Introduction
Chromosomal rearrangements involving the mixed
lineage leukemia (MLL) gene are the most common genetic alterations
in acute leukemia, particularly in infants (1), and the majority of the rearrangements
indicate a poor prognosis (2).
Reciprocal translocation represents the most
frequent form of MLL rearrangement (3,4). All
the chromosomal breaks occur in the 8.3-kb breakpoint cluster
region within the MLL gene between introns 8 and 13 (5). To date, >60 translocation gene
partners have been identified (3,4). In
pediatric and adult acute myeloid leukemia (AML), the most frequent
fusion partners are represented by MLLT3/AF9 (9p22), MLLT10/AF10
(10p12), ELL (19p13.1), MLLT4/AF6 (6q27) and MLLT1/ENL (19p13.3)
(4), however, t(11;22)(q23;q11) is
rare.
In total, eight cases of AML with 11q23
rearrangement involving the 22q11 region as the partner have
previously been reported (6–12);
among these cases, only one was adult AML-M2. The current report
presents a new case of AML-M2 in a 32-year-old male patient and
reviews previous cases in the literature. Patient provided written
informed consent.
Case report
In August 2012, a 32-year-old male was admitted to
the Department of Hematology (Beijing Chaoyang Hospital, Beijing,
China) with asthenia, nausea, vomiting, spontaneous ecchymosis, a
cough and a fever. The patient’s hemoglobin level was 7.2 g/dl,
platelet count was 13×109/liter and white blood cell
count was 2.44×109/liter. Bone marrow smears were
hypercellular containing 61.5% myeloblasts, 7% promonocytes and
2.5% monoblasts. In addition, the blasts in the bone marrow were
positive for peroxidase staining. Immunophenotypic analysis
revealed that the blasts were positive for cluster of
differentiation (CD)117, CD33 and CD64, weakly positive for CD15,
human leukocyte antigen-DR, myeloperoxidase and CD13, and negative
for CD22, CD56, cCD3, CD11b, CD14, CD19, CD34 and CD7. The patient
had been well prior to the development of these symptoms, had no
history of exposure to organic solvents or dye, and had never
received irradiation or anticancer agents.
The diagnosis was determined as AML with maturation
(French-American-British classification of M2). The patient
commenced a standard 7+3 schedule with cytarabine and daunorubicin
as an induction therapy. Following the achievement of complete
remission (CR), the patient underwent consolidation chemotherapy
followed by a medium dose of cytarabine. The patient relapsed
following three cycles of medium-dose cytarabine and subsequently
received salvage therapy, however, the patient succumbed to a
cerebral hemorrhage in March 2013.
A bone marrow sample was processed following
short-term culture (24 h) according to the standard procedures. The
chromosomes were stained by G-banding and the karyotype was
determined according to recommendations from the International
System for Human Cytogenetic Nomenclature (2009) (13).
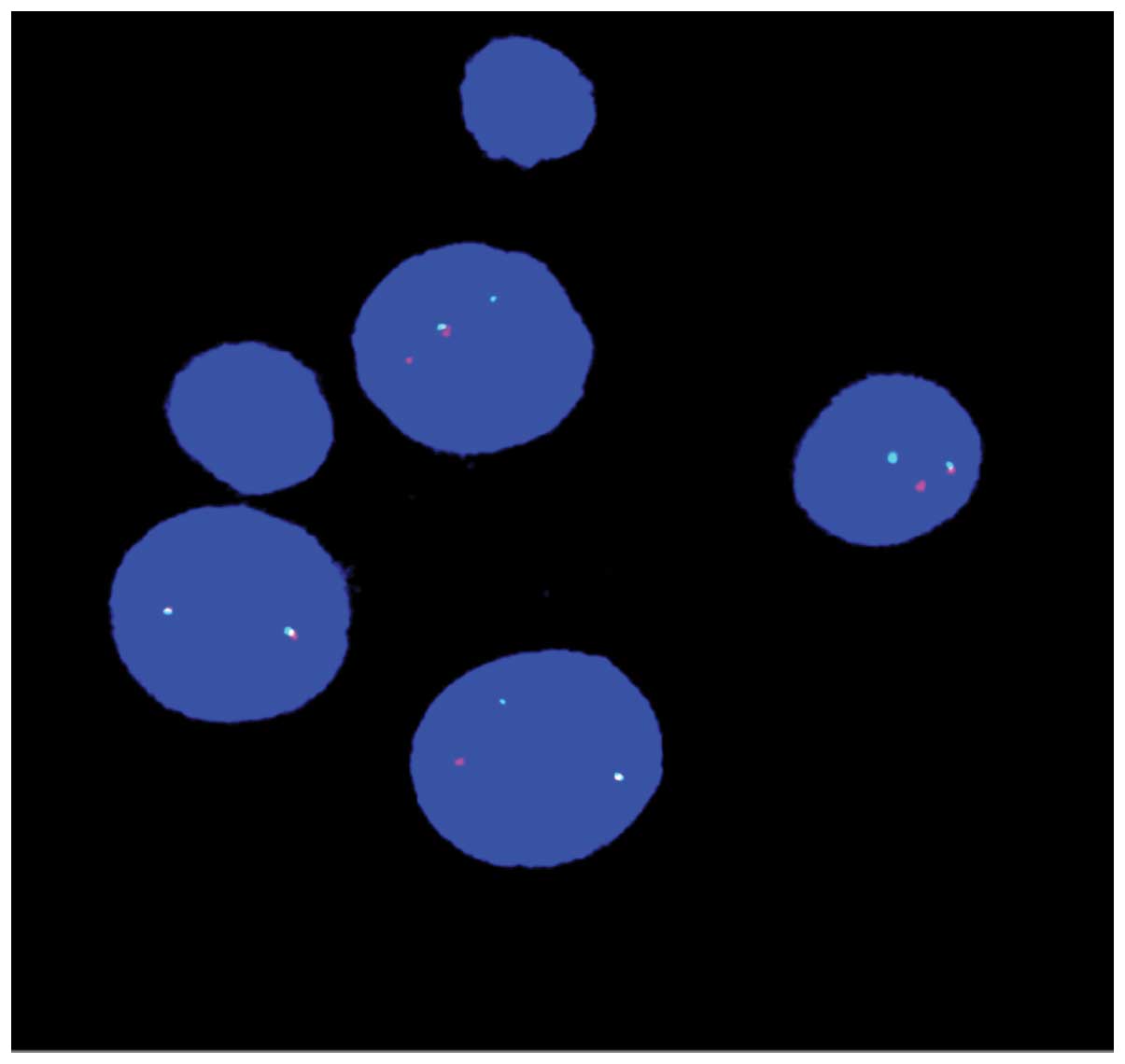
Fluorescent in situ hybridization (FISH) was
performed on 200 interphase cells using the Vysis LSI MLL dual
color break apart translocation probes (Abbott Molecular, Inc., Des
Plaines, IL, USA).
RNA was extracted from the patient’s peripheral
blood cells using the Whole Blood RNA isolation kit (BioChain
Institute, Inc., Newark, CA, USA). In total, 1–2 μg of RNA was
reverse transcribed to cDNA using the Thermo Scientific Maxima
First-Strand cDNA synthesis kit (Thermo Fisher Scientific, Waltham,
MA, USA). cDNA solution (2 μl) was amplified by polymerase chain
reaction (PCR) to a total volume of 20 μl with 0.5 μM of each
primer and 10 μl master mix. The primers used for the first reverse
transcription (RT)-PCR were as follows: Sense, TACAGGACCGCCAAGAA
for MLL-5S; and antisense, TTGGGCAGCTTCACGAAGTC for SEPT-5A. The
nucleotide sequences of the PCR products were determined by a
BigDye Terminator v3.1 cycle sequencing kit (Invitrogen Life
Technologies, Paisley, UK).
The karyotype of the bone marrow cells from the
patient was identified as 46,XY,t(11;22)(q23;q11.2)[13]/46,X,
−Y,+10,t(11;22)(q23;q11.2)[7]/47,XY,+10,t(11;22)(q23;q11.2)[1]/46,XY[1]
in the 22 cells that were examined (Fig. 1). FISH analysis using an
MLL-specific probe showed a split in the MLL gene (Fig. 2). This result indicated that the
gene was involved in this translocation.
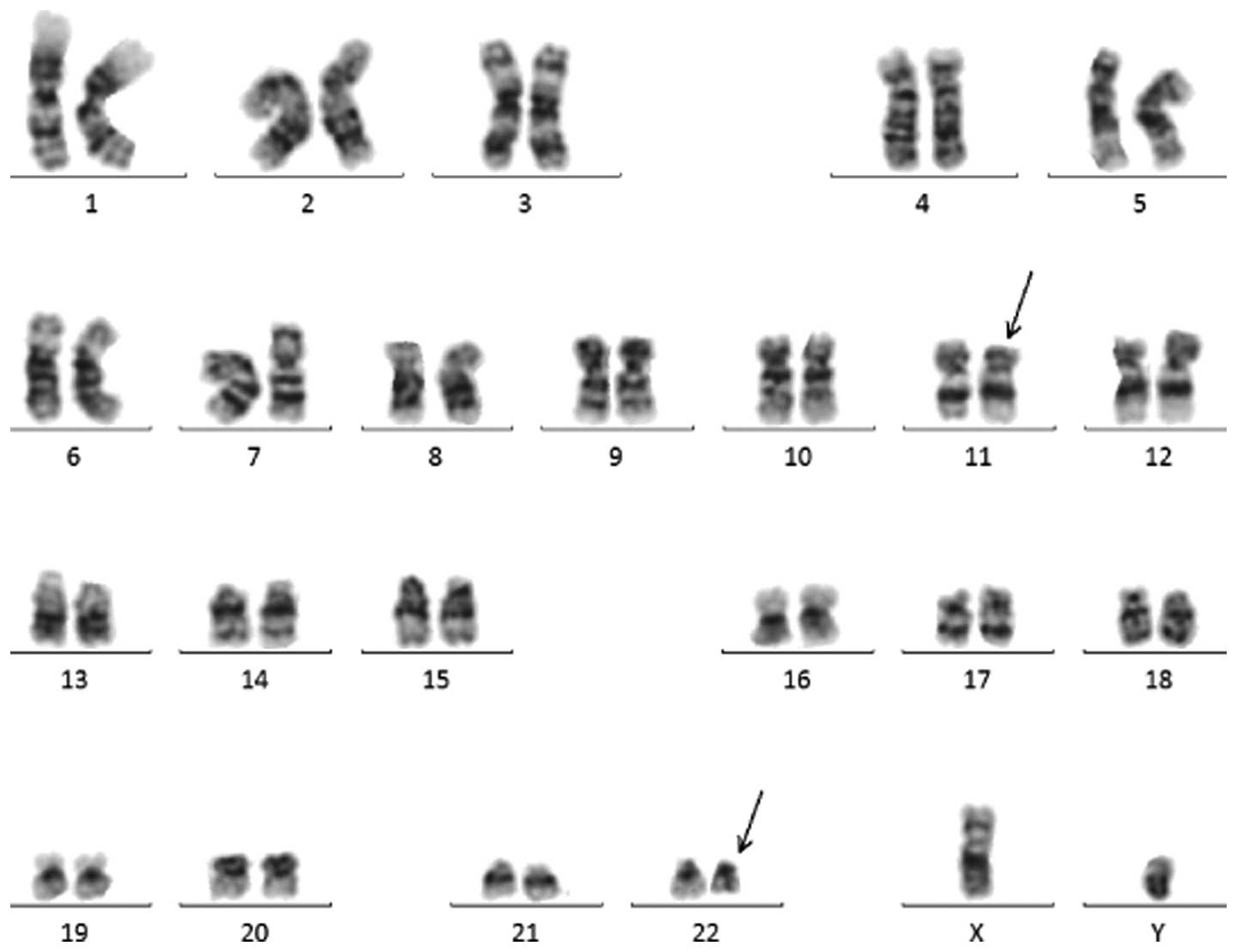 | Figure 1Representative karyotype of the bone
marrow cells was 46,XY,t(11;22)(q23;q11.2)[13]/46,X,-Y,+10,
t(11;22)(q23;q11.2)[7]/47,XY,+10,t(11;22)(q23;q11.2)[1]/46,XY[1].
Arrows indicate the derivative chromosomes 11 and 22. |
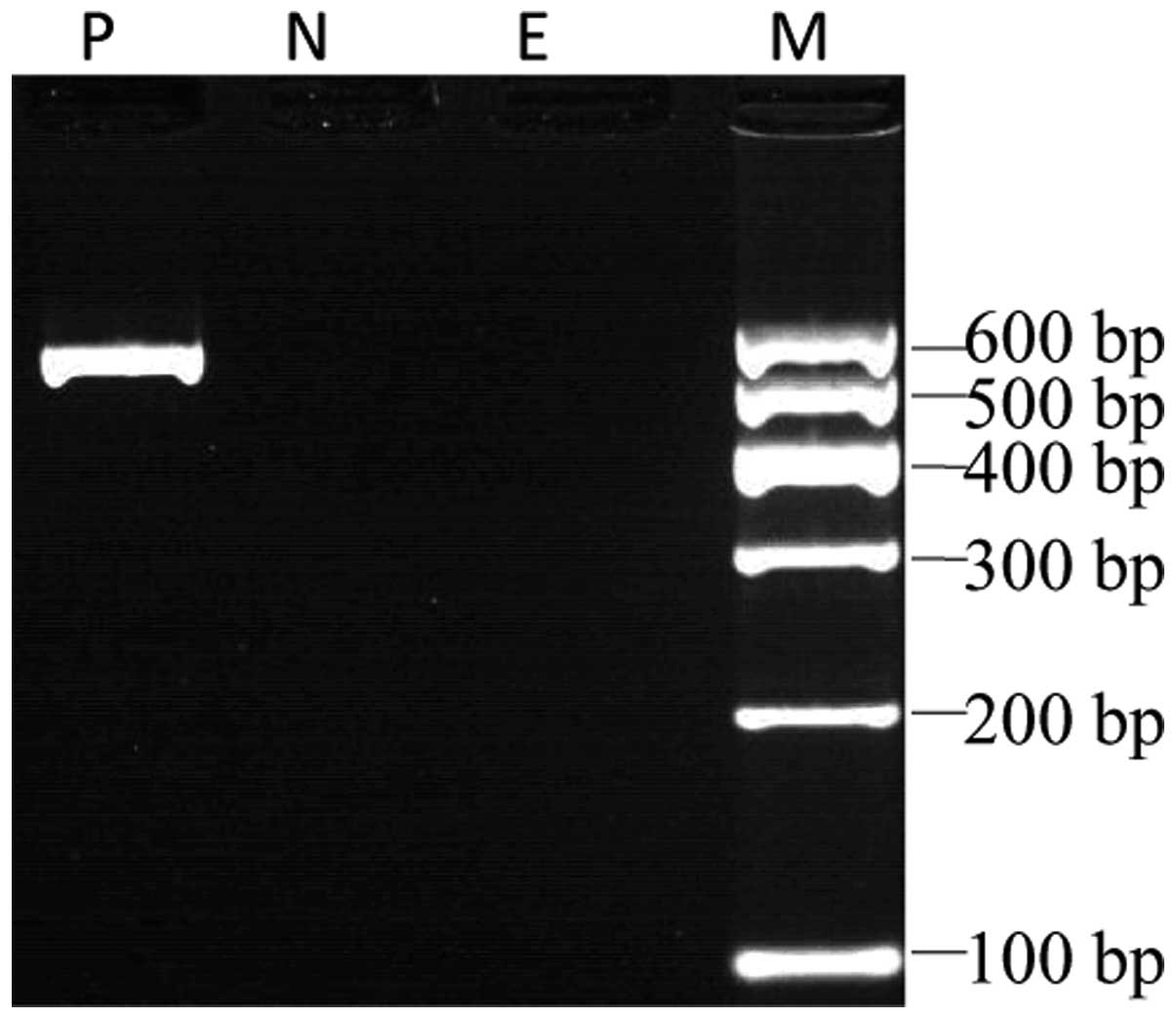
To demonstrate an MLL-septin 5 (SEPT5) fusion
transcript in the current t(11;22)-AML patient, RT-PCR analysis was
performed using the MLL-5S primer and an antisense primer, SEPT-5A
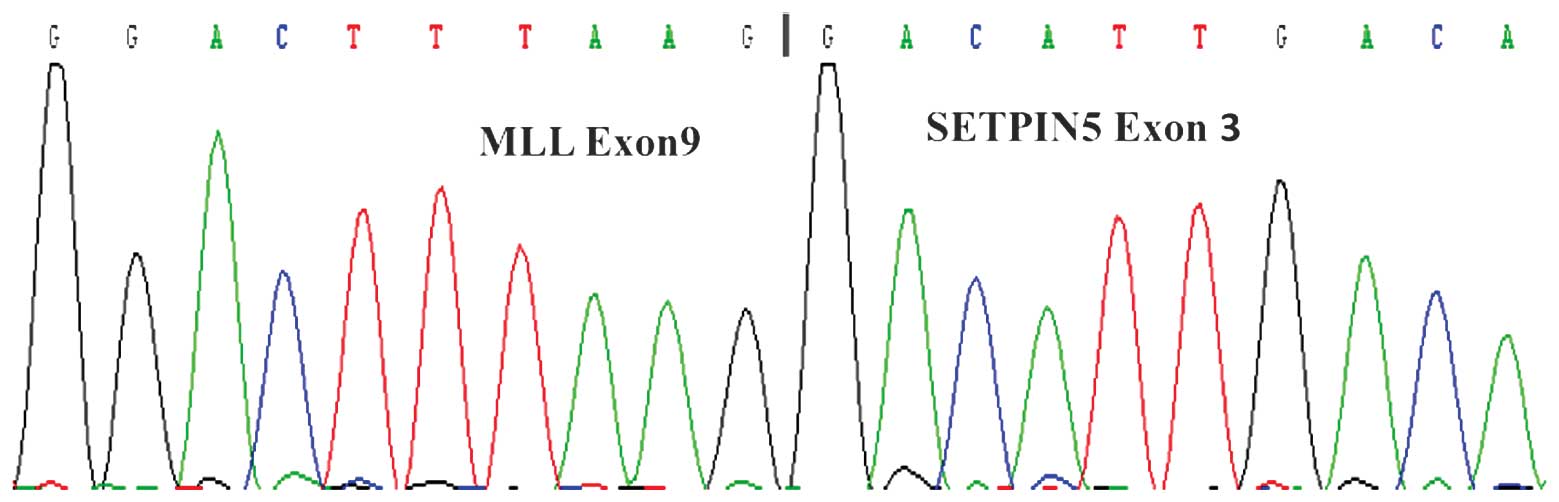
(Fig. 3). Nucleotide sequencing
analysis of the PCR product demonstrated the fusion between MLL
exon 9 and SEPT5 exon 3, and the product was 521 bp in length
(Fig. 4).
Discussion
The present study describes an adult patient who
presented with de novo AML-M2 with t(11;22)(q23;q11.2),
which resulted in fusion of the MLL gene to the SEPT5 gene. The
first case of adult AML-M2 with this translocation was reported in
2001 (8), and to the best of our
knowledge, the current study presents the second case of reported
adult AML-M2 with t(11;22). Although the patient exhibited a
positive response to the induction therapy of cytarabine plus
daunorubicin and achieved CR, the patient relapsed following three
cycles of consolidation chemotherapy. The duration of CR was
extremely short.
Several cases of AML with t(11;22)(q23;q11) have
previously been reported. A 66-year-old female was diagnosed with
AML-M5 with t(11;22)(q23;q11.2) and received two cycles of
combination chemotherapy, including etoposide, cytosine
arabinoside, vinca alkaloids and mitoxantrone (12). Remission was achieved at two months
following chemotherapy, however, the CR lasted only six months. In
an additional report (7), a
39-year-old male was diagnosed with AML-M2 and the karyotype of the
bone marrow cells was 46,XY,t(11;22)(q23;q11) in all 20 cells that
were examined. The patient was treated with idarubicin and
cytarabine and achieved CR. However, the patient relapsed two
months later and eventually succumbed to the disease 12 months
following the first diagnosis without responding to chemotherapy;
the duration of CR was only two months. In addition, a 36-year-old
male was previously diagnosed with AML-M4 with t(11;22)(q23;q11)
(9). The patient was treated with
standard-dose cytarabine and daunorubicin and achieved CR. Although
the patient accepted high-dose cytarabine as a consolidation
therapy, CR was only 8.9 months and overall survival was 20.8
months. More recently, a 23-month-old female was diagnosed with
AML-M5 with t(11;22)(q23;q11). The patient was enrolled in the ELAM
02 protocol (aracytine, mitoxantrone and methotrexate) and achieved
CR following induction chemotherapy with mitoxantrone plus
cytarabine. According to the protocol, the patient received an
additional transplant of bone marrow from a sibling and the
duration of CR was more than two years (6). It appeared that the allogenic bone
marrow transplantation (BMT) overcame the impact of t(11;22)
q23;q11).
To date, only 32 cases of AML with MLL-SEPT fusions
have been reported in the literature (5,6),
including four cases of MLL-SEPT5 fusion (6,8,9). The
current study describes a novel case of AML with MLL-SEPT5 fusion.
Molecular studies demonstrated a fusion of the MLL exon 9 and SEPT5
exon 3. The four previously reported cases of the MLL-SEPT5
rearrangement presented the following fusions: MLL exon 7 and SEPT5
exon 3 for two patients (9); MLL
exon 10 and SEPT5 exon 3 for one patient (6); and MLL exon 6 and SEPT5 exon 4 for one
patient (8). Although the MLL-SEPT5
fusion transcripts identified in the current study are different to
those previously reported, it appeared that the SEPT5 exon 3 had a
greater frequency of involvement. Further studies are required to
understand the impact of such a fusion on prognosis.
In conclusion, although the AML patients with
t(11;22)(q23;q11) have exhibited positive responses to the
induction therapy, the duration of CR is relatively short under
conventional chemotherapy. An allogenic BMT appeared to overcome
the impact of t(11;22)(q23;q11), however, future studies are
required to confirm this.
References
|
1
|
Pui CH, Kane JR and Crist WM: Biology and
treatment of infant leukemias. Leukemia. 9:762–769. 1995.PubMed/NCBI
|
|
2
|
Balgobind BV, Raimondi SC, Harbott J, et
al: Novel prognostic subgroups in childhood 11q23/MLL-rearranged
acute myeloid leukemia: results of an international retrospective
study. Blood. 114:2489–2496. 2009. View Article : Google Scholar
|
|
3
|
Meyer C, Schneider B, Jakob S, et al: The
MLL recombinome of acute leukemias. Leukemia. 20:777–784. 2006.
View Article : Google Scholar
|
|
4
|
Meyer C, Kowarz E, Hofmann J, et al: New
insights to the MLL recombinome of acute leukemias. Leukemia.
23:1490–1499. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cerveira N, Bizarro S and Teixeira MR:
MLL-SEPTIN gene fusions in hematological malignancies. Biol Chem.
392:713–724. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Launay E, Henry C, Meyer C, et al:
MLL-SEPT5 fusion transcript in infant acute myeloid leukemia with
t(11;22)(q23;q11). Leuk Lymphoma. Jun 3–2013.(Epub ahead of
print).
|
|
7
|
Abdou SM, Jadayel DM, Min T, et al:
Incidence of MLL rearrangement in acute myeloid leukemia, and a
CALM-AF10 fusion in M4 type acute myeloblastic leukemia. Leuk
Lymphoma. 43:89–95. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tatsumi K, Taki T, Taniwaki M, et al: The
CDCREL1 gene fused to MLL in de novo acute myeloid
leukemia with t(11;22)(q23;q11.2) and its frequent expression in
myeloid leukemia cell lines. Genes Chromosomes Cancer. 30:230–235.
2001.
|
|
9
|
Megonigal MD, Rappaport EF, Jones DH, et
al: t(11;22)(q23;q11.2) in acute myeloid leukemia of infant twins
fuses MLL with hCDCrel, a cell division cycle gene in the genomic
region of deletion in DiGeorge and velocardiofacial syndromes. Proc
Natl Acad Sci USA. 11:6413–6418. 1998. View Article : Google Scholar
|
|
10
|
Baer MR, Stewart CC, Lawrence D, et al:
Acute myeloid leukemia with 11q23 translocations: myelomonocytic
immunophenotype by multiparameter flow cytometry. Leukemia.
12:317–325. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Marukawa O, Akao Y, Inazawa J, et al:
Molecular cloning of the breakpoint of t(11;22) (q23;q11)
chromosome translocation in an adult acute myelomonocytic
leukaemia. Br J Haematol. 92:687–691. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kobayashi H, Miyachi H, Ogawa T and Jimbo
M: Translocation t(11;22)(q23;q11) in an adult with acute
monoblastic leukemia. Jpn J Med. 29:527–532. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shaffer LG, Slovak ML and Campbell LJ: An
International System for Human Cytogenetic Nomenclature. S. Karger;
Basel: pp. 1382009
|