Introduction
Renal cell carcinoma (RCC) is one of the most common
types of genitourinary cancer, accounting for ~2–3% of all
malignant tumors (1), and with an
incidence of ~209,000 new cases and 102,000 mortalities per year
worldwide. In Europe in 2008, 88,300 patients were diagnosed with
RCC and 39,230 succumbed to the disease (2). With the development of diagnostic
technology, an increasing number of patients with RCC are diagnosed
at an early stage. However, a considerable number of patients with
RCC present with metastasis at the time of diagnosis. Emerging
evidence indicates that the development and progression of RCC are
closely associated with the tumor microenvironment (3).
Exosomes are 30–100 nm-sized membrane vesicles,
which are released into the tumor microenvironment by the majority
of tumor cell types (4). Exosomes
are currently recognized as important mediators of cell-to-cell
communication, which transfer proteins, mRNAs and microRNAs to
neighboring or distant cells to modulate immune function,
angiogenesis, cell proliferation and tumor cell invasion (5–7).
However, whether cancer cell-derived exosomes regulate the
migration and invasion of the cancer cell itself and the underlying
mechanisms have not yet been investigated.
Our previous study indicated that tumor cell-derived
exosomes contributed to proliferation of the tumor cell (8). In the present study, exosomes were
isolated from the 786-0 human renal carcinoma cell line and the
effects of 786-0 cell-derived exosomes on the migration and
invasion of 786-0 cells as well as the underlying mechanisms were
investigated in vitro. Our findings may provide a novel
insight into the mechanisms underlying the development and
progression of RCC.
Materials and methods
Cell culture
The 786-0 human renal cancer cell line was purchased
from the Institute of Cell Research, Chinese Academy of Sciences
(Shanghai, China). The cells were cultured in RPMI-1640 medium
(Gibco, Shanghai, China) supplemented with 10% (v/v) fetal bovine
serum (FBS; Shijiqing Inc, Beijing, China) at 37°C in an atmosphere
of 5% (v/v) CO2.
Isolation and purification of
exosomes
The 786-0 cell-derived exosomes were isolated and
purified as previously described (9). Briefly, cultured supernatants (100 ml)
were harvested and sequentially centrifuged (4°C) at 300, 800 and
10,000 × g for 10, 30 and 30 min, respectively, to remove the
pellet and cell debris. The clarified supernatant was then
centrifuged at 1,000 × g for 30 min in a 100 kDa MWCO Centrifugal
Filter Device (Amicon Drive Systems, Inc., Pineville, NC, USA). The
ultracentrifuge supernatant was underplayed with 30% sucrose/D2O
density cushion, followed by ultracentrifugation at 100,000 × g for
60 min. The cushion was collected and diluted in phosphate-buffered
saline (PBS). The exosomes were further concentrated by
centrifuging at 1,000 × g in a 100 kDa MWCO for 30 min. A membrane
filter (0.22 μm) was used and following sterilization the exosomes
were stored at −80°C. The Bradford method was used to quantify the
total protein concentration of exosomes.
Identification of exosomes
The morphological characteristics of the exosomes
were identified under a transmission electron microscope (TEM;
JEM-2010, JEOL, Ltd., Tokyo, Japan). The exosomes (20 μl) were
resuspended, loaded onto electron microscopy grids and stained with
2% phosphotungstic acid (pH 6.8) for 1 min. The samples were
subjected to analysis under TEM at 80 kV. Western blotting was used
to identify the molecular composition of G250, intercellular
adhesion molecule-1 (ICAM-1) and 70 kDa heat shock protein (Hsp70),
which were purchased from Zhongshan Bio-tech Co., Ltd. (Guangdong,
China).
Wound healing assay
The 786-0 cells were divided into three groups as
follows: Exosomes (Exo), PBS (PBS) and exosomes depression (Exo-D)
groups and pretreated with 100 μg/ml exosomes, 100 μl PBS and 7
mmol/l Amiloride, 5-(N,N-Dimethyl)-, hydrochloride, respectively,
for 24 h. Each group was cultured in triplicate. The cells
(1×105) were seeded in 12-well plates. After the cells
formed a confluent monolayer, a 100 μl tip was used to scratch the
monolayer. The cultured medium was replaced with fresh complete
medium and the cells were incubated at 37°C in an atmosphere of 5%
(v/v) CO2. The wound healing was analyzed under a
microscope and images were captured 24 h following incubation.
Invasion assay
The cells were divided into three groups and
pretreated in triplicate as described above. The cell invasion
assay was performed using transwell chambers with 8 μm pore-sized
membranes coated with Matrigel (Collaborative Research, Inc.,
Bedford, MA, USA) and placed in 24-well plates. The 786-0 cells
(1.5×104) were harvested and loaded into the upper
segment of the chambers. RPMI-1640 medium with 5% FBS was added to
the upper segment of the chamber and the lower chamber contained
10% FBS. Following incubation for 24 h, the upper surfaces of the
transwell chambers were wiped with cotton swabs, and the invading
cells were fixed and stained with crystal violet solution. The
invading cell numbers were counted in five randomly selected
microscope fields.
Cell attachment assays
The 786-0 cells were divided into three groups and
pretreated in triplicate as described above. The cells were
harvested and seeded onto 6-well culture dishes in RPMI-1640 medium
with 10% FBS for 3 h. Cell attachment assays were performed,
non-adherent cells were removed and adherent cells were washed
twice with PBS and fixed in 95% alcohol. The cell numbers were
counted in randomly selected high power fields under an inverted
light microscope.
Western blot analysis
The 786-0 cells were pretreated as previously
described for the wound healing assay. The cells were harvested and
protein was extracted using a Protein Extraction kit according to
the manufacturer’s instructions (Active Motif, Carlsbad, CA, USA).
The protein concentrations were determined by the Bradford protein
assay. Total protein (10 μg) was separated by SDS-PAGE and
transferred onto a polyvinylidene difluoride membrane (Millipore,
Billerica, MA, USA). The membranes were blocked with 5% skimmed
milk in Tris-buffered saline containing 0.1% Tween-20 for 1 h and
incubated with primary antibodies (1:200 dilution) against human
chemokine receptor type 4 (CXCR4) and matrix metalloproteinase-9
(MMP-9) obtained from Tianjin Saier Biotechnology Co., Ltd.
(Tianjin, China) overnight at 4°C. The bound antibodies were
detected with goat anti-rabbit IgG (Zhongshan Bio-tech Co., Ltd.)
and visualized using enhanced chemiluminescence reagents
(Millipore). The relative levels of each target protein to the
control, β-actin, were determined by densitometric analysis using
Image J software.
Statistical analysis
Data are expressed as the mean ± standard deviation.
The differences between the experimental groups were determined by
Student’s t-test using SPSS 19.0 (IBM, Armonk, NY, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
Identification of exosomes derived from
the 786-0 cells
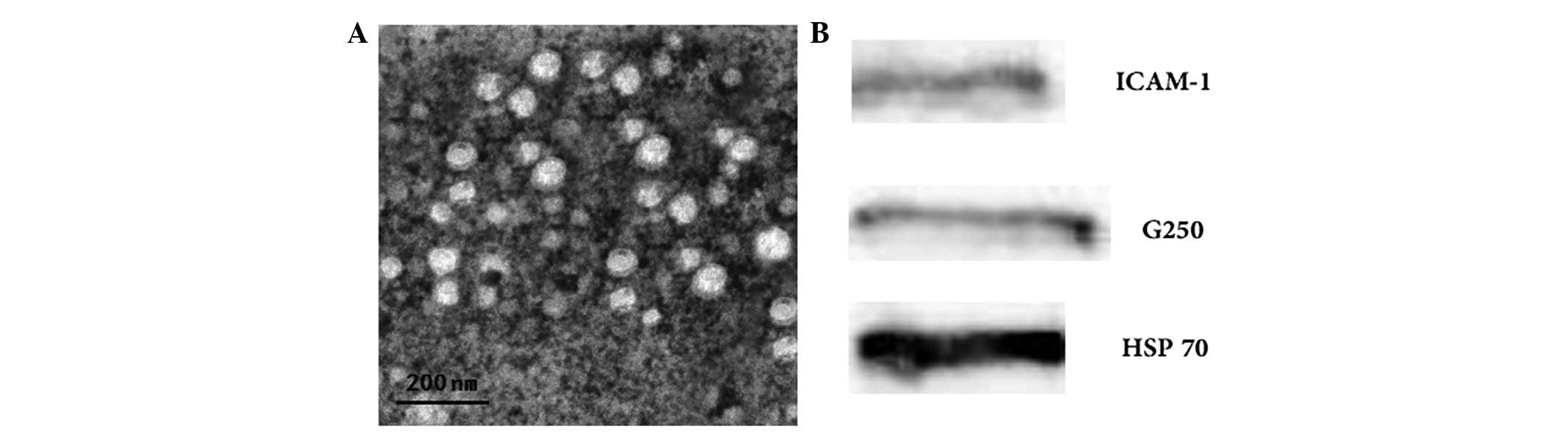
The morphological characteristics of the exosomes
derived from 786-0 cells were identified by TEM. The 786-0
cell-derived exosomes exhibited a typical characteristic of a
cup-shaped or saucer-like structure and ranged from 30 to 100 nm in
diameter (Fig. 1A). Western blot
analysis revealed that 786-0 cell-derived exosomes expressed G250,
ICAM-1 and Hsp70 (Fig. 1B). The
Bradford protein assay found the protein concentration of prepared
exosomes was 1,800.7±275.4 μg/ml.
786-0 cell-derived exosomes enhance 786-0
cell motility
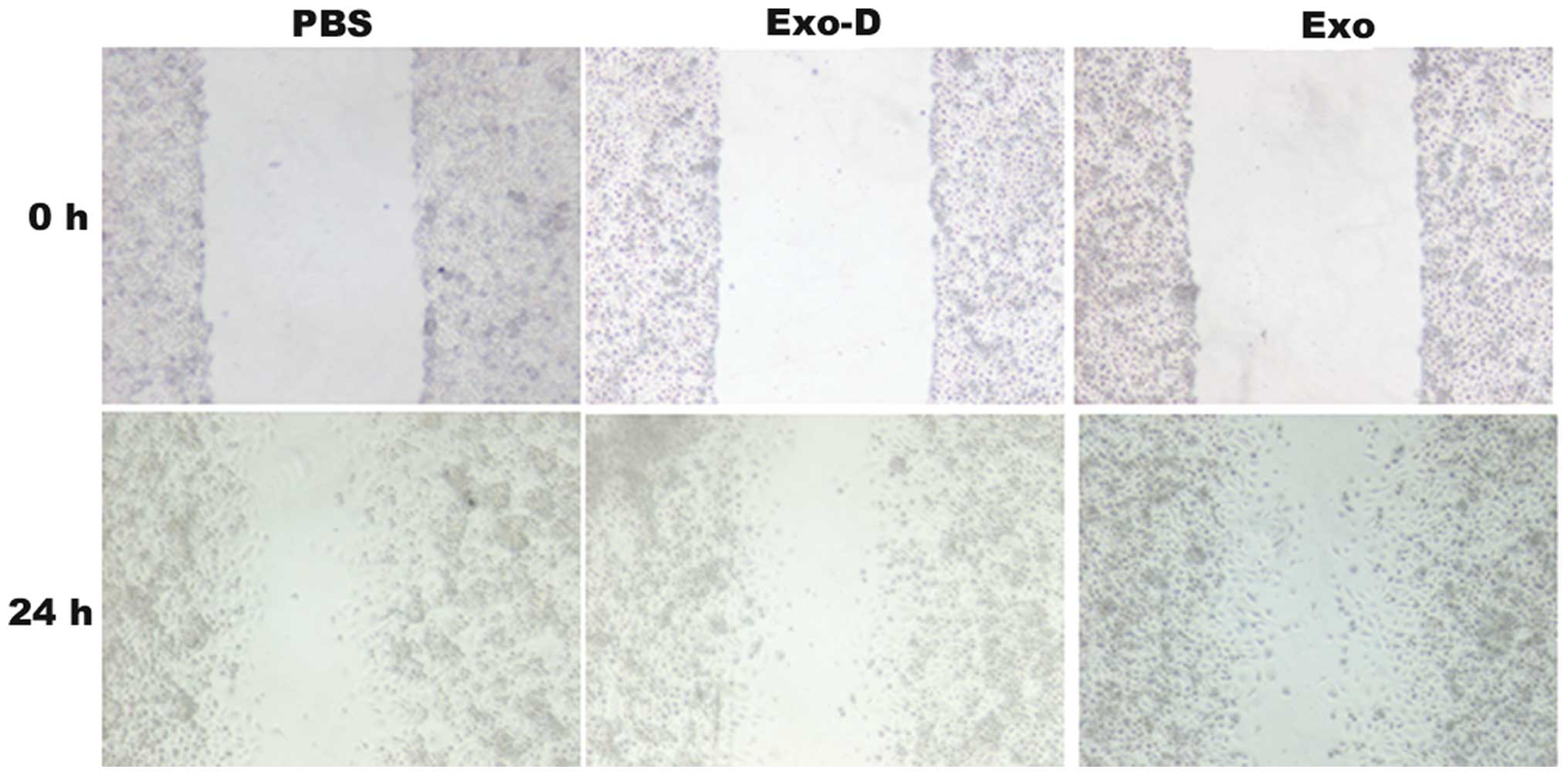
Amiloride, 5-(N,N-Dimethyl)-, hydrochloride was
reported to depress the cell exosome secretion. In the wound
healing assays, the 786-0 cells exhibited an enhanced migratory
capacity to the wounded areas in the Exo group compared with the
PBS and Exo-D groups (Fig. 2). The
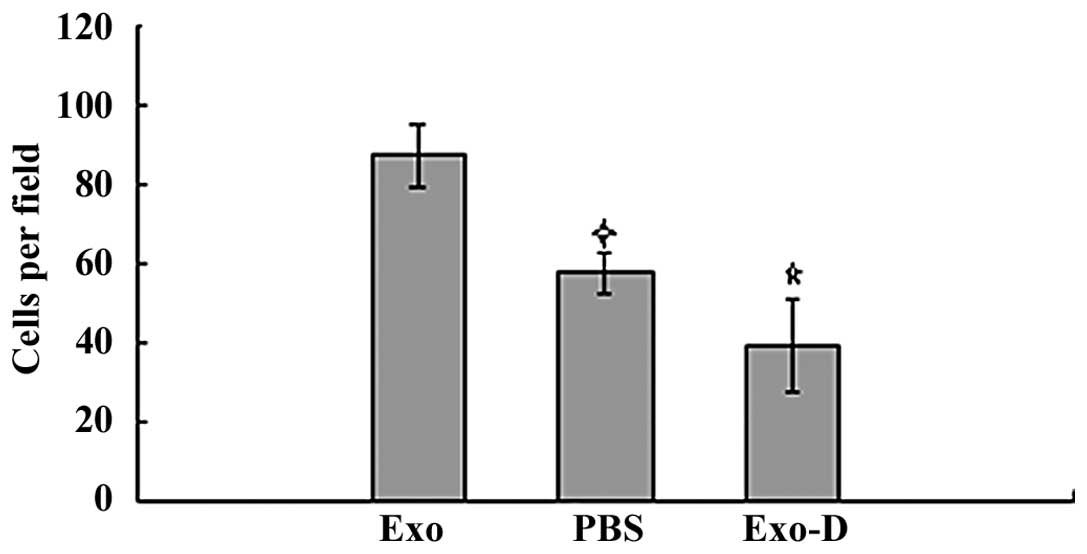
Matrigel invasion assays revealed that the 786-0 cells in the Exo
group had a higher degree of motility compared with those in the
blank control and the Exo-D groups. The invasion ability of cells
in the Exo group (87.5±7.8 cells per field) was significantly
higher compared with that of cells in the PBS (57.6±5.4 cells per
field; P<0.05) and Exo-D (39.3±11.7 cells per field; P<0.05)
groups (Fig. 3).
786-0 cell-derived exosomes enhance 786-0
cell adhesion
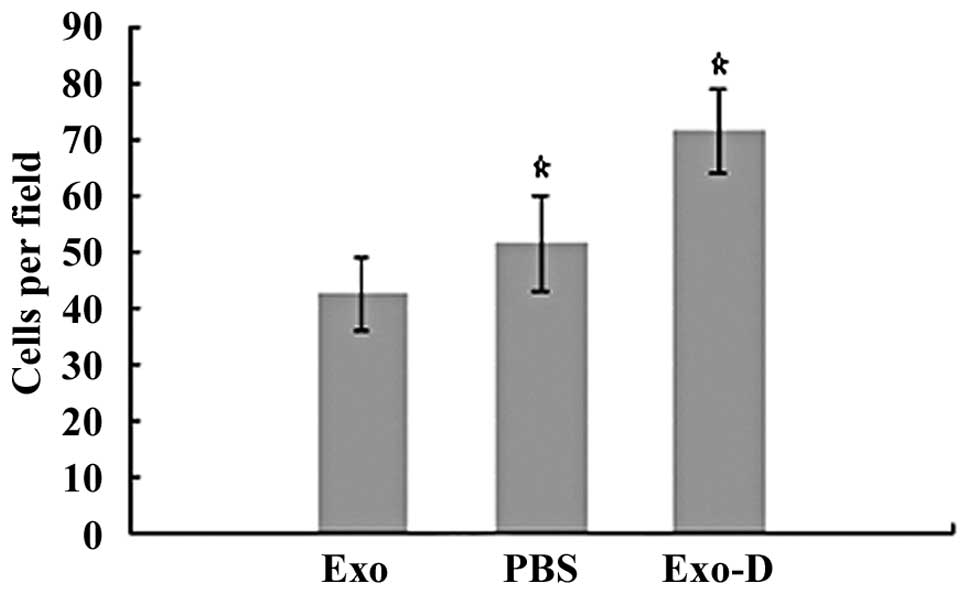
In the cell attachment assays, cells in the Exo, PBS
and Exo-D groups were inoculated in 6-well culture dishes. After
the cells were incubated in 10% FBS/ RPMI-1640 medium for 3 h,
significantly enhanced cell attachment was observed in the Exo-D
group (71.5±7.5 cells per field) compared with attachment in the
Exo (42.5±6.5 cells per field; P<0.05) and PBS (51.5±8.5 cells
per field; P<0.05) groups (Fig.
4). These findings indicated that 786-0 cell-derived exosomes
may decrease the adhesion ability of 786-0 cells.
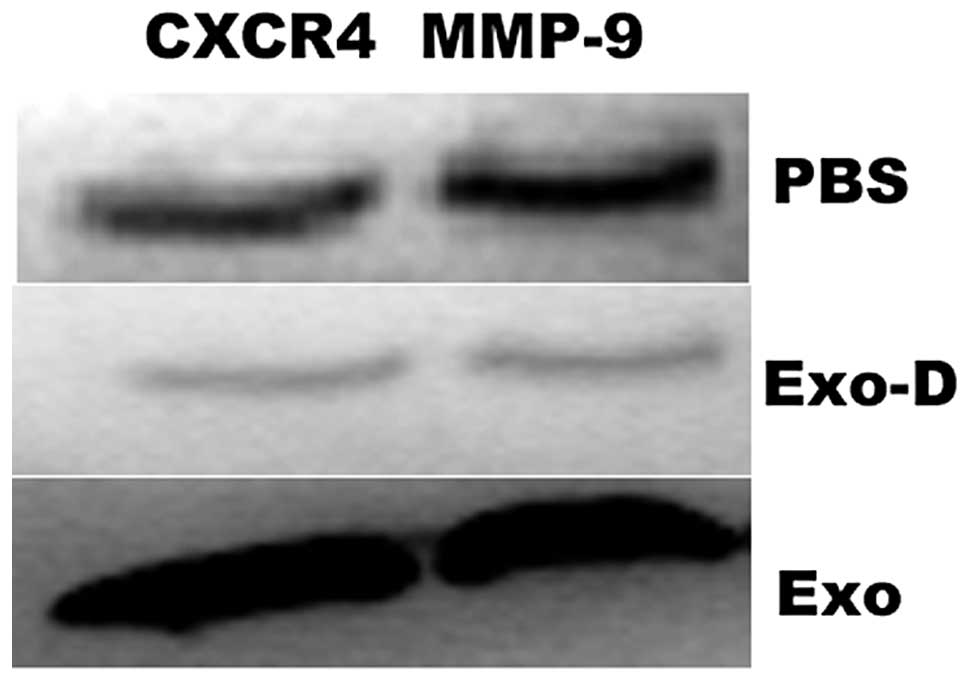
786-0 cell-derived exosomes increase
786-0 cell migration and invasion via the CXCR4 and MMP-9 signaling
pathways
To determine whether exosomes increase 786-0 cell
migration and invasion via the CXCR4 and MMP-9 signaling pathways,
the protein expression of CXCR4 and MMP-9 was examined by western
blott analysis. The protein expression levels of CXCR4 and MMP-9
were identified to be significantly enhanced in the Exo group
compared with the PBS group, however, were significantly reduced in
the Exo-D group (Exo-D) compared with the PBS group (Fig. 5).
Discussion
Surgery is the primary curative therapy for patients
with local RCC. The treatment for metastatic RCC (mRCC) has been a
focus of investigation, however, remains unclear. Analysis of
interleukin-2, vascular endothelial growth factor (VEGF) and
mammalian target of rapamycin inhibitors have provided improvements
in the clinical outcomes of mRCC (10). However, the prognosis for advanced
mRCC patients remains poor, with a five-year survival rate of
<10% (11). Therefore, further
investigations into the underlying mechanisms of mRCC are
required.
Tumor-derived exosomes have a bimodal role in cancer
progression. First, exosomes regulate the local and systemic
environment and contribute to cancer growth and dissemination in
vivo. Second, exosomes may be important in eliciting the
antitumor responses of the immune system (12). Our previous study demonstrated that
tumor-derived exosomes enhanced tumor cell proliferation and
inhibited Jurkat T-cell proliferation in vitro (8). However, whether tumor-derived exosomes
enhanced migration and invasion of the cancer cell itself in
vitro, was not clarified. In the present study, purified
exosomes derived from the 786-0 human RCC cell line exhibited
typical characteristics, including cup-shaped or saucer-like
structures, and ranged between 30 and 100 nm in diameter. Protein
analysis revealed that the 786-0 cell-derived exosomes expressed
G250, ICAM-1 and Hsp70. In addition, treatment with 100 μg/ml
exosomes enhanced cell migration and invasion, but decreased cell
attachment in vitro. However, the underlying mechanisms by
which exosomes enhanced the migration and invasion of 786-0 cells
remains unclear.
The invasion of tumor cells is a complex, multistage
process, which includes the degradation, by proteases, of the
extracellular matrix (ECM) and the basement membrane surrounding
the primary tumor, as well as enhancing cell migration and invasion
(13,14). Numerous molecules have been
associated with cancer metastasis, including MMPs, VEGFs, tumor
necrosis factor, platelet-derived growth factor, transforming
growth factor-β and Twist-related protein 1 (15). Chemokines and their receptors have
been found to contribute to cancer metastasis, particularly stromal
cell-derived factor-1α and its receptor CXCR4 (16,17).
The CXCR4 chemokine receptor is highly expressed in various types
of tumors, including breast, gastric, ovarian, pancreatic, prostate
and renal cancers and acute myeloid leukemia (18). Tumors expressing CXCR4 acquire
properties that enable them to invade tissue barriers, migrate to
secondary organs and form metastases. In patients with RCC, CXCR4
expression has been correlated with a poor overall survival and the
expression of CXCR4 and CXCR7 has been considered as a marker, with
~80% accuracy, for predicting RCC metastasis (19,20).
As CXCR4 has been associated with the metastasis of renal cancers,
CXCR4 expression may be a potential molecular marker for the
increased cell invasion and migration abilities, which are induced
by tumor-derived exosomes.
MMP-9 is a downstream signaling molecule of CXCR4
and is critical for the migration and invasion of cancer cells
(21). MMP-9 belongs to a large
family of MMPs, which are responsible for degrading a wide range of
ECM components (22). Additionally,
MMP-9 is closely associated with tumor invasion and metastasis in a
variety of human tumors, including lung adenocarcinoma,
hepatocellular carcinoma, and prostate and breast cancers (23–26);
furthermore, it was expressed in the serum and urine of patients
with RCC (27). Downregulation of
forkhead box protein M1 reduced the expression and activity of
MMP-2, -9 and VEGF, resulting in the inhibition of migration,
invasion and angiogenesis of renal cancer cell lines (28). In the present study, 786-0 cells
treated with 100 μg/ml 786-0 cell-derived exosomes showed high
migration and invasion capabilities and an increased expression of
CXCR4 compared with the PBS-treated group. These findings indicated
that the CXCR4 and MMP-9 signaling pathways may be involved in the
enhanced migration and invasion capability of 786-0 cells, which
was induced by the 786-0 cell-derived exosomes.
In conclusion, these data demonstrated that
exosomes, which were derived from the 786-0 human RCC cell lines
induced the high migration and invasion capabilities of 786-0 cells
in vitro. The tumor-derived exosomes induced the tumor cell
to highly express CXCR4 and MMP-9. To the best of our knowledge,
this is the first study to report that 786-0 cell-derived exosomes
enhanced migration and invasion of 786-0 cancer cells. However,
further investigations are required to elucidate whether renal
tumor-derived exosomes degrade the ECM and promote renal tumor
invasion in vivo. Our findings may provide novel insights
into the tumor progression of RCC.
Acknowledgements
The present study was supported by a research grant
from the Foundation of National Natural Science Foundation of China
(grant no. 81272572). The authors would like to thank Professors
Luo Chun-Li and Yin Zhi-Kang.
References
|
1
|
Ferlay J, Parkin DM and Steliarova-Foucher
E: Estimates of cancer incidence and mortality in Europe in 2008.
Eur J Cancer. 46:765–781. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics. CA Cancer J Clin. 60:277–300. 2010.
|
|
3
|
Milella M and Felici A: Biology of
metastatic renal cell carcinoma. J Cancer. 2:369–373. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Théry C, Ostrowski M and Segura E:
Membrane vesicles as conveyors of immune responses. Nat Rev
Immunol. 9:581–593. 2009.PubMed/NCBI
|
|
5
|
Balaj L, Lessard R, Dai L, Cho YJ, Pomeroy
SL, Breakefield XO and Skog J: Tumour microvesicles contain
retrotransposon elements and amplified oncogene sequences. Nat
Commun. 2:1802011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Valadi H, Ekström K, Bossios A, Sjöstrand
M, Lee JJ and Lötvall JO: Exosome-mediated transfer of mRNAs and
microRNAs is a novel mechanism of genetic exchange between cells.
Nat Cell Biol. 9:654–659. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kahlert C and Kalluri R: Exosomes in tumor
microenvironment influence cancer progression and metastasis. J Mol
Med (Berl). 91:431–437. 2013. View Article : Google Scholar
|
|
8
|
Yang L, Wu XH, Wang D, Luo CL and Chen LX:
Bladder cancer cell-derived exosomes inhibit tumor cell apoptosis
and induce cell proliferation in vitro. Mol Med Rep. 8:1272–1278.
2013.PubMed/NCBI
|
|
9
|
Zhang Y, Luo CL, He BC, Zhang JM, Cheng G
and Wu XH: Exosomes derived from IL-12-anchored renal cancer cells
increase induction of specific antitumor response in vitro: A novel
vaccine for renal cell carcinoma. Int J Oncol. 36:133–140.
2010.
|
|
10
|
Weiss C, Schulze B, Ottinger A and Rödel
C: To combine or not combine: the role of radiotherapy and targeted
agents in the treatment for renal cell carcinoma. World J Urol. May
8–2013.(Epub ahead of print).
|
|
11
|
Coppin C, Kollmannsberger C, Le L,
Porzsolt F and Wilt TJ: Targeted therapy for advanced renal cell
cancer (RCC): a Cochrane systematic review of published randomised
trials. BJU Int. 108:1556–1563. 2011. View Article : Google Scholar
|
|
12
|
Yang C and Robbins PD: The roles of
tumor-derived exosomes in cancer pathogenesis. Clin Dev Immunol.
2011:8428492011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Samara GJ, Lawrence DM, Chiarelli CJ,
Valentino MD, Lyubsky S, Zucker S and Vaday GG: CXCR4-mediated
adhesion and MMP-9 secretion in head and neck squamous cell
carcinoma. Cancer Lett. 214:231–241. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yu T, Wu Y, Helman JI, Wen Y, Wang C and
Li L: CXCR4 promotes oral squamous cell carcinoma migration and
invasion through inducing expression of MMP-9 and MMP-13 via the
ERK signaling pathway. Mol Cancer Res. 9:161–172. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Park B, Sung B, Yadav VR, Cho SG, Liu M
and Aggarwal BB: Acetyl-11-keto-β-boswellic acid suppresses
invasion of pancreatic cancer cells through the downregulation of
CXCR4 chemokine receptor expression. Int J Cancer. 129:23–33.
2011.
|
|
16
|
Raman D, Baugher PJ, Thu YM and Richmond
A: Role of chemokines in tumor growth. Cancer Lett. 256:137–165.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Orimo A, Gupta PB, Sgroi DC,
Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL
and Weinberg RA: Stromal fibroblasts present in invasive human
breast carcinomas promote tumor growth and angiogenesis through
elevated SDF-1/CXCL12 secretion. Cell. 121:335–348. 2005.
View Article : Google Scholar
|
|
18
|
Manu KA, Shanmugam MK, Rajendran P, Li F,
Ramachandran L, Hay HS, Kannaiyan R, Swamy SN, Vali S, Kapoor S, et
al: Plumbagin inhibits invasion and migration of breast and gastric
cancer cells by downregulating the expression of chemokine receptor
CXCR4. Mol Cancer. 10:1072011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang L, Chen W, Gao L, Yang Q, Liu B, Wu
Z, Wang Y and Sun Y: High expression of CXCR4, CXCR7 and SDF-1
predicts poor survival in renal cell carcinoma. World J Surg Oncol.
10:2122012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gahan JC, Gosalbez M, Yates T, Young EE,
Escudero DO, Chi A, Garcia-Roig M, Satyanarayana R, Soloway MS,
Bird VG and Lokeshwar VB: Chemokine and chemokine receptor
expression in kidney tumors: molecular profiling of histological
subtypes and association with metastasis. J Urol. 187:827–833.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao M, Gao Y, Wang L, Liu S, Han B, Ma L,
Ling Y, Mao S and Wang X: Overexpression of integrin-linked kinase
promotes lung cancer cell migration and invasion via NF-κB-mediated
upregulation of matrix metalloproteinase-9. Int J Med Sci.
10:995–1002. 2013.
|
|
22
|
Jia W, Gao XJ, Zhang ZD, Yang ZX and Zhang
G: S100A4 silencing suppresses proliferation, angiogenesis and
invasion of thyroid cancer cells through downregulation of MMP-9
and VEGF. Eur Rev Med Pharmacol Sci. 17:1495–1508. 2013.
|
|
23
|
Shiau MY, Fan LC, Yang SC, Tsao CH, Lee H,
Cheng YW, Lai LC and Chang YH: Human papillomavirus up-regulates
MMP-2 and MMP-9 expression and activity by inducing interleukin-8
in lung adenocarcinomas. PLoS One. 8:e544232013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dai ZJ, Wang BF, Lu WF, Wang ZD, Ma XB,
Min WL, Kang HF, Wang XJ and Wu WY: Total flavonoids of
Scutellaria barbata inhibit invasion of hepatocarcinoma via
MMP/TIMP in vitro. Molecules. 18:934–950. 2013.
|
|
25
|
Wang X, Lee SO, Xia S, Jiang Q, Luo J, Li
L, Yeh S and Chang C: Endothelial cells enhance prostate cancer
metastasis via IL-6→androgen receptor →TGF-β →MMP-9 signals. Mol
Cancer Ther. 12:1026–1037. 2013.PubMed/NCBI
|
|
26
|
Leifler KS, Svensson S, Abrahamsson A,
Bendrik C, Robertson J, Gauldie J, Olsson AK and Dabrosin C:
Inflammation induced by MMP-9 enhances tumor regression of
experimental breast cancer. J Immunol. 190:4420–4430. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
DI Carlo A: Evaluation of neutrophil
gelatinase-associated lipocalin (NGAL), matrix metalloproteinase-9
(MMP-9) and their complex MMP-9/NGAL in sera and urine of patients
with kidney tumors. Oncol Lett. 5:1677–1681. 2013.
|
|
28
|
Xue YJ, Xiao RH, Long DZ, Zou XF, Wang XN,
Zhang GX, Yuan YH, Wu GQ, Yang J, Wu YT, et al: Overexpression of
FoxM1 is associated with tumor progression in patients with clear
cell renal cell carcinoma. J Transl Med. 10:2002012. View Article : Google Scholar : PubMed/NCBI
|