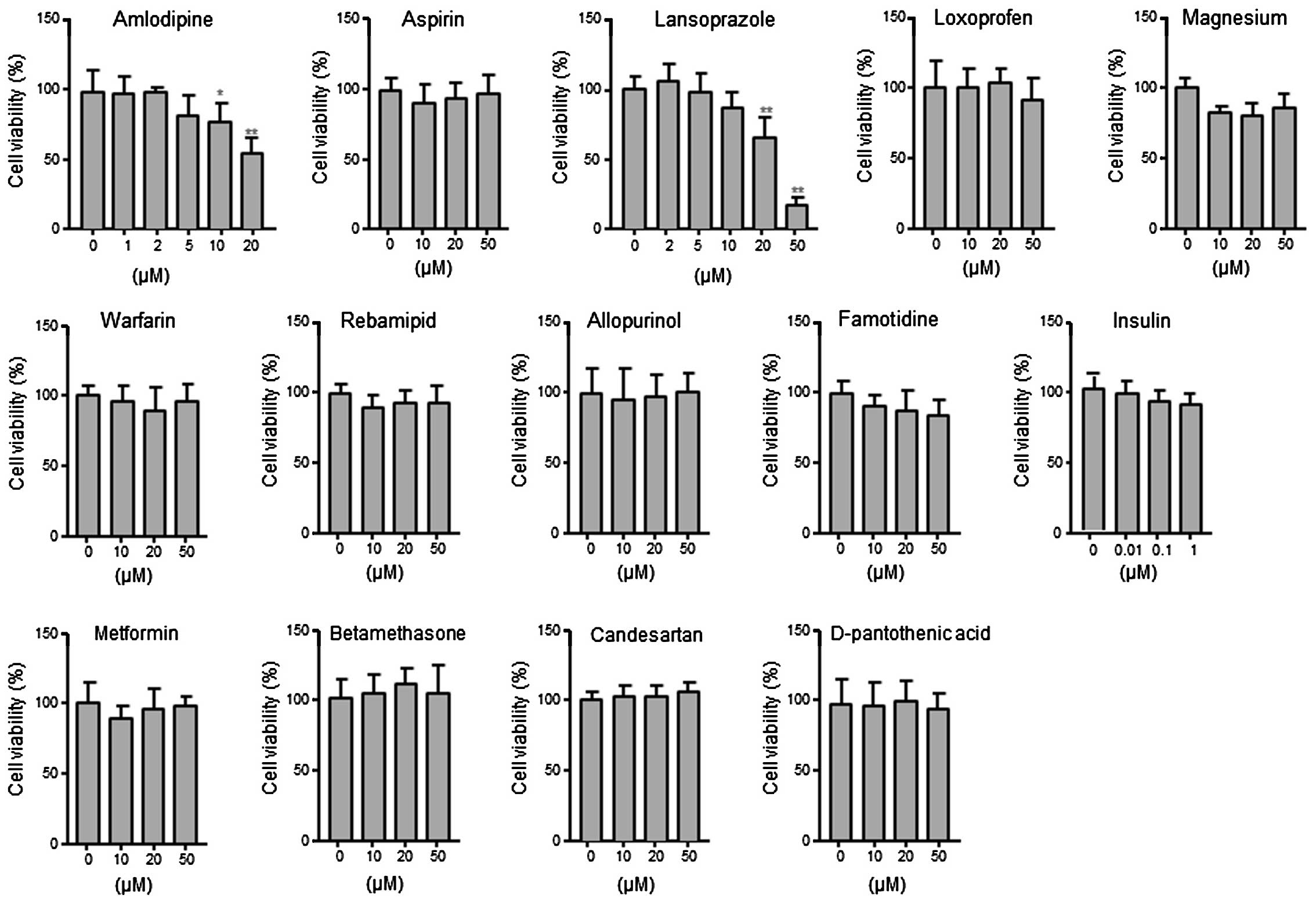
and diabetes). Firstly, the effects of the drugs listed in Table 1 on the viability of androgen
receptor (AR)-positive prostate cancer LNCaP cells were examined.
LNCaP cells were treated with each drug at a concentration of 1–50
μM for 24 h. As shown in Fig. 1, no
significant effect was identified on cell viability at
concentrations of ≤20 μM, with the exception of amlodipine and
lansoprazole. Based on these results, the treatment concentrations
were determined to be 2 μM for amlodipine, 10 μM for lansoprazole
and 20 μM for the other drugs to examine the effects of the drugs
on PSA expression.
 | Table IFrequently used drugs from the
prescriptions received at the pharmacy of Gifu Pharmaceutical
University. |
Table I
Frequently used drugs from the
prescriptions received at the pharmacy of Gifu Pharmaceutical
University.
| Rank | Generic name | Count, n |
|---|
| 1 | Amlodipine | 107 |
| 2 | Aspirin | 106 |
| 3 | Lansoprazole | 100 |
| 4 | Loxoprofen | 94 |
| 5 | Magnesium oxide | 80 |
| 6 | Warfarin | 74 |
| 7 | Rebamipide | 67 |
| 8 | Allopurinol | 65 |
| 9 | Famotidine | 64 |
| 10 | Insulin | 62 |
| 11 | Metformin | 58 |
| 12 | Betamethasone | 57 |
| 13 | Candesartan | 55 |
| 14 | D-pantothenic
acid | 52 |
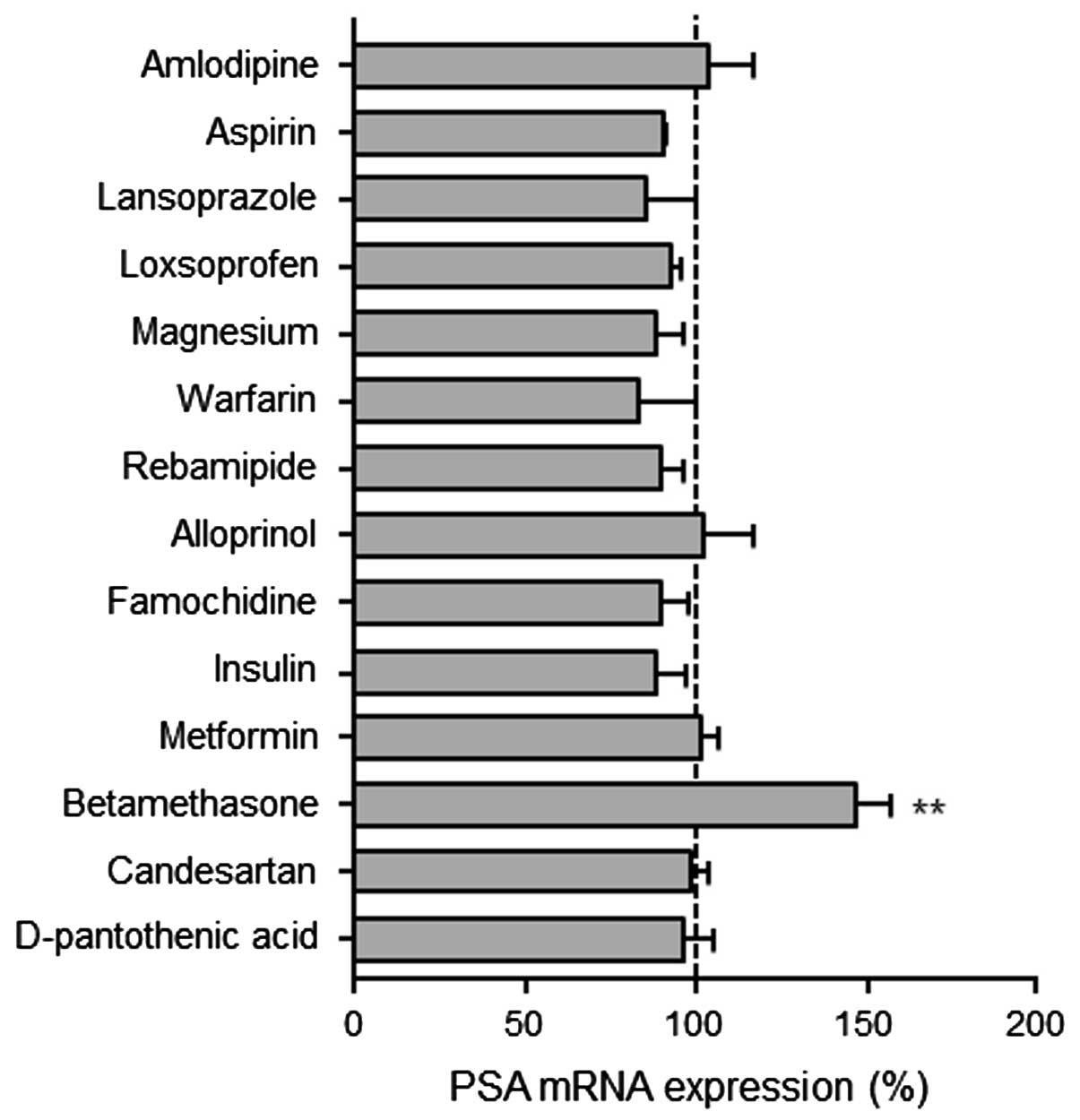
Fig. 2 shows the PSA
mRNA expression in LNCaP cells following treatment with various
drugs for three days. The PSA mRNA expression levels increased
significantly in the betamethasone-treated LNCaP cells, whereas no
significant differences were identified in the levels of PSA mRNA
expression among the cells that were treated with the other drugs.
Furthermore, long durations of drug treatment (nine or 14 days)
showed no significant differences in the PSA expression levels
among the drugs that were tested (data not shown).
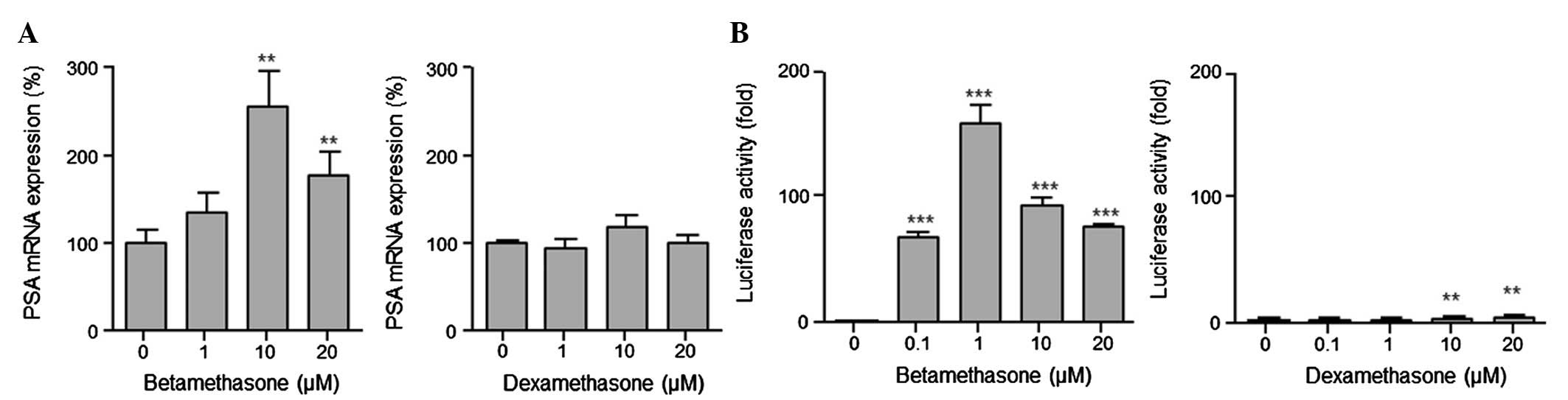
Effect of betamethasone on AR
transcriptional activity in LNCaP cells
LNCaP cells have a point-mutated AR (T877A), which
broadens the ligand specificity (8,9). Since
PSA expression was found to be highly induced by betamethasone (an
agonist against the glucocorticoid receptor), which has a steroid
structure, this induction was considered to be mediated through
transactivation of the mutated AR. As shown in Figs. 2 and 3A (left panel), the PSA induction by
betamethasone in steroid-deprived medium was higher than that in
normal medium. Furthermore, the AR transcriptional activity
measured by the luciferase assay was significantly elevated by
betamethasone treatment in the LNCaP cells (Fig. 3B). Notably, dexamethasone, an
agonist of the glucocorticoid receptor, did not influence the PSA
mRNA expression in the LNCaP cells (Fig. 3A, right panel). Furthermore,
although the induction of AR transcriptional activity by
dexamethasone was observed, the extent was much lower than that by
betamethasone (Fig. 3B, right
panel).
Discussion
The present study examined the drugs most frequently
prescribed at the pharmacy of Gifu Pharmaceutical University and
found that betamethasone, a frequently used drug among elderly
males (rank 12, Table I), increased
the PSA mRNA expression in prostate cancer LNCaP cells.
The significant increase of PSA expression by
betamethasone is through the transcriptional activation of AR.
Betamethasone is a ligand for the glucocorticoid receptor;
therefore, the PSA increase is considered to be the result of the
agonistic effect of this drug on the mutated AR (T877A) in LNCaP
cells. Previously, it has been reported that betamethasone induces
AR transcriptional activation to almost the same level as
dihydrotestosterone when the T877A AR expression vector is
transfected into CV-1 cells, whereas the activation is extremely
low with the transfection of wild-type AR (10). In the present study, betamethasone
treatment did not activate AR transcription in wild-type
AR-transfected PC-3 cells (data not shown). Notably, the induction
of AR transcriptional activity by betamethasone was markedly higher
than that by the typical glucocorticoid receptor agonist,
dexamethasone, in the LNCaP cells that were endogenously expressing
T877A AR. A previous study demonstrated no notable differences in
the AR transcriptional activity between betamethasone and
dexamethasone treatments in T877A AR-transfected CV-1 cells
(10). Therefore, the induction of
AR transcriptional activity by betamethasone may be involved in a
mechanism additional to acting as a ligand to the mutated AR.
It must also be noted that the results from our
experimental model using LNCaP cells may provide insight into the
effect of drugs on PSA test results. However, the serum PSA levels
are not singularly determined by the amount of PSA produced from
the cells. In the present study, aspirin did not affect PSA
expression in LNCaP cells, although, the serum PSA levels in
aspirin users has been identified as lower than those in non-users
(4). The lowering of the serum PSA
levels by aspirin may be mediated through its anti-inflammatory
effect, since chronic inflammation causes the development of
prostate cancer and anti-inflammatory aspirin has been postulated
to have an inhibitory effect on prostate cancer development.
In the present study, the betamethasone that was
prescribed to patients was for external application only. The
circulating concentration of an externally applied drug is
generally markedly lower than that of a drug that is administered
orally. In addition, the PSA increase due to the betamethasone was
identified to be particularly significant in the mutated T877A AR,
which is consistent with the results of a previous study (10). Occasionally, prostate cancer cells
exhibit a mutated AR, particularly in patients who are resistant to
androgen ablation therapy (11).
Therefore, it appears unlikely that the application of
betamethasone to the skin would result in an alternation of serum
PSA levels in healthy individuals. However, betamethasone is
prescribed to patients with hormone-refractory advanced prostate
cancer (12); therefore, in
patients that are orally administering betamethasone, a false PSA
test result may occur.
In conclusion, the PSA test was identified to be
useful for monitoring the disease status in patients with prostate
cancer; however, it may be beneficial to examine whether
betamethasone influences the PSA test results in clinical
cases.
References
|
1
|
Mochtar CA and Andika RS: The value of
prostate-specific antigen in Asia. Ther Adv Urol. 2:77–83. 2010.
View Article : Google Scholar
|
|
2
|
Fowke JH, Motley SS, Barocas DA, et al:
The associations between statin use and prostate cancer screening,
prostate size, high-grade prostatic intraepithelial neoplasia
(PIN), and prostate cancer. Cancer Causes Control. 22:417–426.
2011. View Article : Google Scholar
|
|
3
|
Hamilton RJ, Banez LL, Aronson WJ, et al:
Statin medication use and the risk of biochemical recurrence after
radical prostatectomy: results from the Shared Equal Access
Regional Cancer Hospital (SEARCH) Database. Cancer. 116:3389–3398.
2008. View Article : Google Scholar
|
|
4
|
Murad AS, Down L, Davey Smith G, et al:
Associations of aspirin, nonsteroidal anti-inflammatory drug and
paracetamol use with PSA-detected prostate cancer: findings from a
large, population-based, case-control study (the ProtecT study).
Int J Cancer. 128:1442–1448. 2011. View Article : Google Scholar
|
|
5
|
Chang SL, Harshman LC and Presti JC Jr:
Impact of common medications on serum total prostate-specific
antigen levels: analysis of the National Health and Nutrition
Examination Survey. J Clin Oncol. 28:3951–3957. 2010. View Article : Google Scholar
|
|
6
|
Iguchi K, Toyama T, Ito T, et al:
Antiandrogenic activity of resveratrol analogs in prostate cancer
LNCaP cells. J Andrology. 33:1208–1215. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Iguchi K, Hayakawa Y, Ishii K, et al:
Characterization of the low pH/low nutrient-resistant LNCaP cell
subline LNCaP-F10. Oncol Rep. 28:2009–2015. 2012.PubMed/NCBI
|
|
8
|
Berrevoets CA, Veldscholte J and Mulder E:
Effects of antiandrogens on transformation and transcription
activation of wild-type and mutated (LNCaP) androgen receptors. J
Steroid Biochem Mol Biol. 46:731–736. 1993. View Article : Google Scholar
|
|
9
|
Otsuka T, Iguchi K, Fukami K, et al:
Androgen receptor W741C and T877A mutations in AIDL cells, an
androgen-independent subline of prostate cancer LNCaP cells. Tumour
Biol. 32:1097–1102. 2011. View Article : Google Scholar
|
|
10
|
Chang CY, Walther PJ and McDonnell DP:
Glucocorticoids manifest androgenic activity in a cell line derived
from a metastatic prostate cancer. Cancer Res. 61:8712–8717.
2001.
|
|
11
|
Koochekpour S: Androgen receptor signaling
and mutations in prostate cancer. Asian J Andrology. 12:639–657.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kassi E and Moutsatsou P: Glucocorticoid
receptor signaling and prostate cancer. Cancer Lett. 302:1–10.
2011. View Article : Google Scholar : PubMed/NCBI
|