Introduction
Gastric cancer (GC) is one of the most common types
of malignancies worldwide, with an estimated 934,000 cases reported
globally in 2002, and is the second most common cause of
cancer-related mortality (1). GC is
also a genetic disease developing from a multistep process. Single
or multiple mutations in genes associated with growth control,
invasion and metastasis, form the molecular genetic basis of
malignant transformation and tumor progression (2). Chemotherapy, to a certain extent,
plays a critical role in the treatment of malignant tumors.
However, the identification of predictive biomarkers of resistance
or sensitivity to chemotherapy remains a fundamental challenge in
the selection of patients most likely to benefit from it.
The phosphatidylinositol-3-kinase (PI3K)/Akt pathway
has been shown to be activated in a variety of cancer types.
Studies have shown that the increased expression of PI3K or
phosphoinositide 3-Akt (p-Akt) contributes to gallbladder
carcinogenesis (3) or predicts the
survival of advanced endometrial carcinoma (4). Activation of the PI3K/Akt pathway is
required for the apoptotic evasion (5) and is significantly associated with
increasing tumor grade, decreased apoptosis and clinical outcome in
human gliomas (6). It supports the
development of metastatic cancer and promotes the aggressive
behavior of soft tissue sarcoma (7,8),
indicating the PI3K/Akt pathway as an important biomarker for the
prognosis of cancer patients.
Hypoxia-inducible factor-1α (HIF-1α) plays an
essential role in the adaptive response of cells to hypoxia and is
associated with aggressive tumor behavior. HIF-1α is highly
expressed in small-cell lung cancer and aids in predicting the
overall survival of patients (9),
as well as in selecting patients most likely to benefit from
HIF-1α-targeted therapies (10).
HIF-1α is also overexpressed in mantle cell lymphoma, where it
enhances the aggressive potential and, therefore, this observation
may result in more efficient target therapies (11). Notably, hypoxia induces a biphasic
effect on HIF-1α stabilization with accumulation in early hypoxia,
depending on activation of the PI3K/Akt pathway (12). In hypoxic tumor cells, reactive
oxygen species increase HIF-1α transcription via the PI3K/Akt
pathway (13), and silencing of
HIF-1α suppresses tumorigenicity of renal cell carcinoma through
the regulation of the PI3K/Akt pathway (14). Activation of PI3K/Akt signaling
promotes the progression of hepatocarcinogenesis, while its
blockade controls angiogenesis and tumor growth by regulating the
expression of HIF-1α (15,16).
Notably, the expression of PI3K/Akt is increased in
non-small cell lung cancer treated with adjuvant chemotherapy and
serves as a novel independent prognostic biomarker (17). Deregulation of the PI3K/Akt pathway
is associated with resistance to the chemotherapeutic agent and
confers drug resistance to treatment with paclitaxel in breast
cancer (18,19). Although certain studies have
demonstrated the enhanced effectiveness of targeting tumor cells
with combinations of chemotherapeutic agents and signal
transduction inhibitors (20), the
enhancing effects of blockade of the PI3K/Akt pathway on paclitaxel
in hypoxic GC cells remains unclear. In the present study, the
correlations of PI3K, p-Akt and HIF-1α expression with the
clinicopathological characteristics of patients with GC were
analyzed. Hypoxic GC SGC-7901 cells were pretreated with LY294002
and/or paclitaxel to investigate the enhancing effects of the PI3K
inhibitor on paclitaxel through cell proliferation activity and
apoptosis.
Materials and methods
Materials
The human GC SGC7901 cell line was donated from the
Shanghai Tumor Research Institute (no. 01842; Shanghai, China). The
primers of PI3K and HIF-1α were synthesized by the Shanghai
Biological Engineering Technology Co., Ltd. (Shanghai, China). The
PI3K rabbit-anti-human polyclonal antibody (sc-1637) was purchased
from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA); p-Akt
rabbit-anti-human polyclonal antibody (D9E) and β-actin were
purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA);
and HIF-1α rabbit-anti-human polyclonal antibody (BA0912) was
obtained form Wuhan Boster Biological Engineering Co., Ltd (Wuhan,
China). The GC tissue samples treated with paclitaxel were obtained
from severe combined immune deficiency (SCID) mice orthotopically
implanted with human GC cells from the Gastrointestinal Disease
Laboratory of Shanghai Sixth People’s Hospital (Shanghai,
China).
Drugs and reagents
Paclitaxel was donated by Professor G-Y Fan from the
Kunming Botany Institute (Yunnan, China); the Cell Counting Kit-8
(CCK-8) was purchased from Tongren Chemical Institute (Japan);
Dulbecco’s modified Eagle’s medium (DMEM) and fetal bovine serum
(FBS) were purchased from Thermo Fisher Scientific Inc. (Waltham,
MA, USA); TRIzol® reagent and LY294002 were obtained
from Invitrogen Life Technologies (Carlsbad, CA, USA); M-MLV
Reverse Transcriptase was purchased from Promega Corporation
(Madison, WI, USA); SYBR Green master mix was purchased from Takara
Bio, Inc., (Otsu, Japan); the Annexin V-fluorescein isothiocyanate
cell apoptosis detection kit was obtained from KeyGen Biotech.,
Co., Ltd. (ab14085; Nanjing, China); and the Enhanced
Chemiluminescence (ECL)-PLUS™ western blotting reagents were
purchased from GE Healthcare (Piscataway, NJ, USA).
Clinical samples and data
A total of 45 patients with GC and 36 patients with
chronic gastritis were enrolled in this study at the General
Surgery and Digestive Endoscopy Room from December, 2007 to June,
2008. The pathological staging was determined according to the
American Joint Committee on Cancer tumor-node-metastasis (TNM)
staging system. The use of the tissue samples and clinical data was
approved by the Medical Ethics Committee of Shanghai Jiao Tong
University (Shanghai, China).
Immunohistochemical analysis
The protein expression of PI3K, p-Akt and HIF-1α
were analyzed by immunohistochemical staining. The anti-PI3K, p-Akt
and HIF-1α antibodies were used at 1:100 dilutions. Endogenous
peroxidase was inhibited by incubation with freshly prepared 3%
hydrogen peroxide with 0.1% sodium azide. Non-specific staining was
blocked with 0.5% casein and 5% normal serum. The tissues were
incubated with biotinylated antibodies and horseradish peroxidase
(Cell Signaling Technology, Inc.). Staining was developed with
diaminobenzidine substrate and sections were counterstained with
hematoxylin and eosin. Normal serum or phosphate-buffered saline
(PBS) replaced the antibodies in negative controls. The images were
analyzed with Image-Pro Plus 4.5 System (Media Cybernetics, Inc.,
Rockville, MD, USA). The total optical density value and area of
intracellular fluorescence for each section was measured.
Cell culture and pretreatment
The SGC7901 cells were cultured in DMEM supplemented
with 10% heat-inactivated FBS, 100 U/ml penicillin and 100 μg/ml
streptomycin. The cells were stored in a humidified atmosphere of
5% CO2 at 37°C for 30 min. Under normal oxygen, the
cells were incubated in 20% O2 and 5% CO2
with saturated humidity at 37°C for 30 min. The hypoxic cells were
incubated in an AnaeroPack™ containing 20% CO2 and
<1% O2 at 37°C for 30 min. The cells pretreated under
hypoxic conditions were further treated with the PI3K inhibitor,
LY294002, (40 Mm) for 30 min, followed by paclitaxel (0.1 Mm).
Quantitative polymerase chain reaction
(qPCR)
To quantitatively determine the mRNA expression
levels of PI3K and HIF-1α in GC SGC-7901 cells, qPCR was used.
Total RNA of each clone was extracted with TRIzol reagent according
to the manufacturer’s instructions. Reverse-transcription using
M-MLV, and cDNA amplification using SYBR Green master mix kit, were
performed according to the manufacturer’s protocol. The genes were
amplified using specific oligonucleotide primers and a human
β-actin gene was used as an endogenous control. The PCR primer
sequences were as follows: Forward, 5′-AAGAAGCAAGCAGCTGAG-3′ and
reverse, 5′-CTACAGAGCAGGCATAG-3′ for PI3K; forward,
5′-TCAAAGTCGGACAGCCTC-3′ and reverse, 5′-CCAGCAGTCTACATGC-3′ for
HIF-1α; forward, 5′-CTTCGAGCAAGAGATGGC-3′ and reverse,
5′-CTCCTTCTGCATCCTGTC-3′ for β-actin. Data were analyzed using the
comparative Ct method (2−ΔΔCt). Three separate
experiments were performed for each clone.
Western blot analysis
The hypoxic SGC-7901 cells treated with LY294002
and/or paclitaxel were harvested and extracted using lysis buffer
[Tris-HCl, sodium-dodecyl sulfate (SDS), mercaptoethanol and
glycerol] purchased from Santa Cruz Biotechnology, Inc. Cell
extracts were heated to boiling point for 5 min in loading buffer
and equal amounts of cell extracts were separated on 15% SDS-PAGE
gels. Separated protein bands were transferred into polyvinylidene
fluoride membranes, which were blocked in 5% skimmed milk powder.
The primary antibodies against PI3K, p-Akt and HIF-1α were diluted
according to the manufacturer’s instructions and incubated
overnight at 4°C. Horseradish peroxidase-linked secondary
biotinylated antibodies were added at a dilution ratio of 1:1,000
and incubated at room temperature for 2 h. The membranes were
washed with PBS three times and the immunoreactive bands were
visualized using the ECL-PLUS/Kit according to the manufacturer’s
instructions. The relative protein levels in different cell lines
were normalized to the GAPDH concentration. Three separate
experiments were performed for each clone. Finally, the immune
complexes were developed using an ECL detection kit according to
the manufacturer’s instructions (ECL GST western blotting detection
kit, Pierce Biotechnology, Inc., Waltham, MA, USA) and the
GelGDoc2000 imaging system (Bio-Rad Laboratories GmbH, Munich,
Germany) was employed to analyze the bands, and the protein levels
by the relative optical density.
CCK-8 assay
Cell proliferation following treatment with LY294002
and/or paclitaxel was measured by the CCK-8 assay. The GC SGC-7901
cells were seeded at a density of 2×104 cells/100
μl/well in 96-well plates and left to attach overnight. The medium
was then removed and 200 μl of FBS was added followed by LY294002
and/or paclitaxel. The cells were incubated under these conditions
for 24, 48, 72, 96, 120 and 148 h at 37°C in a humidified
atmosphere of 5% CO2. After the designated time, CCK-8
was added to each well containing 200 μl of the culture medium and
the oligopeptide mixture, and further incubated for 4 h at 37°C.
The amount of formazan dye was measured at 450 nm using the
Multi-Mode Microplate Reader (Nanchang Biotek Medical Device Co.,
Ltd., Nanchang, China). All the experiments were performed in
triplicate and repeated three times.
Cell apoptosis analysis
Cell apoptosis detection was performed using the BD
Accuri™ C6 Flow cytometer (BD Biosciences, San Jose, CA, USA). The
exposure of PBS on the extracellular side of the cell membrane was
quantified by propidium iodide (PI) staining (Invitrogen Life
Technologies). The SGC-7901 cells were placed in six-well plates
and, after 24 h of incubation, the cells were treated with LY294002
and/or paclitaxel for 24 h and then harvested. Following
centrifugation, cell pellets were washed twice with cold PBS. The
cells were then incubated with 5 μl of PI at room temperature for
15 min in the dark. Following incubation, 400 μl of 1X binding
buffer was added to each tube. The cells were immediately analyzed
by flow cytometry.
Statistical analysis
Data are expressed as the means ± standard deviation
where applicable. Statistically significant differences in each
assay were determined by SPSS software, version 20.0 (SPSS, Inc.,
Chicago, IL, USA). Differences in each group were tested for
significance using Student’s t-test or one-way analysis of
variance. P<0.05 was considered to indicate a statistically
significant difference.
Results
Correlations of PI3K, p-Akt and HIF-1α
expression with the clinicopathological characteristics
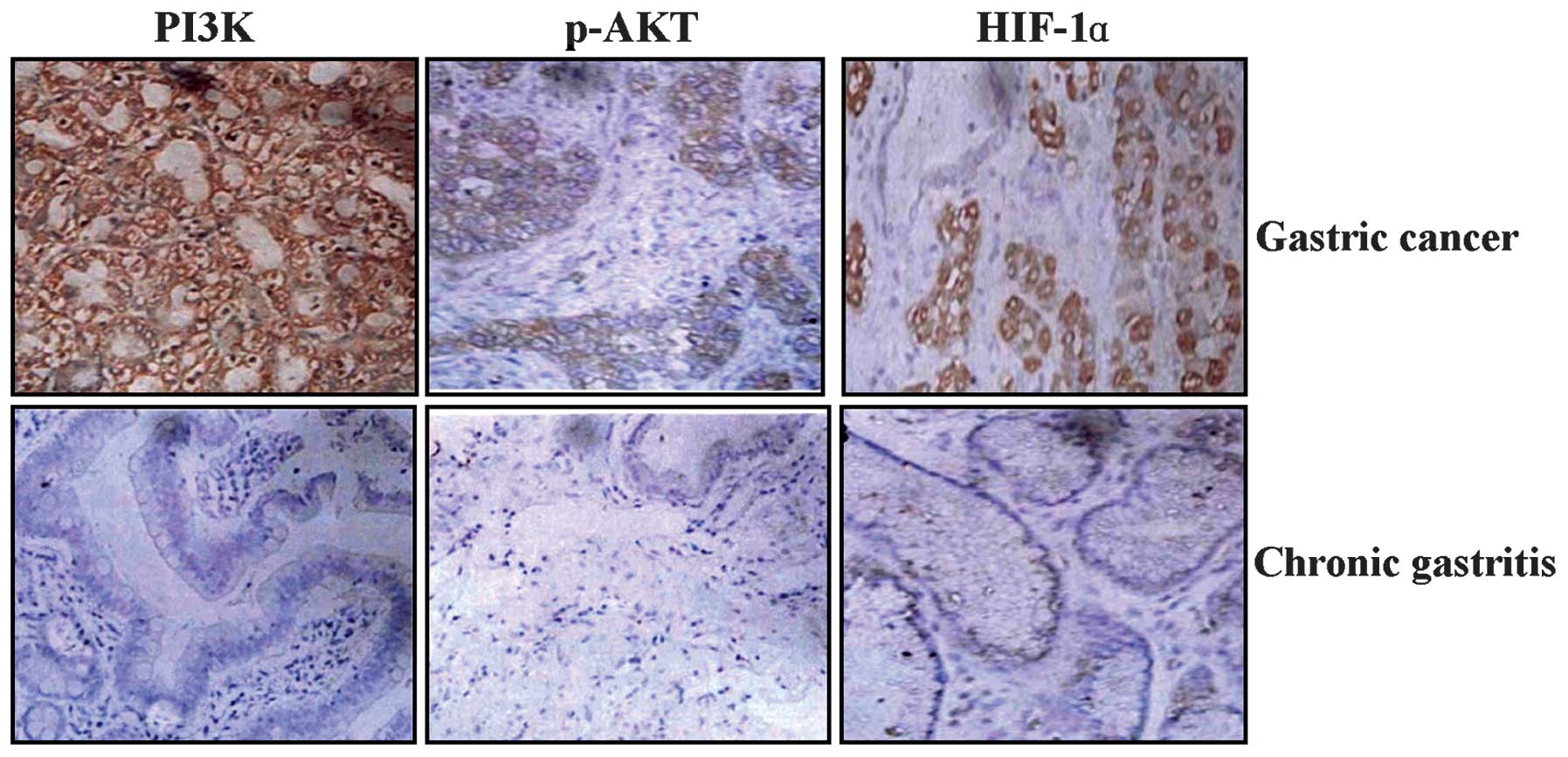
The GC tissue sections were analyzed by
immunohistochemistry (IHC) and Image-Pro Plus 4.5 software (Media
Cybernetics, Inc.). As shown in Fig.
1 and Table I, the positive
expression of PI3K, p-Akt and HIF-1α was predominantly localized in
the cytoplasm of the GC tissue cells, but was not identified in the
chronic gastritis cells. The expression intensities of PI3K, p-Akt
and HIF-1α were significantly increased in GC tissues compared with
chronic gastritis tissues (each P<0.01).
 | Table IExpression of PI3K, p-AKT and HIF-1α
in gastric cancer (Gray values). |
Table I
Expression of PI3K, p-AKT and HIF-1α
in gastric cancer (Gray values).
| Markers | Group | Cases | Gray value | P-value |
|---|
| PI3K | Gastric cancer | 45 | 202.4±10.7 | <0.01 |
| Chronic
gastritis | 36 | 220.3±4.3 | |
| p-AKT | Gastric cancer | 45 | 223.6±11.7 | <0.01 |
| Chronic
gastritis | 36 | 234.0±4.6 | |
| HIF-1α | Gastric cancer | 45 | 200.3±6.9 | <0.01 |
| Chronic
gastritis | 36 | 218.7±4.4 | |
The correlations of PI3K, p-Akt and HIF-1α
expression and various clinical and pathological characteristics
were analyzed. As summarized in Table
II, no significant correlation was found between PI3K, p-Akt
and HIF-1α expression and gender, age, tumor size and peripheral
nerve infiltration in patients with GC (each P>0.05), while
their expression was significantly correlated with TNM staging,
lymph node metastases, lymphatic infiltration and vascular
infiltration (each P<0.01), but inversely correlated with tumor
differentiation (P<0.01).
 | Table IICorrelation of PI3K, p-AKT and HIF-1α
expression with the clinicopathological characteristics of patients
with GC. |
Table II
Correlation of PI3K, p-AKT and HIF-1α
expression with the clinicopathological characteristics of patients
with GC.
| Variables | Cases | PI3K | P-value | p-AKT | P-value | HIF-1α | P-value |
|---|
| Age, years |
| ≤68 | 23 | 201.0±9.2 | 0.052 | 223.7±10.7 | 0.841 | 200.7±6.8 | 0.335 |
| >68 | 22 | 203.9±10.3 | | 223.4±11.7 | | 199.8±7.0 | |
| Gender |
| Male | 32 | 202.9±10.9 | 0.279 | 223.9±11.0 | 0.526 | 200.7±6.6 | 0.142 |
| Female | 13 | 201.2±10.4 | | 223.0±11.7 | | 199.2±7.4 | |
| Tumor size, cm |
| ≤5 | 30 | 202.4±8.4 | 0.926 | 224.2±10.8 | 0.256 | 200.8±5.4 | 0.184 |
| >5 | 15 | 202.9±8.4 | | 200.3±6.9 | | 199.3±9.1 | |
| Degree of
differentiation |
| Well/moderately | 15 | 206.3±10.9 | <0.01 | 227.6±10.6 | <0.01 | 202.1±6.3 | 0.005 |
| Poorly | 30 | 200.4±10.2 | | 221.6±10.9 | | 199.4±7.0 | |
| TNM staging |
| I+II | 20 | 207.7±8.8 | <0.01 | 228.6±9.5 | <0.01 | 204.3±5.6 | <0.01 |
| III+IV | 25 | 198.1±10.3 | | 219.6±10.8 | | 197.1±6.1 | |
| Lymph node
metastases |
| No | 19 | 208.7±8.4 | <0.01 | 228.7±9.6 | <0.01 | 204.3±5.7 | <0.01 |
| Yes | 26 | 197.8±10.2 | | 219.8±10.7 | | 197.3±6.1 | |
| Lymphatic vessel
infiltration |
| − | 11 | 207.6±7.0 | <0.01 | 230.7±8.4 | <0.01 | 205.7±5.3 | <0.01 |
| + | 34 | 200.7±11.2 | | 221.3±11.0 | | 198.5±6.4 | |
| Vascular
infiltration |
| − | 29 | 205.0±9.9 | <0.01 | 226.6±10.6 | <0.01 | 201.6±7.2 | <0.01 |
| + | 16 | 197.6±10.4 | | 218.1±10.0 | | 197.9±5.5 | |
| Perineural
infiltration |
| − | 6 | 203.6±6.6 | 0.324 | 225.7±10.4 | 0.265 | 202.5±4.8 | 0.053 |
| + | 39 | 202.2±11.3 | | 223.2±11.3 | | 199.9±7.1 | |
Effects of LY294002 and/or paclitaxel on
the expression of PI3K, p-Akt and HIF-1α
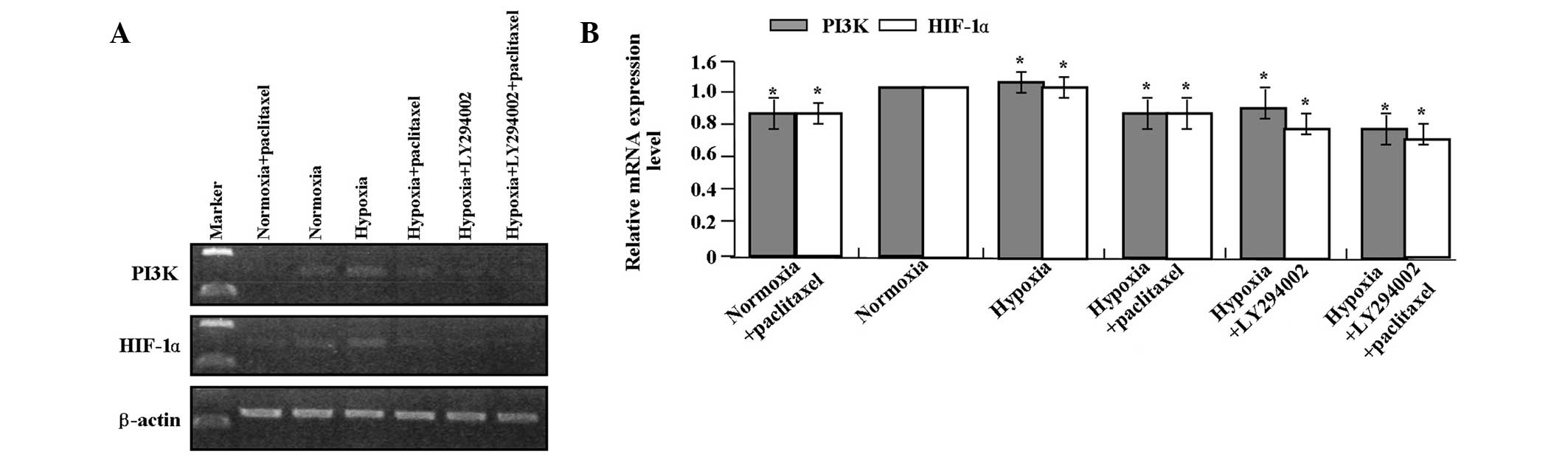
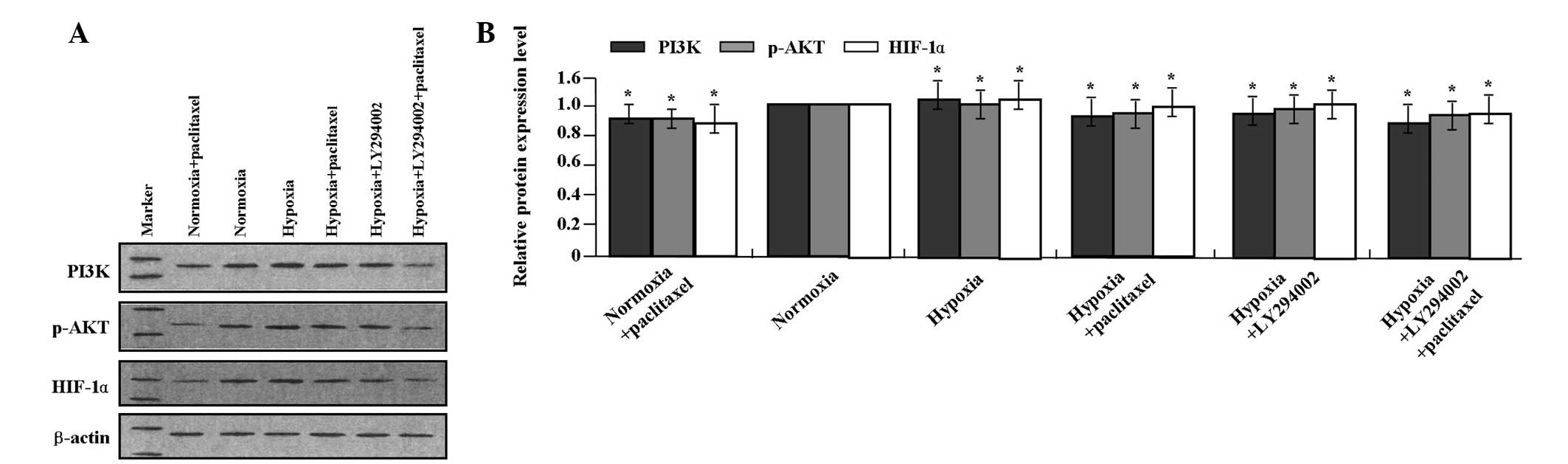
qPCR and western blot analysis were performed to
detect the effects of LY294002 and paclitaxel on the expression of
PI3K, p-Akt and HIF-1α in the GC SGC-7901 cells. LY294002 combined
with paclitaxel markedly inhibited the mRNA (Fig. 2A and B) expression levels of PI3K
and HIF-1α (it was not necessary to detect the expression of p-Akt
as it is downstream of PI3K) and protein (Fig. 3A and B) expression levels of PI3K,
p-Akt and HIF-1α in GC SGC-7901 cells compared with the single
treatment of LY294002 or paclitaxel (each P<0.01). LY294002 or
paclitaxel decreased the expression of PI3K, p-Akt and HIF-1α at
the transcriptional and translational levels compared with the
hypoxic group (each P<0.01).
Effects of LY294002 and/or paclitaxel on
cell proliferation
To determine whether LY294002 and/or paclitaxel
affect the proliferative activity of hypoxic GC cells, the CCK-8
assay was performed to detect cell viability. As summarized in
Table III, LY294002 combined with
paclitaxel significantly decreased the cell viability in SGC-7901
cells compared with the single treatment of LY294002 or paclitaxel
(each P<0.01). In addition, LY294002 or paclitaxel reduced cell
viability compared with the hypoxic group (each P<0.01).
 | Table IIIProliferative activity of gastric
cancer cells (optical density values). |
Table III
Proliferative activity of gastric
cancer cells (optical density values).
| Day |
|---|
|
|
|---|
| Groups | 1 | 2 | 3 | 4 | 5 | 6 |
|---|
| Normoxia | 0.45±0.01 | 0.75±0.02 | 1.25±0.06 | 1.60±0.04 | 1.86±0.04 | 2.12±0.10 |
|
Normoxia+paclitaxel | 0.43±0.02 | 0.60±0.03a | 0.87±0.0a | 1.22±0.04a | 1.54±0.05a | 1.74±0.07a |
| Hypoxia | 0.46±0.03 | 0.68±0.04a | 1.05±0.05a | 1.40±0.07a | 1.65±0.02a | 1.85±0.04a |
|
Hypoxia+paclitaxel | 0.44±0.06 | 0.59±0.04a,b | 0.83±0.05a,b | 1.11±0.05a,b | 1.38±0.07a,b | 1.60±0.10a,b |
|
Hypoxia+LY294002 | 0.45±0.04 | 0.64±0.03a–c | 0.85±0.02a–c | 1.10±0.10a,b | 1.33±0.12a–c | 1.61±0.11a–c |
|
Hypoxia+paclitaxel+LY294002 | 0.42±0.07 | 0.53±0.03a–c | 0.67±0.03a–c | 0.87±0.03a–c | 1.11±0.12a–c | 1.29±0.04a–c |
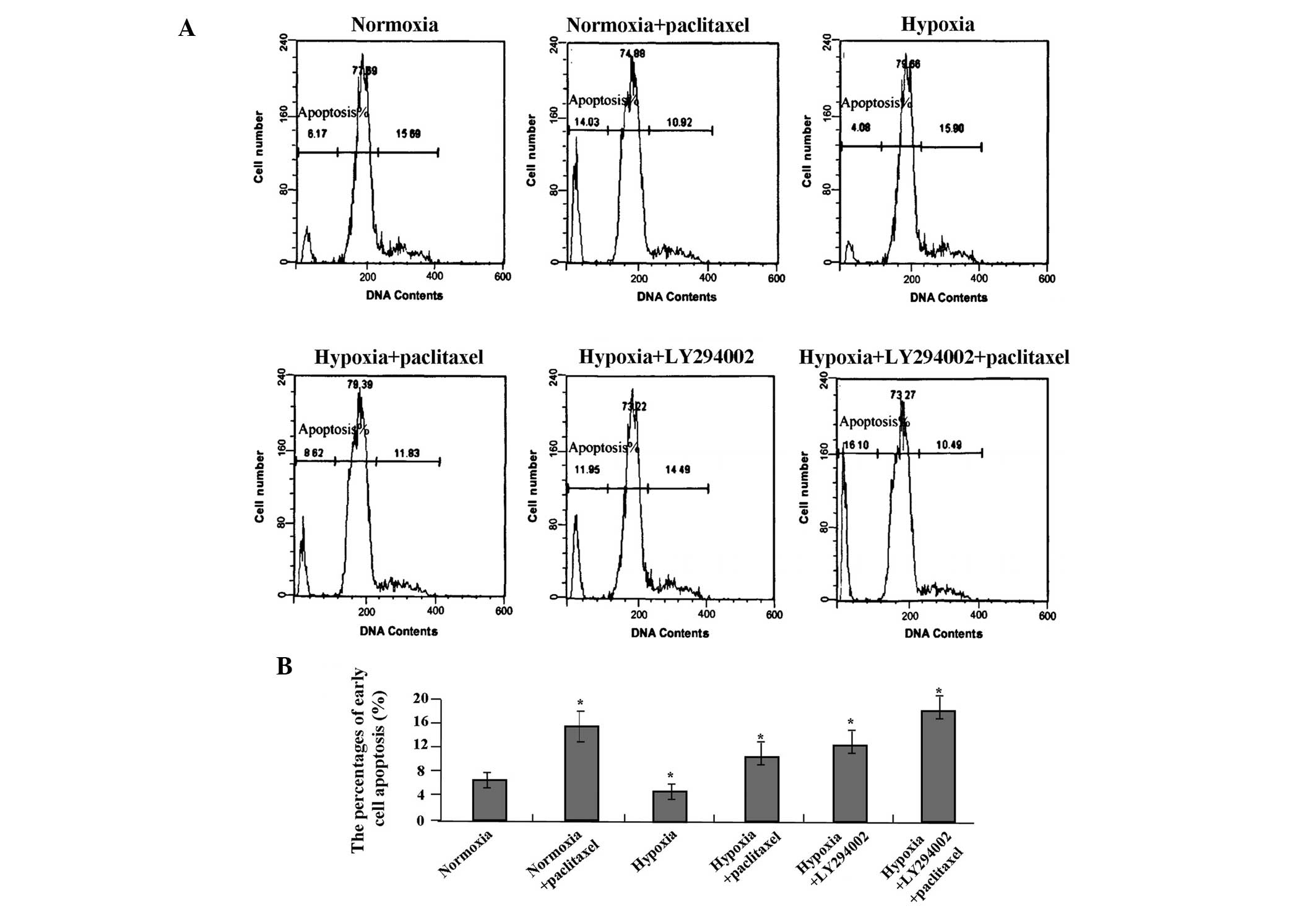
Effects of LY294002 and/or paclitaxel on
cell apoptosis
Cell apoptosis was measured by flow cytometry using
PI staining. Following treatment with LY294002 and/or paclitaxel
for 24 h, LY294002 combined with paclitaxel significantly increased
the percentage of early cell apoptosis compared with the single
treatment of LY294002 or paclitaxel (each P<0.01) (Fig. 4A and B). LY294002 or paclitaxel
increased the percentage of early cell apoptosis compared with the
hypoxic group (both P<0.01).
Effects of paclitaxel on the expression
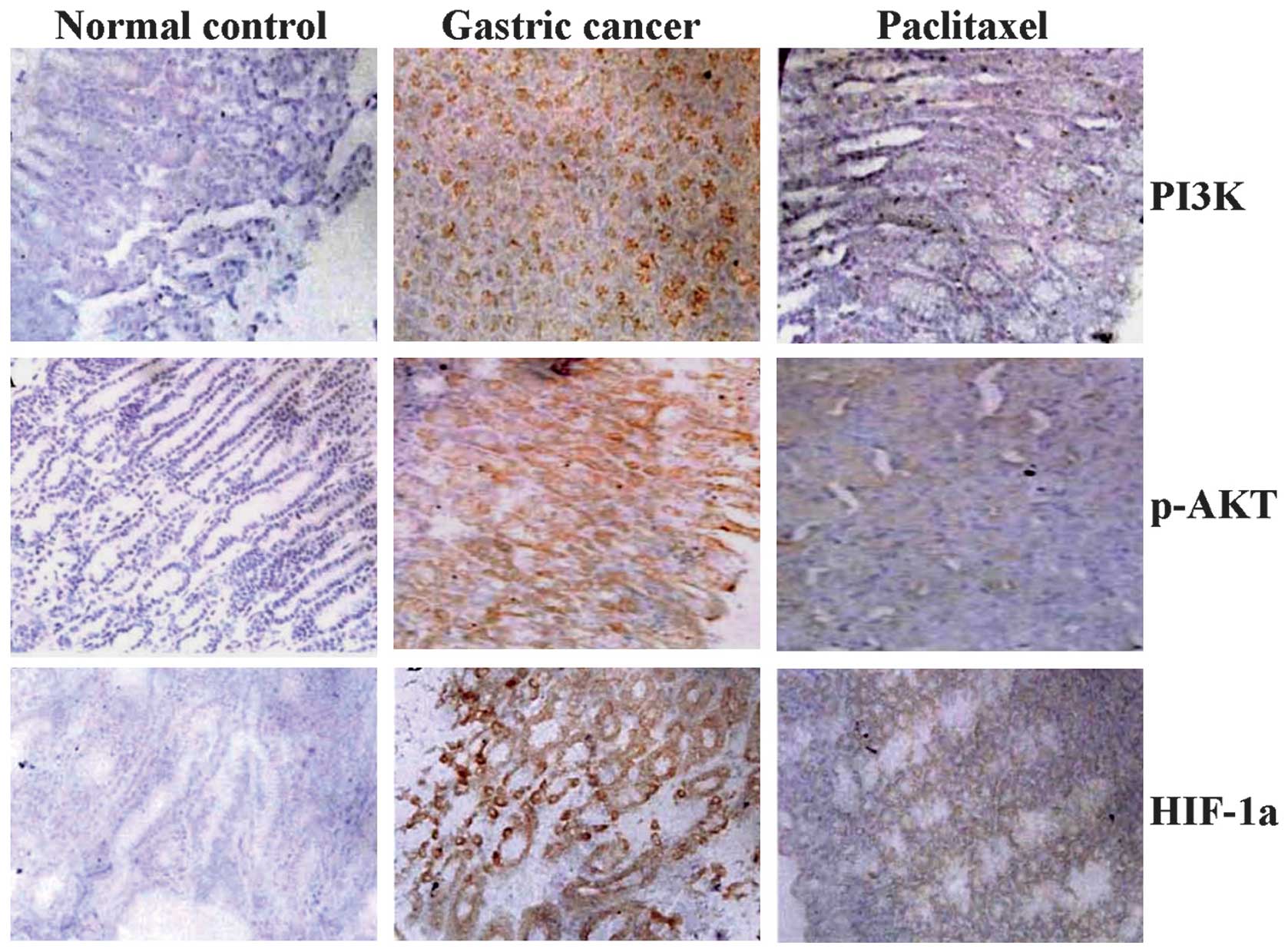
of PI3K, p-Akt and HIF-1α in vivo
SCID mice orthotopically implanted with human GC
tissues were treated with paclitaxel (intraperitoneal
administration of 5 mg/kg) and the effects of paclitaxel on the
expression of PI3K, p-Akt and HIF-1α were assessed by IHC.
Paclitaxel decreased the expression of PI3K, p-Akt and HIF-1α
(Fig. 5) compared with the GC and
normal control groups (each P<0.01).
Discussion
The PI3K/Akt pathway is one of the most important
signaling networks in cancer. Emerging evidence indicates that
activation of this pathway plays a significant role in the
development and progression of certain malignancies. Zhang et
al reported that the PI3K/Akt pathway is expressed in 71.7
(43/60) and 68.3% (41/60) of colon cancer and is closely associated
with serous coat infiltration and lymphatic metastasis (21), serving as an independent prognostic
marker for patients with colorectal cancer (CRC) (22). HIF-1α is a transcription factor
recognized to control the delivery of oxygen and nutrients through
the induction of angiogenesis under hypoxic conditions.
Overexpression of HIF-1α is significantly correlated with
histology, depth of invasion and poor prognosis for patients with
GC, and may be utilized for tumor-specific molecular target-based
therapy (23). In the present
study, the positive expression of PI3K, p-Akt and HIF-1α were
significantly increased in GC tissues compared with chronic
gastritis, and were associated with TNM staging, lymph node
metastases and lymphatic and vascular infiltration, but inversely
correlated with tumor differentiation. This suggested that PI3K,
p-Akt and HIF-1α are potential therapeutic targets for GC.
Inhibiting the molecules involved in the PI3K/Akt or
HIF-1α signal transduction pathway is a possible strategy for the
treatment of cancer. The PI3K inhibitor and conventional
chemotherapy provide an effective approach to inhibiting tumor
growth in ovarian cancer (24).
Paclitaxel is extensively used in chemotherapy for various cancers.
PI3K is involved in low susceptibility of CRC to paclitaxel and
PI3K-targeting agents may enable a new paclitaxel-based
chemotherapy for CRC (25).
Furthermore, the Akt inhibitor increases the therapeutic efficacy
of paclitaxel for patients with ovarian cancer (26). HIF-1α affects the sensitivity of
paclitaxel in lung cancer cells and targeted inhibition of HIF-1α
may overcome the drug resistance of paclitaxel (27). However, an individual study has
reported that pharmacological PI3K/Akt inhibition antagonizes the
efficacy of chemotherapeutic agents primarily effective in the S or
G2 phase of the cell cycle (28).
Our current study indicated that combining the PI3K inhibitor,
LY294002, with paclitaxel significantly decreased cell viability
and increased cell apoptosis in hypoxic GC SGC-7901 cells compared
with the single treatment of LY294002 or paclitaxel. Moreover,
treatment with LY294002 or paclitaxel reduced cell viability and
induced apoptosis compared with the hypoxic GC cells, suggesting
that PI3K/Akt-targeted therapy with paclitaxel is more efficacious
for treating GC with activated PI3K/Akt signaling.
Notably, the PI3K/Akt pathway regulates HIFα
activity (29), and
HIF-1α-dependent transcription is blocked by negative Akt or PI3K
and by the wild-type phosphatase and tensin homolog (30). Direct evidence has also revealed
that activation of the PI3K/Akt/HIF-1α pathway plays a critical
role in mediating hypoxia-induced drug resistance resulting in an
unfavorable treatment outcome of hepatocellular carcinoma (31). However, Manohar et al
(32) reported that the HIF-1α
inhibitor modulates the PI3K/Akt pathway and contributes to
antitumor activity. In the present study, LY294002 combined with
paclitaxel markedly inhibited the expression of PI3K, p-Akt and
HIF-1α in GC SGC-7901 cells compared with the single treatment of
LY294002 or paclitaxel, and LY294002 or paclitaxel decreased the
expression of PI3K and p-Akt in hypoxic conditions, suggesting that
targeting PI3K/Akt signaling in tumor cells may inhibit HIF-1α
expression and increase the therapeutic efficacy of paclitaxel.
In conclusion, our findings suggest that the
increased expression of the PI3K/Akt or HIF-1α pathway is closely
correlated with tumor differentiation, TNM staging, lymph node
metastases, and lymphatic and vascular infiltration. In addition,
inhibition of the PI3K/Akt pathway enhanced the therapeutic
efficacy of paclitaxel in GC cells under hypoxic conditions,
suggesting that the PI3K/Akt or HIF-1α pathway may serve as an
important therapeutic target for the paclitaxel treatment of
cancer.
Acknowledgements
This study was supported by the National Nature
Science Foundation of China (grant nos. 81272752 and 81302093).
References
|
1
|
Jemal A, Bray F, Center MM, et al: Global
cancer statistics. CA Cancer J Clin. 61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Tajima Y, Yamazaki K, Makino R, et al:
Gastric and intestinal phenotypic marker expression in early
differentiated-type tumors of the stomach: clinicopathologic
significance and genetic background. Clin Cancer Res. 12:6469–6479.
2006. View Article : Google Scholar
|
|
3
|
Li Q and Yang Z: Expression of
phospho-ERK1/2 and PI3-K in benign and malignant gallbladder
lesions and its clinical and pathological correlations. J Exp Clin
Cancer Res. 28:652009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Uegaki K, Kanamori Y, Kigawa J, et al:
PTEN-positive and phosphorylated-Akt-negative expression is a
predictor of survival for patients with advanced endometrial
carcinoma. Oncol Rep. 14:389–392. 2005.
|
|
5
|
Sun ZJ, Chen G, Hu X, et al: Activation of
PI3K/Akt/IKK-alpha/NF-kappaB signaling pathway is required for the
apoptosis-evasion in human salivary adenoid cystic carcinoma: its
inhibition by quercetin. Apoptosis. 15:850–863. 2010. View Article : Google Scholar
|
|
6
|
Chakravarti A, Zhai G, Suzuki Y, et al:
The prognostic significance of phosphatidylinositol 3-kinase
pathway activation in human gliomas. J Clin Oncol. 22:1926–1933.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xue G, Restuccia DF, Lan Q, et al:
Akt/PKB-mediated phosphorylation of Twist1 promotes tumor
metastasis via mediating cross-talk between PI3K/Akt and TGF-β
signaling axes. Cancer Discov. 2:248–259. 2012.PubMed/NCBI
|
|
8
|
Valkov A, Kilvaer TK, Sorbye SW, et al:
The prognostic impact of Akt isoforms, PI3K and PTEN related to
female steroid hormone receptors in soft tissue sarcomas. J Transl
Med. 9:2002011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee GW, Go SI, Cho YJ, et al:
Hypoxia-inducible factor-1α and excision repair cross-complementing
1 in patients with small cell lung cancer who received front-line
platinum-based chemotherapy: a retrospective study. J Thorac Oncol.
7:528–534. 2012.
|
|
10
|
Park S, Ha SY, Cho HY, et al: Prognostic
implications of hypoxia-inducible factor-1α in epidermal growth
factor receptor-negative non-small cell lung cancer. Lung Cancer.
72:100–107. 2011.
|
|
11
|
Argyriou P, Papageorgiou SG, Panteleon V,
et al: Hypoxia-inducible factors in mantle cell lymphoma:
implication for an activated mTORC1→HIF-1α pathway. Ann Hematol.
90:315–322. 2011.PubMed/NCBI
|
|
12
|
Mottet D, Dumont V, Deccache Y, et al:
Regulation of hypoxia-inducible factor-1alpha protein level during
hypoxic conditions by the phosphatidylinositol
3-kinase/Akt/glycogen synthase kinase 3beta pathway in HepG2 cells.
J Biol Chem. 278:31277–31285. 2003. View Article : Google Scholar
|
|
13
|
Koshikawa N, Hayashi J, Nakagawara A and
Takenaga K: Reactive oxygen species-generating mitochondrial DNA
mutation up-regulates hypoxia-inducible factor-1alpha gene
transcription via phosphatidylinositol 3-kinase-Akt/protein kinase
C/histone deacetylase pathway. J Biol Chem. 284:33185–33194. 2009.
View Article : Google Scholar
|
|
14
|
Xu K, Ding Q, Fang Z, et al: Silencing of
HIF-1alpha suppresses tumorigenicity of renal cell carcinoma
through induction of apoptosis. Cancer Gene Ther. 17:212–222. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tanaka H, Yamamoto M, Hashimoto N, et al:
Hypoxia-independent overexpression of hypoxia-inducible factor
1alpha as an early change in mouse hepatocarcinogenesis. Cancer
Res. 66:11263–11270. 2006. View Article : Google Scholar
|
|
16
|
Fang J, Ding M, Yang L, et al:
PI3K/PTEN/AKT signaling regulates prostate tumor angiogenesis. Cell
Signal. 19:2487–2497. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shi Y, Chen L, Li J, et al: Prognostic and
predictive values of pERK1/2 and pAkt-1 expression in non-small
cell lung cancer patients treated with adjuvant chemotherapy.
Tumour Biol. 32:381–390. 2011. View Article : Google Scholar
|
|
18
|
McCubrey JA, Steelman LS, Abrams SL, et
al: Roles of the RAF/MEK/ERK and PI3K/PTEN/AKT pathways in
malignant transformation and drug resistance. Adv Enzyme Regul.
46:249–279. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liang K, Lu Y, Li X, et al: Differential
roles of phosphoinositide-dependent protein kinase-1 and akt1
expression and phosphorylation in breast cancer cell resistance to
Paclitaxel, Doxorubicin, and gemcitabine. Mol Pharmacol.
70:1045–1052. 2006. View Article : Google Scholar
|
|
20
|
Abrams SL, Steelman LS, Shelton JG, et al:
Enhancing therapeutic efficacy by targeting non-oncogene addicted
cells with combinations of signal transduction inhibitors and
chemotherapy. Cell Cycle. 9:1839–1846. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang Y, Liu X, Zhang J, et al: The
expression and clinical significance of PI3K, pAkt and VEGF in
colon cancer. Oncol Lett. 4:763–766. 2012.PubMed/NCBI
|
|
22
|
Kim JG, Chae YS, Sohn SK, et al: Clinical
significance of genetic variations in the PI3K/PTEN/AKT/mTOR
pathway in Korean patients with colorectal cancer. Oncology.
79:278–282. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Isobe T, Aoyagi K, Koufuji K, et al:
Clinicopathological significance of hypoxia-inducible factor-1
alpha (HIF-1α) expression in gastric cancer. Int J Clin Oncol.
18:293–304. 2013.
|
|
24
|
Hu L, Hofmann J, Lu Y, et al: Inhibition
of phosphatidylinositol 3′-kinase increases efficacy of paclitaxel
in in vitro and in vivo ovarian cancer models. Cancer Res.
62:1087–1092. 2002.
|
|
25
|
Xu R, Nakano K, Iwasaki H, et al: Dual
blockade of phosphatidylinositol 3′-kinase and mitogen-activated
protein kinase pathways overcomes paclitaxel-resistance in
colorectal cancer. Cancer Lett. 306:151–160. 2011.
|
|
26
|
Kim SH, Juhnn YS and Song YS: Akt
involvement in paclitaxel chemoresistance of human ovarian cancer
cells. Ann N Y Acad Sci. 1095:82–89. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zeng L, Kizaka-Kondoh S, Itasaka S, et al:
Hypoxia inducible factor-1 influences sensitivity to paclitaxel of
human lung cancer cell lines under normoxic conditions. Cancer Sci.
98:1394–1401. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fekete M, Santiskulvong C, Eng C and
Dorigo O: Effect of PI3K/Akt pathway inhibition-mediated G1 arrest
on chemosensitization in ovarian cancer cells. Anticancer Res.
32:445–452. 2012.PubMed/NCBI
|
|
29
|
Shafee N, Kaluz S, Ru N and Stanbridge EJ:
PI3K/Akt activity has variable cell-specific effects on expression
of HIF target genes, CA9 and VEGF, in human cancer cell lines.
Cancer Lett. 282:109–115. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhong H, Chiles K, Feldser D, et al:
Modulation of hypoxia-inducible factor 1alpha expression by the
epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP
pathway in human prostate cancer cells: implications for tumor
angiogenesis and therapeutics. Cancer Res. 60:1541–1545. 2000.
|
|
31
|
Jiao M and Nan KJ: Activation of PI3
kinase/Akt/HIF-1α pathway contributes to hypoxia-induced
epithelial-mesenchymal transition and chemoresistance in
hepatocellular carcinoma. Int J Oncol. 40:461–468. 2012.
|
|
32
|
Manohar SM, Padgaonkar AA, Jalota-Badhwar
A, et al: A novel inhibitor of hypoxia-inducible factor-1α P3155
also modulates PI3K pathway and inhibits growth of prostate cancer
cells. BMC Cancer. 11:3382011.
|