Introduction
As a result of marked improvement in systemic
chemotherapy against unresectable and/or recurrent colorectal
cancer, the median survival time of patients with metastatic
colorectal cancer has improved to >20 months with administration
of fluorouracil (5-FU), irinotecan and oxaliplatin (1). According to the Japanese guidelines
for the treatment of unresectable and/or recurrent colorectal
cancer (2), 5-FU, leucovorin and
oxaliplatin combination chemotherapy (FOLFOX), capecitabine and
oxaliplatin combination chemotherapy (CapeOX), and 5-FU, leucovorin
and irinotecan combination chemotherapy (FOLFIRI), are recommended
as first-line chemotherapy regimens. Additionally, these regimens
plus anti-vascular endothelial growth factor monoclonal antibody
(bevacizumab) or anti-epidermal growth factor receptor monoclonal
antibody have improved the median survival time of patients. At
present, combination chemotherapy with the oral fluoropyrimidine
S-1 has been shown to be effective against metastatic colorectal
cancer (3–9). This report presents a case that
demonstrates the efficacy of S-1 and irinotecan (IRIS) plus
bevacizumab combination therapy against lung metastases of rectal
cancer and discusses the relevant literature. Patient provided
written informed consent.
Case report
A 72-year-old male visited a local physician with a
20-day history of progressive abdominal distension and bloody
stool. The following day the patient was referred to Ibaraki
Medical Center (Ami, Japan) with a diagnosis of rectal cancer.
Patient medical history was otherwise unremarkable. On physical
examination, all findings were unremarkable with the exception of
slight pallor in the palpebral conjunctiva. Hematological
investigations revealed anemia (hemoglobin levels, 9.6 g/dl;
hematocrit, 28.4%). Other laboratory tests and serum levels of
carcinoembryonic antigen and carbohydrate antigen 19-9 were all
within normal limits. Colonoscopy revealed the entire circumference
of an elevated tumor with central depression and erosion at the
lower rectum. A biopsy specimen from the tumor was indicative of a
moderately differentiated adenocarcinoma. Abdominal computed
tomography (CT) revealed thickening of the rectal wall with
regional lymph node swelling but no liver metastasis. Chest CT
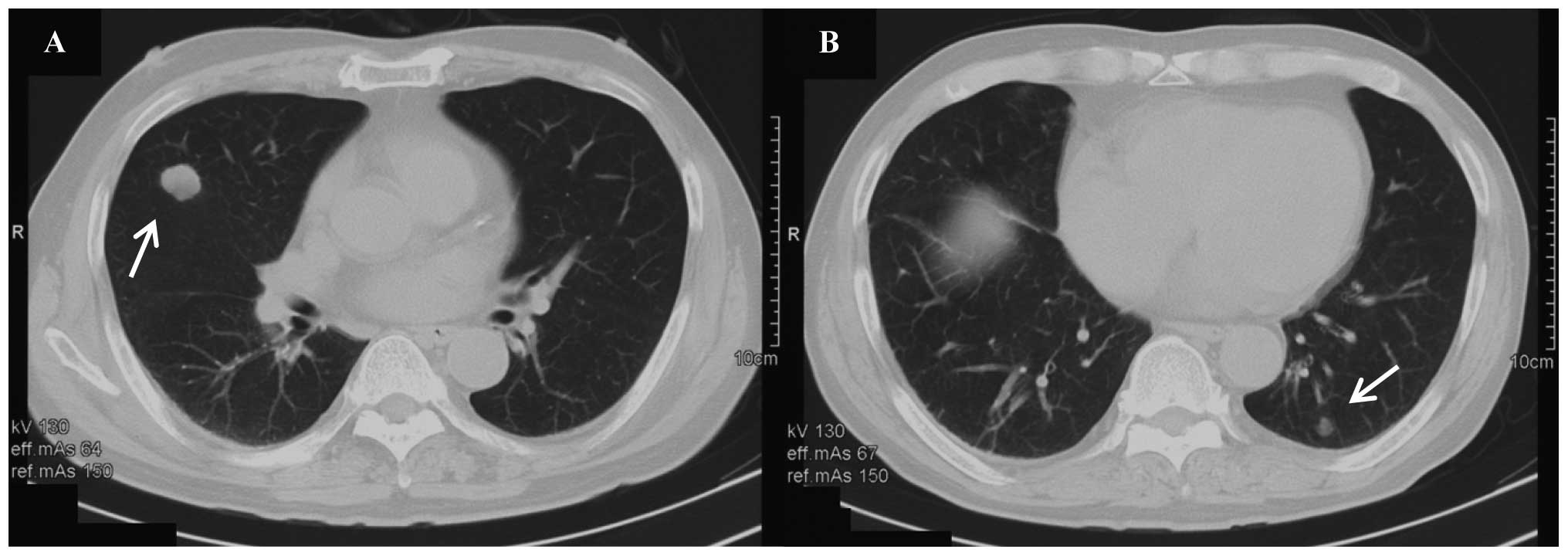
revealed two metastatic lung tumors measuring 25 mm (in the middle
lobe of the right lung) and 10 mm in diameter (in the lower lobe of
the left lung) (Fig. 1). A
diagnosis of rectal cancer with multiple lung metastases was made,
and abdominoperineal resection with lymph node dissection was
performed in October 2009. Light microscopy revealed that the tumor
had infiltrated the deep tissue layer through the muscularis
propria layer of the rectum and that there were cancer metastases
in 12 of the 14 lymph nodes. The tumor was diagnosed as stage IVA
(T3, N2b, M1a) according to the International Union Against Cancer
Tumor Node Metastasis classification (7th edition) (10). The patient refused resection of the
lung metastases and placement of a peripherally inserted central
venous (CV) port, and was hesitant to be treated with oxaliplatin
and capecitabine due to potential peripheral neuropathy and
hand-foot syndrome as side effects. On providing informed consent,
the patient was administered IRIS plus bevacizumab combination
therapy against the lung metastases. S-1 (100 mg/body) was
administered orally on days 1–14 of a 28-day cycle, and irinotecan
(125 mg/m2) and bevacizumab (7.5 mg/kg) were
administered by intravenous infusion on days one and 15. Following
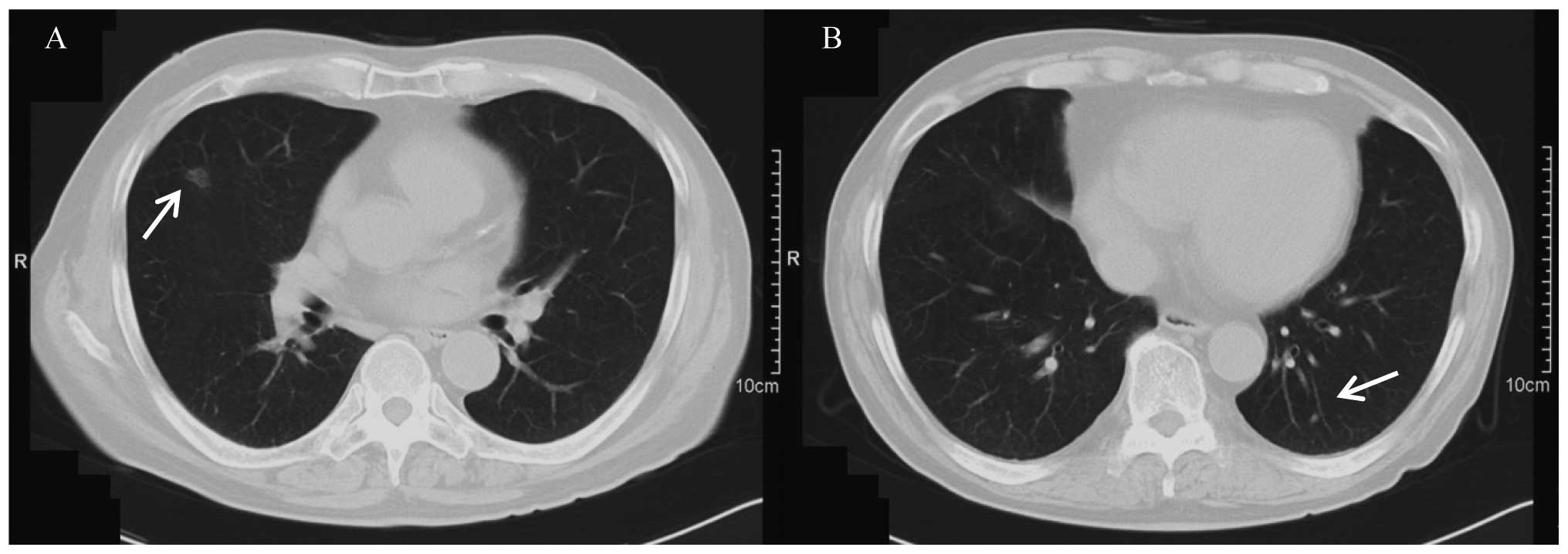
three courses of therapy, the metastatic right lung tumor decreased
in size to ~10 mm in diameter, and the left lung tumor had
decreased in size to ~3 mm in diameter (Fig. 2). Following six courses of therapy,
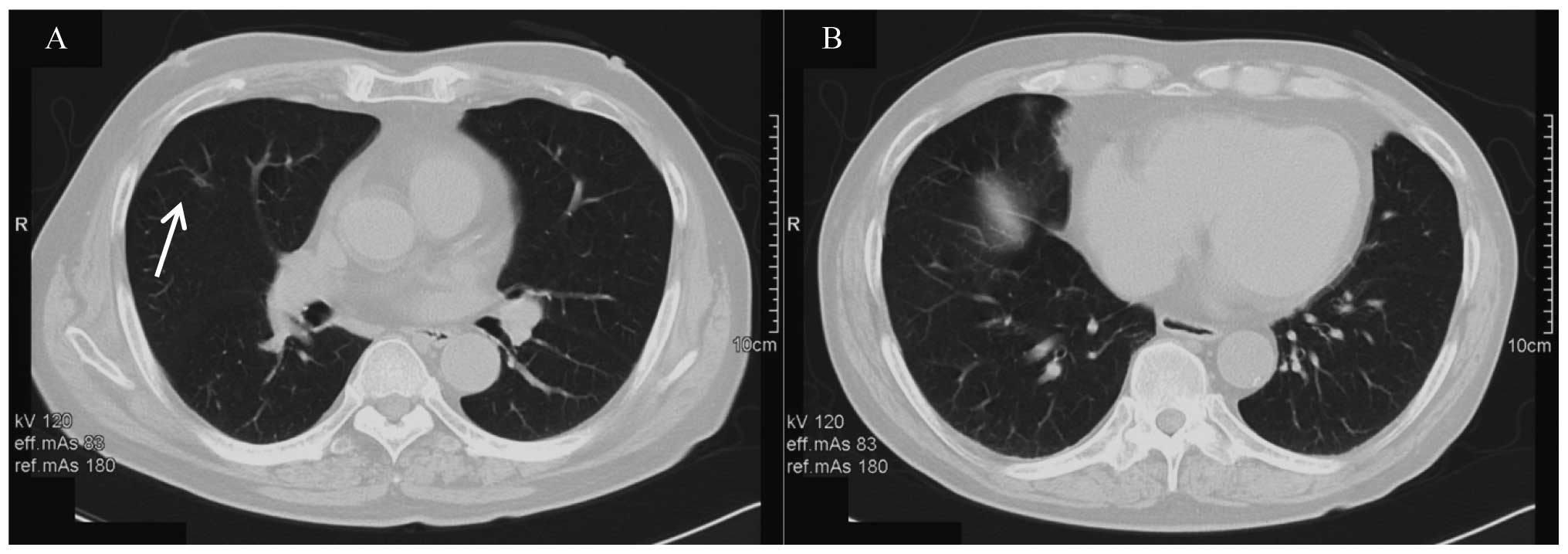
the metastatic right lung tumor had become scar tissue and no
metastases could be detected in the left lung (Fig. 3). Following nine courses of therapy,
no metastatic lung tumors could be identified (Fig. 4). The response was declared
clinically complete. The patient refused additional treatment
following nine courses of therapy, and there was no recurrence 36
months after the final course of therapy.
Discussion
Multidisciplinary therapies, including systemic
chemotherapy against unresectable and/or recurrent colorectal
cancer, have improved, and FOLFOX, CapeOX and FOLFIRI combination
chemotherapies plus bevacizumab or anti-EGFR monoclonal antibody
can improve the survival times of patients. In the present case,
the patient received IRIS plus bevacizumab combination therapy
against lung metastases of rectal cancer. Regarding IRIS
chemotherapy, Muro et al (3)
reported that IRIS was not inferior to FOLFIRI in terms of
progression-free survival when administered as second-line
chemotherapy for patients with metastatic colorectal cancer.
Colucci et al (4) and
Tournigand et al (5)
identified no significant differences in the median survival times
of patients regardless of whether FOLFOX or FOLFIRI was selected as
a first-line chemotherapy. As the noninferiority of IRIS with
regard to FOLFIRI as a second-line chemotherapy has been proven,
IRIS is considered to be a first-line chemotherapy for metastatic
colorectal cancer. Futhermore, according to the European Society
for Medical Oncology (11), IRIS is
recommended as an additional therapeutic option for first-line
chemotherapy in metastatic colorectal cancer.
The effectiveness of IRIS plus bevacizumab
combination therapy as a first-line therapy for metastatic
colorectal cancer has been reported. In a phase II study, Komatsu
et al (12) reported that
the response rate (complete response + partial response) was 57.7%
and the disease control rate (complete response + partial response
+ stable disease) was 90.4%. Kato et al (13) reported that the response rate was
62.0 versus 72% and progression-free survival time was 324 versus
345 days for FOLFIRI plus bevacizumab versus IRIS plus bevacizumab,
respectively. In terms of side effects and safety, Yamada et
al (14) reported that the IRIS
plus bevacizumab regimen was tolerated: Grade 3/4 neutropenia was
observed in 26% patients, grade 3/4 anorexia was observed in 12%
and grade 3/4 diarrhea was observed in 8%. In the present case, the
efficacy of IRIS plus bevacizumab combination therapy was confirmed
by the decrease in size of the metastatic right and left lung
tumors from ~25 mm to 10 mm and ~10 mm to 3 mm in diameter,
respectively, following three courses of therapy. The tumor
regression rate was ~62.8%, and the right lung tumor became scar
tissue following six courses of therapy. There were no metastatic
tumors in the lungs following nine courses of therapy. The patient
exhibited grade 1 depilation, but there were no problems with
tolerability.
Treatment of lung metastases from colorectal cancer
is a debated issue. The overall survival of patients with
completely resectable lung metastases is better than that of
patients with unresectable lung metastases (15–17).
The Japanese guidelines for treatment of lung metastases from
colorectal cancer recommend surgery if the primary lesion and lung
metastases are completely resectable and the performance status of
the patient suggests surgery may be tolerated (2). According to the Japanese guidelines,
resection was recommended for the lung metastases in the present
case; there were two lung metastases and the performance status of
the patient was good. However, the patient selected chemotherapy
against the lung metastases, refusing pneumonectomy and the
adjuvant chemotherapy that would be required following surgery if
the pneumonectomy was performed. The patient selected IRIS plus
bevacizumab combination first-line therapy for the following
reasons: i) High antitumor effectiveness has been reported in a
previous study (although it was a phase II study) (13); ii) a CV port is not necessary; and
iii) unlike 5-FU therapy, patients may be released from the
infusion pump while at home. With regard to the lack of requirement
for a CV port and infusion pump, CapeOX plus bevacizumab therapy
was an option for first-line therapy in the present case. However,
hand-foot syndrome as a side effect of capecitabine and peripheral
neuropathy as a side effect of oxaliplatin are frequently observed,
and management of these side effects is essential (18,19).
In the present case, the patient was hesitant to be treated with
oxaliplatin and capecitabine due to these side effects. Therefore,
better tolerance of IRIS plus bevacizumab combination therapy than
of CapeOX plus bevacizumab combination therapy can be expected in
cases of metastatic colorectal cancer.
In conclusion, IRIS plus bevacizumab combination
therapy is well tolerated and efficacious as first-line
chemotherapy for metastatic colorectal cancer. As a CV port is not
required and patients can be released from the infusion pump while
at home, IRIS plus bevacizumab combination therapy could contribute
to improved quality of life in patients with metastatic colorectal
cancer.
Acknowledgements
The authors would like to thank Enago (www.enago.jp) for the English language review.
References
|
1
|
Grothey A, Sargent D, Goldberg RM and
Schmoll HJ: Survival of patients with advanced colorectal cancer
improves with the availability of fluorouracil-leucovorin,
irinotecan, and oxaliplatin in the course of treatment. J Clin
Oncol. 22:1209–1214. 2004. View Article : Google Scholar
|
|
2
|
Watanabe T, Itabashi M, Shimada Y, et al:
Japanese Society for Cancer of the Colon and Rectum (JSCCR)
guidelines 2010 for the treatment of colorectal cancer. Int J Clin
Oncol. 17:1–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Muro K, Boku N, Shimada Y, et al:
Irinotecan plus S-1 (IRIS) versus fluorouracil and folininc acid
plus irinotecan (FOLFIRI) as second-line chemotherapy for
metastatic colorectal cancer: a randomised phase 2/3
non-inferiority study (FIRIS study). Lancet Oncol. 11:853–860.
2010. View Article : Google Scholar
|
|
4
|
Colucci G, Gebbia V, Paoletti G, et al:
Phase III randomized trial of FOLFIRI versus FOLFOX4 in the
treatment of advanced colorectal cancer: a multicenter study of the
Gruppo Oncologico Dell’Italia Meridionale. J Clin Oncol.
23:4866–4875. 2005.PubMed/NCBI
|
|
5
|
Tournigand C, André T, Achille E, et al:
FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced
colorectal cancer: a randomized GERCOR Study. J Clin Oncol.
22:229–237. 2004. View Article : Google Scholar
|
|
6
|
Choi YH, Kim TW, Kim KP, et al: A Phase II
study of clinical outcomes of 3-week cycles of irinotecan and S-1
in patients with previously untreated metastatic colorectal cancer:
Influence of the UGT1A1 and CYP2A6 polymorphisms on clinical
activity. Oncology. 82:290–297. 2012. View Article : Google Scholar
|
|
7
|
Kaneko J, Isogai J, Aoyagi H, et al: A
case of unresectable multiple hepatic metastases from colorectal
cancer successfully treated with IRIS (S-1, CPT-11) therapy. Gan To
Kagaku Ryoho. 37:2576–2578. 2010.(In Japanese).
|
|
8
|
Ueda S, Minami Y, Hara Y, Kawano T and
Maekawa K: A case of recurrent rectal cancer with multiple lung
metastases successfully treated with S-1 and CPT-11 combination
chemotherapy. Gan To Kagaku Ryoho. 36:1725–1727. 2009.(In
Japanese).
|
|
9
|
Samejima J, Iwasaki H, Hatori S, et al:
Effectiveness of S-1 plus CPT-11 therapy for an elderly patient
with recurrent colon cancer. Gan To Kagaku Ryoho. 36:1371–1373.
2009.(In Japanese).
|
|
10
|
Sobin LH, Gospodarowicz MK and Wittekind
C: TNM Classification of Malignant Tumors. 7th edition.
Wiley-Blackwell; NY: 2009
|
|
11
|
Schmoll HJ, Van Cutsem E, Stein A, et al:
ESMO Consensus Guidelines for management of patients with colon and
rectal cancer. A personalized approach to clinical decision making.
Ann Oncol. 23:2479–2516. 2012. View Article : Google Scholar
|
|
12
|
Komatsu Y, Yuki S, Sogabe S, et al: Phase
II study of combined chemotheraphy with irinotecan and S-1 (IRIS)
plus bevacizumab in patients with inoperable recurrence or advanced
colorectal cancer. Acta Oncol. 51:867–872. 2012. View Article : Google Scholar
|
|
13
|
Kato S, Andoh H, Gamoh M, et al: Safety
verification trial of mFOLFIRI and sequential IRIS + bevacizumab as
first- or second-line therapies for metastatic colorectal cancer in
Japanese patients. Oncology. 83:101–107. 2012.PubMed/NCBI
|
|
14
|
Yamada Y, Yamaguchi T, Matsumoto H, et al:
Phase II study of oral S-1 with irinotecan and bevacizumab (SIRB)
as first-line therapy for patients with metastatic colorectal
cancer. Invest New Drugs. 30:1690–1696. 2012. View Article : Google Scholar
|
|
15
|
Goya T, Miyazawa N, Kondo H, Tsuchiya R,
Naruke T and Suemasu K: Surgical resection of pulmonary metastases
from colorectal cancer. 10-year follow-up. Cancer. 64:1418–1421.
1989.PubMed/NCBI
|
|
16
|
McCormack PM, Burt ME, Bains MS, Martini
N, Rusch VW and Ginsberg RJ: Lung resection for colorectal
metastases. 10-year results. Arch Surg. 127:1403–1406.
1992.PubMed/NCBI
|
|
17
|
Ike H, Shimada H, Ohki S, Togo S,
Yamaguchi S and Ichikawa Y: Results of aggressive resection of lung
metastases from colorectal carcinoma detected by intensive
follow-up. Dis Colon Rectum. 45:468–473. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Farid M, Chowbay B, Chen X, et al: Phase I
pharmacokinetic study of chronomodulated dose-intensified
combination of capecitabine and oxaliplatin (XELOX) in metastatic
colorectal cancer. Cancer Chemother Pharmacol. 70:141–150. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Matsuo K, Higuchi M, Sasaki Y, et al:
Analysis of a case of oxaliplatin - induced persistence sensory
neuropathy. Gan To Kagaku Ryoho. 37:551–554. 2010.(In
Japanese).
|